Abstract
Background
Cutaneous melanoma spreads preferentially through the lymphatic route and sentinel lymph node (SLN) status is regarded as the most important predictor of survival.
Aims
To evaluate whether tumour lymphangiogenesis and the expression of vascular endothelial growth factor C (VEGF‐C) is related to the risk of SLN metastasis and to clinical outcome in a case–control series of patients with melanoma.
Methods
Forty five invasive melanoma specimens (15 cases and 30 matched controls) were investigated by immunostaining for the lymphatic endothelial marker D2‐40 and for VEGF‐C. Lymphangiogenesis was measured using computer assisted morphometric analysis.
Results
Peritumorous lymphatic vessels were more numerous, had larger average size, and greater relative area than intratumorous lymphatics. The number and area of peritumorous and intratumorous lymphatics was significantly higher in melanomas associated with SLN metastasis than in non‐metastatic melanomas. No significant difference in VEGF‐C expression by neoplastic cells was shown between metastatic and non‐metastatic melanomas. Using logistic regression analysis, intratumorous lymphatic vessel (LV) area was the most significant predictor of SLN metastasis (p = 0.04). Using multivariate analysis, peritumorous LV density was an independent variable affecting overall survival, whereas the intratumorous LV area approached significance (p = 0.07).
Conclusions
This study provides evidence that the presence of high peritumorous and intratumorous lymphatic microvessel density is associated with SLN metastasis and shorter survival. The intratumorous lymphatic vessel area is the most significant factor predicting SLN metastasis. The tumour associated lymphatic network constitutes a potential criterion in the selection of high risk patients for complementary treatment and a new target for antimelanoma therapeutic strategies.
Keywords: D2‐40; lymphangiogenesis; melanoma, vascular endothelial growth factor C; sentinel lymph node
Despite early detection, both the incidence of and mortality from cutaneous melanoma are still increasing in most Western countries.1,2 Because about half of the patients develop the first metastasis within the regional lymph node,3 it is thought that the tumour spreads preferentially through the lymphatic system. The detection of micrometastases by sentinel lymph node (SLN) biopsy has prognostic value, and is regarded as the most important predictor of survival in patients without clinical evidence of nodal metastasis.4,5
“Recent investigations have shown that in many human tumours neoplastic cells induce de novo lymphangiogenesis through the sprouting and proliferation of new lymphatic vessels”
In the past, little attention has been paid to the contribution of tumour induced lymphangiogenesis to the metastatic process, because of the absence of specific markers for lymphatic vessels. Recently, however, the identification of molecular markers and growth factors specific for lymphatic vessels and the introduction of novel techniques for the isolation/characterisation of lymphatic endothelial cells, have increased interest in the field.6
In early studies, the lymphatic system was considered to be a passive conducting system for the diffusion of neoplastic cells.7,8,9,10 More recent investigations have shown that in many human tumours neoplastic cells induce de novo lymphangiogenesis through the sprouting and proliferation of new lymphatic vessels.11,12 The number of tumour associated lymphatics seems to correlate with lymph node metastasis in a large variety of human tumours.13,14,15,16,17 In addition, diverse animal tumour models have provided evidence that increased concentrations of lymphangiogenetic factors, including vascular endothelial growth factor C (VEGF‐C), promote new lymphatic vessel growth and lymphatic tumour spread to regional lymph nodes.18
Few data are available on the association between lymphatic microvessel density and prognosis in melanoma. Although a positive correlation between the number of lymphatics and lymph node macrometastasis has been reported,19,20 other studies showed that increased lymphatic vessel density (LVD) correlated with improved patient survival.21 Thus, at present the prognostic role of lymphangiogenesis in cutaneous melanoma remains controversial. Apart from methodological differences, it is possible that the discrepancy results from the relative ability of different molecular markers to identify lymphatic vessels. None of the previous studies analysed the correlation of lymphangiogenesis with SLN status.
In our present study, we have investigated the hypothesis that lymphangiogenesis correlates with the presence of SLN metastasis and with overall survival. For this purpose, we have used a recently developed antibody, D2‐40, which specifically labels the lymphatic endothelium, to characterise lymphatic vessels in a case–control matched series of cutaneous melanomas.
Materials and methods
Specimen selection
Patients were identified retrospectively from the melanoma unit database, department of dermatology, Hospital Clinic, Barcelona, Spain. All patients were submitted to SLN biopsy, according to previously described procedures,22 and gave informed consent.
In total, 15 patients affected by primary cutaneous melanoma with metastasis to SLN were matched with a group of 30 patients without SLN metastasis. Each case was paired with two controls and closely matched by tumour thickness and presence of ulceration, in addition to, when possible, Clark's level and histotype. As shown in table 1, cases and controls did not differ significantly with regard to mean age, sex, site, or additional histopathological parameters, including tumour growth phase, presence of regression, and inflammatory infiltrate. Histopathological slides of primary tumours were reviewed for the following parameters: tumour thickness, Clark's level, presence of ulceration, histotype, histological regression, vascular invasion, inflammatory infiltrate, and tumour growth phase. Table 1 summarises the clinical and pathological characteristics of the whole series. Most of the tumours were located on the extremities and trunk, whereas acral and head and neck melanomas were less common. This distribution reflects the fact that cutaneous melanomas occurring on the head and neck region are most frequently diagnosed as lentigo maligna melanomas, which, in our experience, are very seldom submitted to SLN biopsy. Conversely, the low number of acral melanomas simply reflects the overall anatomical distribution of cutaneous melanomas. Indeed, acral lentiginous melanomas in Western countries are rare tumours, representing approximately 6% of all cutaneous melanomas. This distribution is similar to other case–control series published in the literature on this specific subject.19
Table 1 Clinical and pathological characteristics of the whole series.
| SLN+ (n = 15) | SLN– (n = 30) | p Value | |
|---|---|---|---|
| Mean (range) age (years) | 63.3 (41–86) | 55.3 (29–88) | − |
| Sex | 0.20 | ||
| Male | 11 (73.3%) | 16 (53.3%) | |
| Female | 4 (26.7%) | 14 (46.7%) | |
| Site | 0.60 | ||
| Upper extremity | 2 (13.3%) | 7 (23.3%) | |
| Lower extremity | 4 (26.7%) | 7 (23.3%) | |
| Trunk | 8 (53.3%) | 11 (36.7%) | |
| Head and neck | 0 (0.0%) | 3 (10.0%) | |
| Acral regions | 1 (6.7%) | 2 (6.7%) | |
| Mean (range) thickness (mm) | 3.0 (0.8–6.2) | 2.9 (0.8–8) | − |
| Level | 0.19 | ||
| III | 10 (66.7%) | 21 (70.0%) | |
| IV | 5 (33.3%) | 5 (16.7%) | |
| V | 0 (0.0%) | 4 (13.3%) | |
| Ulceration | 0.64 | ||
| Absent | 11 (73.3%) | 22 (73.3%) | |
| Present | 4 (26.7%) | 8 (26.7%) | |
| Histotype | 0.93 | ||
| SSM | 9 (60.0%) | 17 (56.7%) | |
| NM | 5 (33.3%) | 10 (33.3%) | |
| ALM | 1 (6.7%) | 3 (10.0%) | |
| Regression | 0.32 | ||
| Absent | 10 (66.7%) | 24 (80.0%) | |
| Present | 5 (33.3%) | 6 (20.0%) | |
| Vascular invasion | 0.15 | ||
| Absent | 14 (93.3%) | 30 (100%) | |
| Present | 1 (6.7%) | 0 (0.0%) | |
| Tumour growth phase | 0.64 | ||
| Radial | 1 (6.7%) | 5 (16.7%) | |
| Vertical | 14 (93.3%) | 25 (83.3%) | |
| Inflammatory infiltrate | 0.53 | ||
| Absent/sparse | 6 (40.0%) | 16 (53.3%) | |
| Moderate/pronounced | 9 (60.0%) | 14 (46.7%) |
ALM, acral lentiginous melanoma; NM, nodular melanoma; SSM, superficial spreading melanoma.
The median follow up time for the 45 patients was 42 months (range, 1.4–63.3; SD, 15.7). Among the patients with metastatic disease, six developed subsequent regional lymph node and distant metastases, nine only locoregional relapse, and five distant metastasis without locoregional relapse. For distant metastasis, the most common sites were lung, the central nervous system, liver, and bone. Fourteen patients died of metastatic melanoma.
Immunohistochemistry
Paraffin wax embedded sections (4 μm in thickness) were dewaxed in Bio‐Clear (Bio‐Optica, Milan, Italy) and hydrated with a graded series of ethanol concentrations and water. After inactivation of endogenous peroxidase activity with 3.0% hydrogen peroxidase in distilled water for 20 minutes, slides were submitted to antigen retrieval with microwave pretreatment (Microwave MicroMED T/T Mega; Milestone, Bergamo, Italy) in Tris/EDTA/citrate buffer (pH 7.8) for 20 minutes. After blocking non‐specific antigen with normal horse serum (UltraVision, Labvision, Freemont, California, USA), the sections were incubated overnight at 4°C with primary goat polyclonal antibody anti‐VEGF‐C (sc‐7133; Santa Cruz Biotechnology Inc, Santa Cruz, California, USA) at a 1/100 dilution. VEGF‐C is a tumour lymphangiogenesis factor that binds to and specifically activates Flt‐4 and Flk‐1. The gene that encodes VEGF‐C has been localised to chromosome 4q34. Staining was achieved using a biotin conjugated antigoat secondary antibody (goat immunoglobulins; Dako A/S, Glostrup, Denmark) at a 1/100 dilution and streptavidin–peroxidase (UltraVision). The bound antibodies were visualised using 3,3′ diaminobenzidine (BioGenex, San Ramon, California, USA) as chromogen. Nuclei were lightly counterstained with Mayer's haematoxylin. A negative control was included with each run by substituting the primary antibody with non‐immune rabbit serum. As positive control, a sample of melanoma metastatic to a lymph node was used. The control sections were treated in parallel with the samples in the same run.
For D2‐40 immunostaining, slides were treated with normal goat serum (Ultravision kit; Labvision), followed by incubation with the monoclonal antibody D2‐40 (Signet Laboratories, Dedham, Massachusetts, USA; prediluted) overnight at 4°C. The D2‐40 antibody has been generated against an oncofetal antigen expressed in fetal testis and in testicular germ cell tumours, which has been successively recognised as a selective marker of lymphatic endothelium not reacting with blood vessel endothelium.23,24,25,26 This was followed by incubation with the secondary antibody (Ultravision kit; Labvision). The reaction was visualised using 3,3′ diaminobenzidine and the nuclei were lightly counterstained with haematoxylin.
VEGF‐C immunostained sections were independently assessed by three observers (DM, AF, and MS). The results were expressed according to semiquantitative criteria as follows: negative staining (score 0), 1–20% positive cells (score 1+), 21–50% positive cells (score 2+), and more than 50% positive cells (score 3+). The staining intensity was scored on a scale as weak, moderate, or strong. The level of concordance, expressed as percentage of agreement between the observers, was 88.8% (40 of 45 specimens). In the remaining five cases, unanimous concordance was reached upon revision and discussion.
Computer assisted morphometric analysis of tumour lymphatics
To determine LVD and lymph vessel size within and surrounding the tumour, sections stained with the D2‐40 antibody were examined using a Leica DM LB microscope (Leica Ltd, Cambridge, UK) at ×200 magnification. Two distinct sets of measurements were performed in each tumour section: three fields with the highest LVD (hot spots) were identified (1) within the tumour mass and (2) within an area of 500 μm from the tumour border. In each selected field the outline of each individual stained vessel was identified and traced using a computer aided image analysis system (Quantimetl Leica) to determine (1) LVD, defined as the number of vessel/mm2; (2) the average vessel area (LVA); and (3) the relative lymphatic vascular area, defined as the proportion of the vascular area in a field that is positively stained.19 The mean values of the measurements in three fields were used for statistical analysis.
Statistical analysis
The Mann‐Whitney test or Kruskal‐Wallis test were used to examine the association of lymphatic vessel parameters with clinicopathological parameters. Within different tissue samples, intratumorous and peritumorous lymphangiogenesis parameters were compared by means of a paired value Wilcoxon test. Multivariate logistic regression analysis was used to determine which clinicopathological factors were predictive of lymph node metastasis. Overall survival curves and disease free survival curves were obtained using the Kaplan–Meier method and compared using the log rank test. A p value < 0.05 was considered significant. Multivariate analysis (Cox proportional hazards model) was used to determine which variables had an independent effect on clinical outcome. All statistical tests were performed using SPSS software (release 10.0, SPSS Inc, Chicago, Illinois, USA).
Results
D2‐40 immunostaining
Immunohistochemistry for D2‐40 showed consistent staining of endothelial cells of lymphatic vessels, whereas endothelial cells rimming blood vessels were negative. Overall, a variable number of D2‐40 positive, irregularly shaped lymphatic vessels were detected in all cases. All the stained vessels were typically thin walled, lined by a single layer of attenuated endothelial cells lacking pericytes, and appeared to be partially collapsed. A few of them contained lymphocytes. Lymphatic vessels were seen both in the tumour mass concentrated in discrete hotspots within sheets of tumour cells and in the peritumorous area, indicating that D2‐40 positive vessels did not have a specific distribution pattern. Intratumorous lymphatics often showed numerous small or ill defined lumina that differed from the larger peritumorous lymphatics, which showed more dilated open lumina (figs 1, 2). In some cases, lymphatic vessels were seen in close association with mononuclear cells within the tumour mass and, more frequently, in the peritumorous stroma (fig 3). Tumour emboli were also seen within the D2‐40 positive peritumorous lymphatic channels (fig 4). No D2‐40 immunostaining was identified in tumour cells. In the adjacent normal skin, scattered D2‐40 positive vessels were present throughout the dermis and adipose tissue. Small sized lymphatic vessels were mostly confined to the superficial dermis, whereas the vessels located in the deep dermis, at the border between the dermis and the subcutis, and in the subcutaneous septa, often showed wider lumina and thicker connective tissue walls.

Figure 1 D2‐40 positive intratumorous lymphatics show numerous small lumina compressed by melanoma cells (arrows).

Figure 2 D2‐40 positive peritumorous lymphatics showed more dilated open lumina compared with intratumorous lymphatics (arrows).

Figure 3 D2‐40 positive lymphatic vessels are seen in close association with mononuclear cells within the tumour mass and, more frequently, in the peritumorous stroma (arrows).
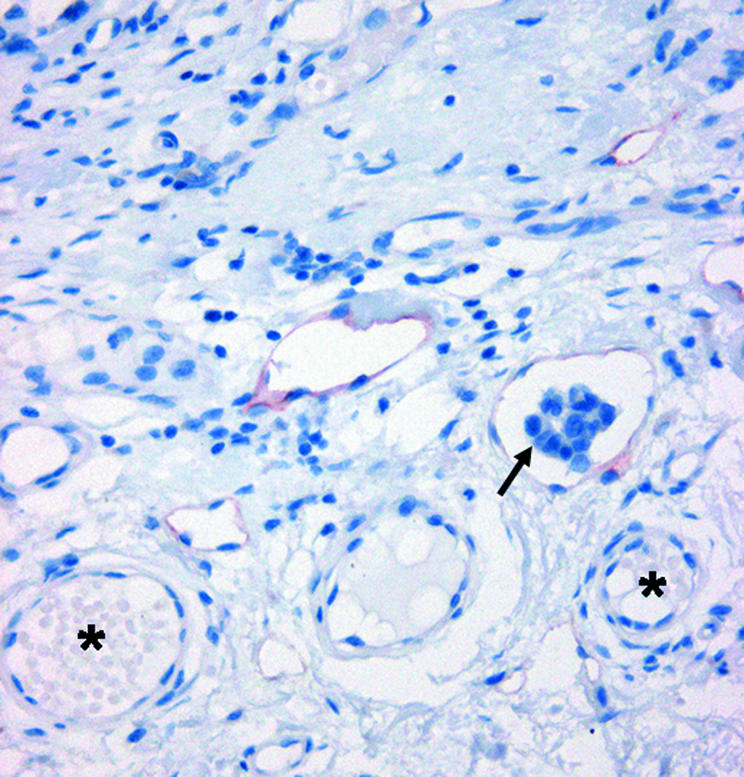
Figure 4 Tumour embolus within a D2‐40 positive peritumorous lymphatic channel (arrow). Note that adjacent capillary vessels containing red blood cells are unstained (asterisks).
D2‐40 computer assisted morphometric analysis
For the whole series, the mean intratumorous and peritumorous LVDs were 23.6 and 30.8, respectively. The mean intratumorous and peritumorous LVAs were 979.1 μm2 and 2617.4 μm2, respectively. Computer assisted morphometric analysis showed that peritumorous lymphatic vessels were more numerous, had a larger average size, and occupied a greater relative area than intratumorous lymphatics (p = 0.002, p < 0.0001, and p < 0.0001, respectively; Wilcoxon signed ranks test).
Correlation with clinicopathological parameters
Interestingly, tumours showing a moderate/pronounced inflammatory infiltrate showed a significantly higher LVD, both at the intratumorous (p = 0.04; Mann‐Whitney test) and peritumorous level (p = 0.05; Mann‐Whitney test). In addition, there was a significant correlation between LVA and ulceration and between LVA and tumour growth phase. Indeed, ulcerated melanomas were associated with a higher intratumorous and peritumorous LVA compared with non‐ulcerated melanomas (p < 0.0001 and p = 0.008, respectively; Mann‐Whitney test). Similarly, vertical growth phase melanomas were associated with a higher intratumorous and peritumorous LVA in comparison with radial growth phase melanomas (p = 0.003 and p = 0.006, respectively; Mann‐Whitney test).
No significant correlation was found between lymphangiogenesis parameters determined within or around melanomas and other clinicopathological variables, including thickness, level, and histotype (table 2).
Table 2 Lymphangiogenesis parameters related to conventional clinicopathological variables in 45 cutaneous melanomas.
| Variable | N | Mean (SD) intratumorous LVD | p Value | Mean (SD) intratumorous LVA (μm2) | p Value | Mean (SD) peritumorous LVD | p Value | Mean (SD) peritumorous LVA (μm2) | p Value |
|---|---|---|---|---|---|---|---|---|---|
| Age | 0.006 | 0.02 | 0.11 | 0.18 | |||||
| ⩽58 | 21 | 17.27 (11.04) | 588.73 (649.48) | 27.59 (14.07) | 2442.80 (2206.05) | ||||
| >58 | 24 | 29.27 (15.53) | 1320.81 (1472.52) | 33.72 (14.14) | 2770.22 (1616.62) | ||||
| Sex | 0.32 | 0.18 | 0.44 | 0.92 | |||||
| Male | 27 | 25.27 (15.38) | 1105.51 (1307.89) | 29.35 (12.73) | 2514.19 (1580.06) | ||||
| Female | 18 | 21.27 (13.87) | 789.66 (1050.43) | 33.11 (16.47) | 2772.27 (2339.09) | ||||
| Site | 0.15 | 0.56 | 0.84 | 0.80 | |||||
| Upper extremity | 9 | 21.08 (13.66) | 631.48 (463.89) | 28.50 (15.09) | 2114.46 (1373.86) | ||||
| Lower extremity | 11 | 21.65 (14.38) | 726.06 (600.08) | 28.78 (14.77) | 2194.92 (1269.03) | ||||
| Trunk | 19 | 22.07 (15.16) | 917.26 (1065.21) | 31.53 (13.98) | 3085.74 (2433.10) | ||||
| Head and neck | 3 | 34.40 (8.40) | 2775.79 (3056.31) | 35.53 (6.92) | 2289.12 (1540.16) | ||||
| Acral regions | 3 | 38.17 (17.70) | 1545.78 (1919.60) | 36.63 (23.35) | 3037.79 (1854.07) | ||||
| Thickness (mm) | 0.65 | 0.25 | 0.88 | 0.16 | |||||
| ⩽1.0 | 6 | 21.08 (16.95) | 655.12 (617.05) | 34.41 (19.28) | 2209.67 (1155.04) | ||||
| 1.01–2.0 | 12 | 19.68 (9.37) | 568.96 (601.02) | 29.68 (13.88) | 3111.75 (2541.82) | ||||
| 2.01‐4.0 | 19 | 27.15 (15.59) | 1137.77 (1517.26) | 30.15 (14.80) | 1967.76 (1278.24) | ||||
| >4.0 | 8 | 23.31 (18.36) | 1460.86 (1309.97) | 31.63 (11.66) | 3724.71 (2061.08) | ||||
| Level | 0.53 | 0.57 | 0.58 | 0.82 | |||||
| III | 31 | 23.84 (14.73) | 1052.39 (1398.92) | 30.18 (13.57) | 2711.43 (2007.76) | ||||
| IV | 10 | 26.82 (15.86) | 935.76 (663.60) | 35.00 (16.12) | 2381.03 (1234.00) | ||||
| V | 4 | 14.45 (11.26) | 520.24 (450.96) | 25.80 (16.62) | 2479.90 (2774.74) | ||||
| Ulceration | 0.15 | 0.0001 | 0.25 | 0.008 | |||||
| Absent | 33 | 21.48 (13.78) | 636.47 (738.69) | 29.57 (15.02) | 2409.99 (1983.74) | ||||
| Present | 12 | 29.69 (16.30) | 1921.60 (1713.72) | 34.40 (11.91) | 3187.86 (1578.02) | ||||
| Histotype | 0.25 | 0.73 | 0.79 | 0.43 | |||||
| SSM | 26 | 20.42 (12.73) | 850.57 (967.93) | 30.61 (13.89) | 2700.39 (2188.45) | ||||
| NM | 15 | 26.86 (16.50) | 1112.61 (1505.47) | 29.98 (14.52) | 2328.31 (1444.93) | ||||
| ALM | 4 | 32.78 (18.03) | 1314.65 (1364.09) | 35.80 (19.13) | 3162.30 (1534.18) | ||||
| Regression | 0.82 | 0.30 | 0.49 | 1.0 | |||||
| Absent | 34 | 23.20 (14.45) | 927.65 (1259.40) | 29.97 (14.40) | 2393.58 (1214.22) | ||||
| Present | 11 | 25.11 (16.35) | 1138.43 (1077.02) | 33.60 (14.25) | 3309.30 (3209.70) | ||||
| Tumour growth phase | 0.44 | 0.003 | 0.38 | 0.006 | |||||
| Radial | 6 | 17.73 (13.09) | 331.32 (403.12) | 25.51 (11.28) | 1447.87 (1130.39) | ||||
| Vertical | 39 | 24.58 (14.95) | 1078.84 (1262.75) | 31.68 (14.64) | 2797.36 (1938.43) | ||||
| Inflammatory infiltrate | 0.04 | 0.96 | 0.05 | 0.82 | |||||
| Absent/sparse | 22 | 18.99 (12.62) | 995.73 (1336.99) | 26.64 (12.80) | 2544.79 (1446.31) | ||||
| Moderate/pronounced | 23 | 28.14 (15.53) | 963.33 (1102.90) | 34.89 (14.73) | 2686.90 (2281.23) | ||||
| SLN | 0.04 | 0.001 | 0.003 | 0.007 | |||||
| Negative | 30 | 19.01 (10.30) | 717.97 (1182.75) | 26.86 (13.50) | 2075.86 (1450.36) | ||||
| Positive | 15 | 32.98 (18.07) | 1501.56 (1121.29) | 38.86 (12.69) | 3700.55 (2255.86) | ||||
| Status | 0.30 | 0.06 | 0.07 | 0.13 | |||||
| Alive | 31 | 21.90 (13.33) | 900.62 (1327.46) | 28.24 (13.07) | 2282.78 (1497.54) | ||||
| Dead | 14 | 27.57 (17.45) | 1153 (912.77) | 36.65 (15.6) | 3358.43 (2483.89) |
ALM, acral lentiginous melanoma; LVA, lymphatic vessel area; LVD, lymphatic vessel density; NM, nodular melanoma; SLN, sentinel lymph node; SSM, superficial spreading melanoma.
Correlation with SLN status
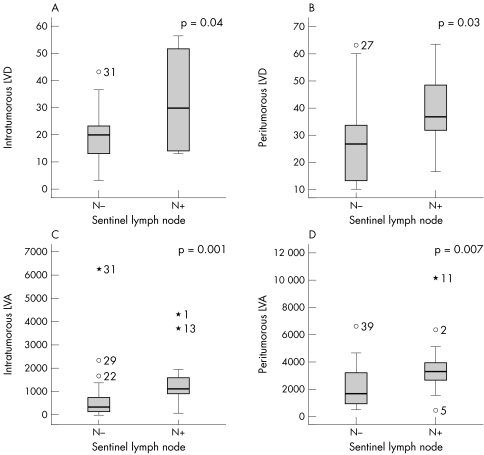
Cases associated with SLN metastasis showed a significantly higher LVD both in the tumour mass and in the peritumorous area (p = 0.04 and p = 0.003, respectively; Mann‐Whitney test; fig 5A, B). The same positive correlation was found when the LVA was considered as a parameter to describe lymphangiogenesis (p = 0.001 for intratumorous lymphatics and p = 0.007 for peritumorous lymphatics; Mann‐Whitney test; fig 5C, D). Similarly, the relative lymphatic vascular area both within and around the tumour was higher in metastatic tumours than in non‐metastatic melanomas (p = 0.001 and p = 0.009, respectively; Mann‐Whitney test). Intratumorous lymphatic vessels showed a higher average vessel size in metastatic melanomas than in non‐metastatic tumours (p = 0.03, Mann‐Whitney test). Conversely, there was no significant difference in average vessel size around the tumour between metastatic and non‐metastatic melanomas (p = 0.3, Mann‐Whitney test).
Figure 5 Tumours associated with sentinel lymph nodes showed a significantly higher lymphatic vessel density (LVD) both in (A) the tumour mass and (B) the peritumorous area (p = 0.04 and p = 0.003, respectively), in addition to significant (C) intratumorous and (D) peritumorous lymphatic vessel area (LVA; p = 0.001 and p = 0.007, respectively), in comparison with non‐metastatic melanomas.
To determine which of the parameters studied best predicted the presence of SLN metastasis, we performed logistic regression analysis including lymphangiogenesis parameters. Because our study was originally constructed as a case–control study, the variables for which the cases were matched were not included in the analysis. The results showed that melanomas with high intratumorous LVA (median value as cutoff) have a significantly higher risk of SLN metastasis (p = 0.04; odds ratio, 20.12; 95% confidence interval (CI), 1.12 to 358.39), whereas other lymphangiogenetic parameters failed to reach significance.
Survival analysis
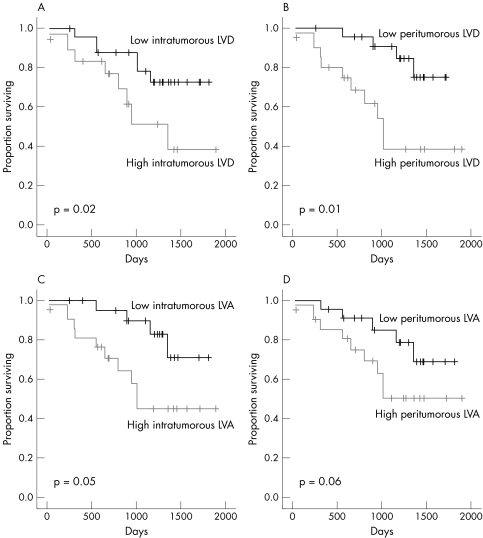
We then explored the effect of lymphangiogenesis parameters on overall survival using the median value as the cutoff value. Figure 6 shows the Kaplan–Meier estimates of cause specific survival according to intratumorous and peritumorous LVD and LVA. High intratumorous and peritumorous LVD were significantly associated with shorter overall survival (p = 0.02 and p = 0.01, respectively).
Figure 6 Kaplan–Meier estimates of cause specific survival according to (A) intratumorous lymphatic vessel density (LVD; ⩽ 20 v > 20) and (B) peritumorous LVD (⩽ 30 v > 30). Kaplan–Meier estimates of cause specific survival by (C) intratumorous lymphatic vessel area (LVA; ⩽ 652 μm2v > 652 μm2) and (C) peritumorous LVA (⩽ 2310.49 μm2v > 2310.49 μm2).
Using multivariate analysis (Cox proportional hazards model), peritumorous LVD was the only independent prognostic variable affecting overall survival (p = 0.01; hazard ratio (HR), 12.2; 95% CI, 1.62 to 92.02), whereas male sex (p = 0.05; HR, 6.7; 95% CI, 0.94 to 48.28) and intratumorous LVA (p = 0.07; HR, 10.93; 95% CI, 0.75 to 157.85) approached significance.
VEGF‐C immunostaining
As assessed by immunohistochemistry, 40 of 45 melanoma samples expressed cytoplasmic VEGF‐C, whereas the reaction was judged technically not evaluable in the other five cases. Overall, 28 of 40 cases expressed VEGF‐C in more than 20% of the neoplastic cells. However, there was considerable heterogeneity in the intensity of staining and expression patterns. In most cases, melanoma cells were moderately positive for VEGF‐C (fig 7), but in a few cases staining in the melanoma cells was weak. Adjacent VEGF‐C positive and negative melanoma cells had similar cell morphology. Melanoma cells close to areas with an inflammatory infiltrate had similar VEGF‐C expression to those not in close proximity to areas of inflammation. In some invasive melanomas we found that neoplastic melanocytes showed a progressive decrease in the intensity of VEGF‐C immunoreactivity as they descended into the deeper portions of the reticular dermis.
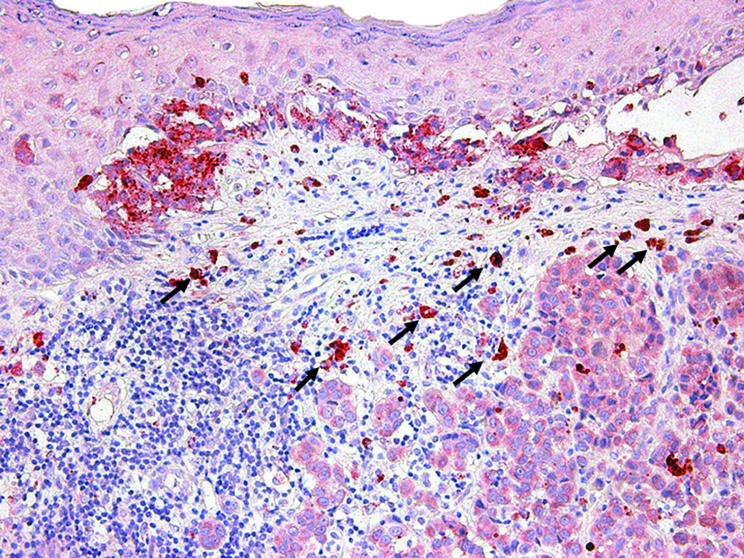
Figure 7 Immunohistochemical staining for vascular endothelial growth factor C (VEGF‐C) within the neoplastic cells of a thin cutaneous melanoma, both within the in situ and invasive components. Note that VEGF‐C expression is also detected within epidermal keratinocytes overlying melanoma cells, scattered dermal fibroblasts, and macrophages (arrows).
VEGF‐C expression was also detected in epidermal keratinocytes overlying melanoma cells, scattered dermal fibroblasts, and tumour associated macrophages in the context of the inflammatory responses in and around the tumour. VEGF‐C staining in tumour associated macrophages was stronger than in the adjacent melanoma cells (fig 8).
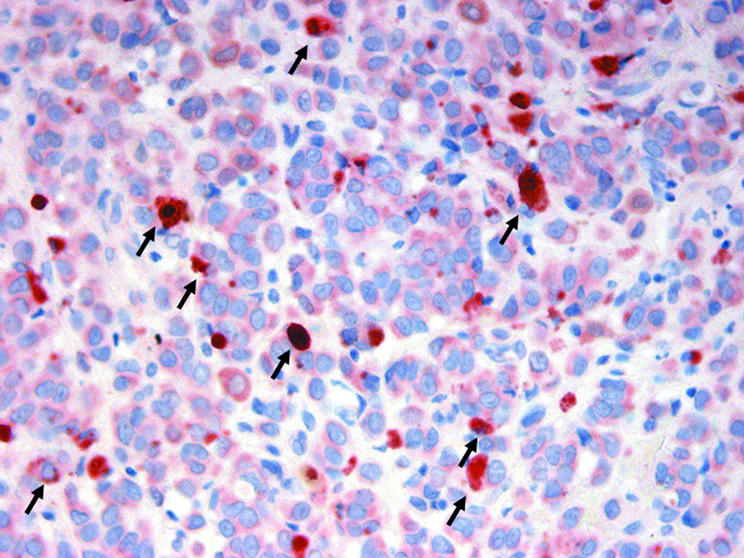
Figure 8 Vascular endothelial growth factor C immunostaining was stronger in tumour associated macrophages (arrows) than in adjacent melanoma cells.
VEGF‐C overexpression was seen more frequently in melanomas metastatic to the SLN, but this did not reach significance. No significant correlation was found between VEGF‐C expression and other clinicopathological parameters (data not shown).
Discussion
In our present study, we found that melanomas with positive SLN showed higher peritumorous and intratumorous LVD and LVA compared with non‐metastatic melanomas. Furthermore, our analysis showed that intratumorous LVA was the best predictor of metastasis to the SLN. A role for lymphangiogenesis in the metastatic process has been proposed.13,14,15,16,17 Although the mechanism has not been fully elucidated, it is possible that the increase in lymphatic surface area increases the chances of intravasation and subsequent dissemination of neoplastic cells. We found that tumour emboli within lymphatics are seen only occasionally and, in general, they are more common within lymphatic vessels in the peritumorous area. However, morphological observation by histology is limited by the fact that it detects events at a fixed time point. Thus, we cannot exclude the possibility that peritumorous neoplastic emboli may originate from the initial invasion of intratumorous lymphatics. Lymphatics in the peritumorous area have been proposed to play a more relevant role in the process of lymphatic neoplastic dissemination.13,27,28 In addition, studies in experimental animals have shown that the lymphatic vessels in the central portion of tumours are often compressed and non‐functional, whereas those at the periphery are enlarged and perfused.10 We found that peritumorous lymphatics were more numerous and of larger size than intratumorous lymphatics, but we have no information with regard to their functional state. As in other types of human tumours, where intratumorous and peritumorous lymphatics seem to interconnect, it is possible that in melanoma the neoplastic spread to lymph nodes involves both intratumorous and peritumorous lymphatics.14
The possible clinical relevance of lymphangiogenesis in melanoma has been widely discussed.7,9,19,20,21,22,23,24,25,26,27,28,29,30 A few studies19,20,21,30 have analysed lymphangiogenesis and melanoma prognosis. In these studies,19,20,30 with one exception,21 LVD was associated with tumour progression and poor prognosis. Dadras et al selected 19 non‐metastatic melanomas closely matched with 18 patients with early lymph node macrometastasis and found that the incidence of intratumorous lymphatic endothelial hyaluronan receptor 1 (LYVE‐1) positive lymphatics was significantly higher in metastatic melanomas and correlated with poor disease free survival.19 In the same study, metastatic melanomas had significantly larger lymphatic vessels, and a relative LVA of > 1.5% was significantly associated with poor disease free and overall survival.19 The study by Shields et al on a series of 21 melanomas stained with LYVE‐1 reported that LVD was significantly increased in tumours that developed metastases.20 Valencak et al evaluated the lymphatic network with the use of podoplanin antibody in 120 melanomas and found that patients with a high LVD had a significantly shorter overall and disease free survival by univariate analysis, although multivariate analysis showed that it had less prognostic significance than blood vessel density.30 In contrast, Straume et al evaluated both intratumorous and peritumorous LVD by LYVE‐1 and podoplanin in a large series of 175 nodular melanomas and provided evidence that increased LVD was associated with an improved survival rate by multivariate analysis.21 These discrepancies have raised discussion on methodological differences among the various studies, focused on LVD detection by the hot spot selection versus the median absolute LVD, computer assisted morphometric analysis versus non‐computerised counting, selection of early versus late stage melanomas, different clinical end points, and the use of different markers.31
To date, several markers for the lymphatic endothelium have been proposed, including basement membrane components, constituents of junctional complexes such as desmoplakin, and enzymes such as 5′ nucleotidase. In addition, several cell surface molecules are thought to be specifically expressed, including PAL‐E, VEGF receptor 3, podoplanin, and LYVE‐1. However, several of the lymphatic markers require further characterisation to demonstrate their specificity, and some have proved to be unreliable.32 In our present study, we used the recently developed monoclonal antibody D2‐40, which specifically reacts with a fixation resistant epitope on lymphatic endothelium and has previously been shown to recognise lymphatic vessels in neoplastic tissues, including melanoma.25,26,29 It should be noted that D2‐40 is a selective marker of the lymphatic endothelium; however, it is not tissue specific. Indeed, it was previously reported to react with an oncofetal antigen expressed in fetal testis and on the surface of testicular germ cell tumours23 and, recently, it has been identified as a mesothelial marker in malignant mesotheliomas.33 Our study is the first case–control study designed to analyse the risk of melanoma SLN metastasis and overall survival using this antibody and computer assisted morphometric analysis to evaluate lymphangiogenesis.
Take home messages
In cutaneous melanoma, the presence of high peritumorous and intratumorous lymphatic microvessel density was associated with sentinel lymph node (SLN) metastasis and shorter survival
The intratumorous lymphatic vessel area was the most significant factor predicting SLN metastasis
There was no significant difference in vascular endothelial growth factor C expression by neoplastic cells between metastatic and non‐metastatic melanomas
The tumour associated lymphatic network might constitute a potential criterion in the selection of high risk patients for complementary treatment and a new target for antimelanoma therapeutic strategies
“The number of lymphatic vessels directly correlated with the intensity of the inflammatory infiltrate in and around the tumour”
We found that ulcerated melanomas were associated with a significantly higher intratumorous and peritumorous LVA compared with non‐ulcerated melanomas. It is conceivable that in ulcerated melanomas the enlargement of the lymphatic vessels may result from the release of VEGFs as a consequence of hypoxia in the context of ischaemia. Previous reports that LVD tended to decrease with increasing tumour thickness20,21 were not confirmed by our investigation, in which we failed to demonstrate a significant correlation between lymphangiogenetic parameters and tumour thickness. With regard to the regional distribution of cutaneous lymphatic vessels, we found that LVD was higher in the head and neck region than in the trunk and upper and lower extremities, as described previously,34 and this heterogeneous distribution may affect the chance of neoplastic dissemination. In agreement with previous observations,21 in our series the number of lymphatic vessels directly correlated with the intensity of the inflammatory infiltrate in and around the tumour. Increasing evidence supports the contribution of peritumorous stromal macrophages to tumour lymphangiogenesis through the production of specific growth factors (VEGF‐C and VEGF‐D) for lymphatic endothelia.28 Apart from promoting lymphangiogenesis, VEGF‐C may activate lymphatics to promote tumour cell chemotaxis, lymphatic intravasation, and hence tumour cell dissemination.6 Neoplastic cells also contribute to the production of VEGF‐C in the tumour microenvironment. In our study, there was a tendency (without reaching significance) for VEGF‐C expression to be seen more frequently in melanomas metastatic to SLN than non‐metastatic melanomas. These results agree with observations of Dadras et al, who reported that VEGF‐C expression by neoplastic cells was equally detected in both metastatic and non‐metastatic melanomas.19 Studies in mice have shown that injection with tumour cells overexpressing VEGF‐C increased metastatic spread to the lymph nodes.11,18,35,36 Furthermore, the in vitro and in vivo preferential expression of VEGF‐C in lymph node derived compared with cutaneous derived metastatic tumour cells reinforced the hypothesis that VEGF‐C expression in melanoma may be predictive of lymph node metastasis.37
In conclusion, the detection of tumour lymphangiogenesis allows the identification of patients with melanoma who are more susceptible to metastatic spread via the lymphatic route and at higher risk of developing SLN metastasis. Thus, our findings support the notion that the tumour associated lymphatic network constitutes a potential new target for the development of antimelanoma therapeutic strategies.
Acknowledgements
This work was supported in part by grants 03/0019 and V2003‐REDC03/03 and /07 from Fondo de Investigaciones Sanitarias; grant RO‐1 CA 83115 (fund 538226 from National Cancer Institute).
Note added in proof
During the process of publication of our study a new article by SS Dadras et al (Mod Pathol 2005;18:1232–42) reported that the lymphatic vascular area was the most sensitive prognostic marker for sentinel lymph node metastasis, in agreement with our observations.
Abbreviations
CI - confidence interval
HR - hazard ratio
LVD - lymphatic vessel density
LVA - lymphatic vessel area
LYVE‐1 - lymphatic endothelial hyaluronan receptor 1
SLN - sentinel lymph node
VEGF‐C - vascular endothelial growth factor C
References
- 1.Rigel D S. Malignant melanoma: perspectives on incidence and its effects on awareness, diagnosis, and treatment. CA Cancer J Clin 199646195–198. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Murray T, Samuels A.et al Cancer statistics 2003. CA Cancer J Clin 2003535–26. [DOI] [PubMed] [Google Scholar]
- 3.Meier F, Will S, Ellwanger U.et al Metastatic pathways and time courses in the orderly progression of cutaneous melanoma. Br J Dermatol 200214762–70. [DOI] [PubMed] [Google Scholar]
- 4.Gershenwald J E, Thompson W, Mansfield P F.et al Multi‐institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol 199917976–983. [DOI] [PubMed] [Google Scholar]
- 5.WHO WHO declares lymphatic mapping to be the standard of care for melanoma. Oncology 199913288 [Google Scholar]
- 6.Pepper M S, Skobe M. Lymphatic endothelium: morphological, molecular and functional properties. J Cell Biol 2003163209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Waal R M W, van Altena M C, Erhard H.et al Lack of lymphangiogenesis in human primary cutaneous melanoma. Am J Pathol 19971501951–1957. [PMC free article] [PubMed] [Google Scholar]
- 8.Pepper M S. Lymphangiogenesis and tumor metastasis: myth or reality? Clin Cancer Res 20017462–468. [PubMed] [Google Scholar]
- 9.Clarijs R, Ruiter D J, de Waal R M. Lymphangiogenesis in malignant tumours: does it occur? J Pathol 2001193143–146. [DOI] [PubMed] [Google Scholar]
- 10.Padera T P, Kadambi A, Di Tomaso E.et al Lymphatic metastasis in the absence of functional intratumor lymphatics. Science 20022961883–1886. [DOI] [PubMed] [Google Scholar]
- 11.Skobe M, Hamberg L M, Hawighorst T.et al Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor‐C in melanoma. Am J Pathol 2001159893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stacker S A, Achen M G, Jussila L.et al Lymphangiogenesis and cancer metastasis. Nat Rev Cancer 20022573–583. [DOI] [PubMed] [Google Scholar]
- 13.Schoppmann S F, Birner P, Studer P.et al Lymphatic microvessel density and lymphovascular invasion assessed by anti‐podoplanin immunostaining in human breast cancer. Anticancer Res 2001212351–2355. [PubMed] [Google Scholar]
- 14.Beasley N J P, Prevo R, Banerji S.et al Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res 2002621315–1320. [PubMed] [Google Scholar]
- 15.Nakamura Y, Yasuoka H, Tsujimoto M.et al Flt‐4‐positive vessel density correlates with vascular endothelial growth factor‐D expression, nodal status, and prognosis in breast cancer. Clin Cancer Res 200395313–5317. [PubMed] [Google Scholar]
- 16.Franchi A, Gallo O, Massi D.et al Tumor lymphangiogenesis in head and neck squamous carcinoma. Cancer 2004101973–978. [DOI] [PubMed] [Google Scholar]
- 17.Choi W W, Lewis M M, Lawson D.et al Angiogenic and lymphangiogenic microvessel density in breast carcinoma: correlation with clinicopathologic parameters and VEGF‐family gene expression. Mod Pathol 200518143–152. [DOI] [PubMed] [Google Scholar]
- 18.Karpanen T, Egeblad M, Karkkainen M J.et al Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res 2001611786–1790. [PubMed] [Google Scholar]
- 19.Dadras S S, Paul T, Bertoncini J.et al Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol 20031621951–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shields J D, Borsetti M, Rigby H.et al Lymphatic density and metastatic spread in human malignant melanoma. Br J Cancer 200490693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Straume O, Jackson D G, Akslen L A. Independent prognostic impact of lymphatic vessel density of low‐grade lymphangiogenesis in cutaneous melanoma. Clin Cancer Res 20039250–256. [PubMed] [Google Scholar]
- 22.Vidal‐Sicart S, Pons F, Puig S.et al Identification of the sentinel lymph node in patients with malignant melanoma: what are the reasons for mistakes? Eur J Nucl Med Mol Imaging 200330362–366. [DOI] [PubMed] [Google Scholar]
- 23.Marks A, Sutherland D R, Bailey D.et al Characterization and distribution of an oncofetal antigen (M2A antigen) expressed on testicular germ cell tumours. Br J Cancer 199980569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahn H J, Bailey D, Marks A. Monoclonal antibody D2‐40, a new marker of lymphatic endothelium, reacts with Kaposi's sarcoma and a subset of angiosarcomas. Mod Pathol 200215434–440. [DOI] [PubMed] [Google Scholar]
- 25.Kahn H J, Marks A. A new monoclonal antibody, D2‐40, for detection of lymphatic invasion in primary tumors. Lab Invest 2002821255–1257. [DOI] [PubMed] [Google Scholar]
- 26.Fogt F, Zimmerman R L, Ross H M.et al Identification of lymphatic vessels in malignant, adenomatous and normal colonic mucosa using the novel immunostain D2‐40. Oncol Rep 20041147–50. [DOI] [PubMed] [Google Scholar]
- 27.Birner P, Schindl M, Obermair A.et al Lymphatic microvessel density as a novel prognostic factor in early‐stage invasive cervical cancer. Int J Cancer 20019529–33. [DOI] [PubMed] [Google Scholar]
- 28.Schoppmann S F, Birner P, Stockl J.et al Tumor‐associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol 2002161947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giorgadze T A, Zhang P J, Pasha T.et al Lymphatic vessel density is significantly increased in melanoma. J Cutan Pathol 200431672–677. [DOI] [PubMed] [Google Scholar]
- 30.Valencak J, Heere‐Ress E, Kopp T.et al Selective immunohistochemical staining shows significant prognostic influence of lymphatic and blood vessels in patients with malignant melanoma. Eur J Cancer 200440358–364. [DOI] [PubMed] [Google Scholar]
- 31.Straume O, Akslen L A. Lymphatic vessel density and prognosis in cutaneous melanoma [letter]. Br J Cancer 2004911224–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sleeman J P, Krishnan J, Kirkin V.et al Markers for the lymphatic endothelium: in search of the holy grail? Microsc Res Tech 20015561–69. [DOI] [PubMed] [Google Scholar]
- 33.Chu A Y, Litzky L A, Pasha T L.et al Utility of D2‐40, a novel mesothelial marker, in the diagnosis of malignant mesothelioma. Mod Pathol 200518105–110. [DOI] [PubMed] [Google Scholar]
- 34.Lubach D, Ludemann W, Berens von Rautenfeld D. Recent findings on the angioarchitecture of the lymph vessel system of human skin. Br J Dermatol 1996135733–737. [PubMed] [Google Scholar]
- 35.Mandriota S J, Jussila L, Jeltsch M.et al Vascular endothelial growth factor‐C‐mediated lymphangiogenesis promotes tumour metastasis. EMBO J 200120672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattila M M, Ruohola J K, Karpanen T.et al VEGF‐C induced lymphangiogenesis is associated with lymph node metastasis in orthotopic MCF‐7 tumors. Int J Cancer 200298946–951. [DOI] [PubMed] [Google Scholar]
- 37.Schietroma C, Cianfarani F, Lacal P M.et al Vascular endothelial growth factor‐C expression correlates with lymph node localization of human melanoma metastases. Cancer 200398789–797. [DOI] [PubMed] [Google Scholar]