Abstract
Background
Although the presence of tumour infiltrating lymphocytes (TIL) is a constant feature in melanomas, their immunophenotypic characterisation is still incomplete. We hypothesise that the transition from normal skin to benign naevi (BN) to melanocytic dysplastic naevi (MDN) to radial growth phase cutaneous malignant melanoma (RGP‐CMM) to vertical growth phase cutaneous malignant melanoma (VGP‐CMM) is associated with alterations in TIL. This study attempted to test this hypothesis and to characterise TIL in the melanocytic skin lesions.
Methods
In total, 74 lesions (12 BN, 12 MDN, 13 RGP‐CMM, 26 VGP‐CMM, and 11 metastatic melanomas) were examined using immunoperoxidase staining methods and antibodies targeting leukocyte common antigen (LCA+), T (CD3+) and B (CD20+) lymphocytes, and resting cytotoxic T cells (TIA‐1+).
Results
Histologically, the transitions from normal skin to BN to MDN to RGP‐CMM to VGP‐CMM was associated with a gradual increase in the numbers of TIL (total, parenchymal, stromal, perivascular, and epidermal TIL, as well as TIL at the base of the lesions). The numbers of TIL were higher at the stroma than at the parenchyma. Similarly, immunostaining revealed that these transitions were associated with a gradual increase in the staining values (staining intensity, percentage of positive cells, and immunoreactivity score) for LCA+, CD20+, CD3+, and TIA‐1+cells. The number of CD3+ cells was higher than that of CD20+ cells. All these differences between the normal skin and the lesional ones reached statistical significance (p<0.01). The majority of CD3+ cells were TIA‐1+ T cells with cytotoxic potential. Compared with primary melanomas, there was a decrease in TIL in metastatic melanomas.
Conclusions
The gradual increase in TIL during melanoma tumorigenesis may reflect increased antigenicity of the tumour cells. Although both humoral and cell mediated immunity are involved in melanomagenesis, the latter seems to have the major role. The immune profile of MDN suggests their intermediacy between BN and CMM.
The melanocytic tumours encompass the full range of lesional steps from benign naevi to metastatic malignancy. Five lesional steps are recognised during melanomagenesis: (a) common acquired melanocytic naevus (BN), (b) dysplastic naevus (MDN), (c) radial growth phase of primary cutaneous malignant melanoma (RGP‐CMM), (d) vertical growth phase of primary cutaneous malignant melanoma (VGP‐CMM), and (e) metastatic melanoma.1,2,3 The rise in the incidence of melanoma makes finding an effective therapy essential. Several reports have suggested potential prognostic and therapeutic ramifications for tumour infiltrating lymphocytes (TIL) in melanoma.4 In melanomas, the tumour cells, surrounding stroma, and overlying epidermis are infiltrated by various types of TIL (mostly cytotoxic T cells, CTL). TIL are responsible for tumour cell killing and regression in this neoplasm. These roles are supported by several experimental findings: (a) the transfer of high numbers of TIL can mediate tumour rejection in animal models,5 (b) TIL numbers and cytokine secretion are critical for T cell induced melanoma regression,6 and (c) the presence of TIL is a powerful predictor of survival in melanomas.7 In melanoma, cell mediated immunity plays the determining role. The essence of this role relies on the integrity of T cell subsets including both CTL and helper T cells. The CTL are characterised by the presence of cytotoxic cytoplasmic granules such as T cell restricted intracellular antigen (TIA‐1). Alternatively, CD4 cells can produce several cytokines.8
To date, despite of the unfolding reports about TIL in melanoma, the immunophenotypic characterisation of TIL in the entire spectrum of the melanocytic skin lesions (especially BN and MDN) is still incomplete. Most previous studies did not define TIL by their location in the melanocytic lesions. In this investigation we hypothesised that the transition from normal skin to BN to MDN to RGP‐CMM to VGP‐CMM is associated with alterations in TIL in response to altered antigenicity of the tumour cells. To explore our hypothesis and to fill this existing gap in the literature, we carried out this investigation. To achieve our goals, we examined TIL in 74 lesions representing the entire spectrum of the melanocytic lesions, using histological and immunoperoxidase staining methods.
MATERIALS AND METHODS
Tissue specimens
Formalin fixed, paraffin embedded tissue specimens comprising 12 BN, 12 MDN, 13 RGP‐CMM, 26 VGP‐CMM and 11 metastatic melanomas (Met‐CMM) were obtained from the Department of Pathology, Assiut University Hospitals,. Histological diagnosis of hematoxylin and eosin stained sections was confirmed by all four authors. Immunostaining for S‐100 and HM45 was used to confirm the diagnosis in selected cases.
Histological features of the melanocytic skin lesions
The diagnosis of BN, MDN, and CMM followed criteria from other groups.9,10,11,12 The dysplastic naevi were defined clinically by size >5 mm, irregular borders, and variegated appearance. Histologically, MDN were defined as compound naevi with pattern and /or cytological atypia. The criteria used for diagnosis of MDN included: (a) marked lentiginous proliferation of melanocytes at the dermoepidermal junction, extending at least three rete ridges beyond lateral margins of dermal component; and (b) cell nests with atypical architectural and cytological appearance. The atypical architectural features of the included irregular size and shape of the cell nests and bridging of adjacent rete ridges. The atypical cytological features were nuclear hyperchromasia, prominent nucleoli, and dusty melanin pigment. The dysplastic naevus cells are both spindled and parallel to surface or epithelioid in shape. The dermis is fibrotic, with lamellar fibrosis or concentric eosinophilic fibrosis, neovascularisation, fusion of rete ridges, and perivascular inflammatory cell infiltrate present. The latter is considered a characteristic feature of MDN.13
Diagnosis of melanoma was established by identification of one of its four major clinical/histopathological subtypes (superficial spreading, nodular, lentigo maligna, and acral lentiginous melanomas).14,15,16,17 VGP‐CMM were histologically identified by the presence of one or more expansile dermal nodules of anaplastic melanocytes. When the tumour had mixed features, it was classified according to the prevailing type. The levels of invasion were categorised following the guidelines of Clark et al: levels I (intraepithelial growth), II (invasion into the papillary dermis), III (tumour cells filling and expanding the papillary dermis, and or invasion through the papillary dermis to the interface with reticular dermis), IV (invasion into the reticular dermis), and V (invasion into the subcutaneous tissues).18,19.
Breslow thickness (mm) was measured using an ocular micrometer. The vertical dimension was measured from the deepest part of invasion to the top of the granular layer or the most superficial cells under the zone of ulceration.20
Histological evaluation of the tumour infiltrating lymphocytes
The tumour infiltrating lymphocytes were evaluated histologically using the following method. The lymphocytes were counted in serial sections and in at least five different fields at ×400 magnification. Cells were counted in several sites including tumour parenchyma, stroma, base (tumour host interface), perivascular areas, and overlying epidermis. Counting of TIL was performed by two different observers, and the areas of highest density were chosen for counting. In metastatic melanomas, counting of TIL was performed only in the tumour parenchyma. Results are expressed as mean (SEM).21,22,23,24.
Immunohistochemical evaluation of the tumour infiltrating lymphocytes
Immunostaining was carried out as previously described.25 Briefly, sections mounted on glass slides were dewaxed and rehydrated through graded alcohol concentrations to water. Endogenous peroxidase activity was blocked with 0.6% H202. Sections were then immersed in the retrieval solution (10 mol/l sodium citrate buffer, pH 6.0, for LCA, CD3, and CD20) and subjected to heat induced antigen retrieval for 15 minutes. The slides, in plastic Coplin jars containing retrieval solution, were microwaved in a microwave set at high (∼750 W) for four cycles of 5 minutes each. Non‐specific protein binding was blocked with 10 minutes' exposure to 10% normal goat serum. Sections were then incubated with mouse monoclonal antibodies for 30 minutes at room temperature (Clones 2b11, L26, and UCHL1 for LCA, CD20 and CD3, respectively; Dako Corp, CA, USA). Anti‐TIA‐1 antibody was provided by Immunotech, Marseille, France. For TIA‐1 staining, the slides, in plastic Coplin jars containing retrieval solution (EDTA, pH 8) were microwaved in a microwave set at high (∼600 W) for 2 minutes then three cycles of 5 minutes each at ∼100 W. Non‐specific protein binding was blocked with 10 minutes exposure to 10% normal goat serum. Sections were then incubated overnight with mouse monoclonal antibodies at 4°C. A secondary staining system (LSAB2; Dako) was used according to the manufacturer's instructions. Sections were next treated with peroxidase labelled streptavidin for 30 minutes at room temperature and incubated with 14‐diaminobenzidine and 0.06% H202 for 5 minutes. They were counterstained with hematoxylin, dehydrated in alcohol, cleared in xylene, and coverslipped. The slides were independently evaluated by two observers (MRH, DAHE).
Positive and negative controls
The positive control specimens consisted of lymph nodes with reactive lymphoid hyperplasia (LCA, CD3, CD20, and TIA‐1).26 Additional sections, running in parallel but with omission of the primary antibody served as the negative controls.26
Semiquantification of LCA, CD3, CD20 and TIA‐1+staining
The average weighted score (immunoreactivity score; IRS) was evaluated by multiplying the percentage of positive cells and the staining intensity. First, the percentage of positive cells was scored (0 = <5%, 1 = 5–25%, 2 = 25–50%, 3 = 50–75%, and 4 = >75%). Second, the staining intensity was scored (1 = weak, 2 = medium, and 3 = intense) as described previously.27,28.
The presence of cells with clear and unequivocal brownish membranous staining identified LCA, CD20, and CD3 positive cells. Reactivity to TIA‐1 appeared as cytoplasmic granular staining.26
Statistical analysis
Analysis of variance and Spearman's correlation coefficient were used. Differences were considered statistically significant at p<0.05. We are aware of the assumptions of homogeneity of variance and normality in the one way analysis of variance. Analysis was performed with SAS software (version 8.1; SAS Institute Inc, Cary, NC, USA).
RESULTS
Histological examination of RGP‐CMM revealed that all of them were melanomas in situ. Examination of VGP‐CMM revealed 2/26 (7.7%), 4/26, (15.4%), 8/26 (30.7%), and 12/26 (46.2%) of cases to be Clark levels II, III, IVm and V, respectively. They included six cases of superficial spreading and 20 of nodular melanomas with mean (SEM) Breslow thickness of 5.7 (0.5) mm (thick melanomas). When the cytological features were examined, the epithelioid cell type was seen in all RGP‐CMM and in 9/26 (34.6%) of VGP‐CMM. In VGP‐CMM, mixed cell type (epithelioid, spindle, rhabdoid) was present in 16/26 (61.5%), and spindle cell type in 1/26 (3.9%). Recurrence and metastasis were seen in two cases of VGP‐CMM (2/26, 7.7%).
Histological examination of 11 Met‐MM revealed that the cell type was mixed (epithelioid and spindle) in 7/11 (64% and epithelioid in 4/11 (36%). All the Met‐MM involved lymph nodal tissues. Cervical lymph nodes were the commonest site for Met‐MM (6/11; 54.6%), followed by inguinal (3/11; 27.3%), and axillary and the popliteal nodes (1/11; 9.1% each).
Evaluation of the immunohistochemical results revealed that the positive and negative controls for LCA, CD3, CD20, and TIA‐1 proteins were positive and negative, respectively, indicating the validity of our results. The presence of TIL was a constant feature in the entire spectrum of the melanocytic skin lesions. The transitions among the sequential phases of melanoma was associated with a gradual increase in the number of tumour infiltrating lymphocytes and the staining values of LCA+, CD20+, CD3+, and TIA‐1+ lymphocytes. The numbers of CD3+ was higher than CD20+. The numbers of TIL were higher at the stroma than the parenchyma .The differences oin TIL values between normal and lesional skin reached statistical significance. In our series, SEM was small in virtually all categories, owing to the homogeneity of the study groups.
The lack of significant variability in the primary data is what leads to “perfect” Spearman correlation scores in every relationship examined.
There was a gradual increase in the number of tumour infiltrating lymphocytes with transitions from normal skin to BN to MDN to RGP‐CMM to VGP‐CMM. This transition was associated with a statistically significant increase (p<0.01) in the mean (SEM) counts of total TIL: (3.9 (0.6) v. 26.8 (1.3) v. 39 (3.6) v. 67.4 (4.3) v. 87.9 (2), respectively for each stage. In addition, these transitions were associated with similar increases in TIL in the parenchyma, stroma, base, perivascular areas, and epidermis of these lesions. Similarly, compared with BN, the values in CMM were statistically significantly higher. In contrast, these values, although higher in RGP‐CMM than MDN and higher in MDN than BN, did not reach statistical significances (table 1, figs 1 and 2).
Table 1 Histological evaluation of tumour infiltrating lymphocytes in the melanocytic skin lesions .
| Material | Total TIL | Positioning of TIL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parenchyma | Stroma | Base | Perivascular | Epidermis | SIL | |||||||||
| Normal skin | 3.9 (0.6) | – | 3.9 (0.6) | – | – | – | 3.9 (0.6) | |||||||
| BN | 26.8 (1.3) | 28.2 (2.9) | 35 (1.9) | 50 (1.2) | 14.6 (3.3) | 5.8 (3.1) | 33.2 (1.4) | |||||||
| DN | 39 (3.6) | 40.8 (7.3) | 53.3 (2.2) | 71 (1.8) | 24.6 (7.8) | 5.8 (5.8) | 49.5 (3.1) | |||||||
| CMM* | 81.1 (2.5) | 78.4 (3.5) | 83.3 (3.5) | 90.8 (1.5) | 70.8 (3.9) | – | 82.4 (2.4) | |||||||
| RGP‐CMM | 67.4 (4.3) | 64.6 (5.9) | 65.2 (8.1) | 80.2 (1.5) | 52.6 (6.3) | – | 63.4 (4.3) | |||||||
| VGP‐CMM | 87.9 (2) | 85.3 (3.8) | 92.4 (1.5) | 96 (1.1) | 80 (3.9) | 78 (1.2) | 87.9 (2) | |||||||
| Met‐MM | 39.6(3.7) | 39.6 (3.7) | – | – | – | – | – | |||||||
Data are mean (SEM). BN, benign naevi; MDN, dysplastic naevi; MM*; cutaneous malignant melanoma referred to total count of RGP‐CMM and VGP‐CMM; RGP‐CMM, radial growth phase cutaneous malignant melanoma; VGP‐CMM, vertical growth phase cutaneous malignant melanoma; Met‐MM, metastatic malignant melanoma.
Figure 1 (A) percentage of positive cells (histological evaluation), (B) percentage of positive cells (immunohistochemical evaluation), (C) positioning of LCA+ and CD20+ cells and (D) positioning of CD3+ and TIA‐1+ cells.
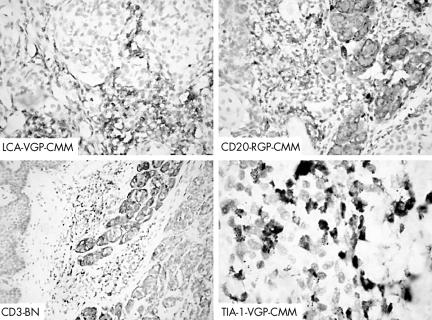
Figure 2 Immunoreactivity for LCA, CD20, CD3 and TIA‐1 in the melanocytic skin lesions. BN, benign naevi; RGP‐CMM, radial growth phase cutaneous malignant melanoma; VGP‐CMM, vertical growth phase cutaneous malignant melanoma. Original magnification: LCA, CD20, ×400; CD2, ×200; TIA‐1, ×1000.
A gradual increase in the staining values of LCA+, CD20+, CD3+, and TIA‐1+lymphocytes occurred during the transition from normal skin to BN to MDN to RGP‐CMM to VGP‐CMM. The transition was associated with a statistically significant gradual increase in all staining values (staining intensity, percentage positive cells, and IRS, p<0.01). Firstly, these transitions were associated with a statistically significant increase (p<0.01) in the total percentage of positive cells (LCA+: 3.9 (0.6 10) v.27.6 (1.6) v.40.7 (3.6) v .66.7 (3.9) v.88.6 (2.0) v.41.4 (4.1; CD20+: 0.8 (0.1) v.5.6 (0.9vs.7.3 (2.2) v.12 (3.8) v.14 (2.5; CD3+: 3 (0.2) v.21.8 (1.1) v.33 (2.5) v.52.7 (4.2) v.75 (1.7); and TIA‐1+: 3 (0.2) v.13.3 (0.8) v.21.7 (1.7) v.46.5 (4.0) v.67 (1.7)). In addition, these transitions were associated with similar increase of total percentage of positive cells in the parenchyma, stroma, base, perivascular areas, and epidermis of these lesions (tables 2–4, figs 1, 2).
Table 2 Immunohistochemical evaluation of positive cells in the melanocytic skin lesions.
| Material | LCA | CD20 | CD3 | TIA‐1 | ||||
|---|---|---|---|---|---|---|---|---|
| Normal skin | 3.9 (0.6) | 0.8 (0.1) | 3 (0.2) | (0.2) | ||||
| BN | 27.6 (1.6) | 5.6 (0.9) | 21.8 (1.1) | 13.3 (0.8) | ||||
| DN | 40.7 (3.6) | 7.3 (2.2) | 33 (2.5) | 21.7 (1.7) | ||||
| RGP‐CMM | 66.7 (3.9) | 12 (3.8) | 52.7 (4.2) | 46.5 (4.0) | ||||
| VGP‐CMM | 88.6 (2.0) | 14 (2.5) | 75 (1.7) | 67 (1.7) | ||||
| Met‐MM | 41.4 (4.1) | 11.4 (3.2) | 29.5 (1.6) | 20.6 (1.5) |
Data are mean % (SEM). BN, benign naevi; MDN, dysplastic naevi; RGP‐CMM, radial growth phase melanoma; VGP‐CMM, vertical growth phase melanoma; Met MM, metastatic malignant melanoma. The differences among the TIL values in normal and lesional skin was statistically significant (p<0.01)
Table 3 Immunohistochemical evaluation of percentage of LCA+ and CD20+ cells in the melanocytic skin lesions.
| Material | LCA | CD20 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | S | B | V | E | SILs | P | S | B | V | E | SILs | |
| Normal skin | – | 3.9 | – | – | – | 3.9 | – | 0.8 | – | – | – | 0.8 |
| (0.6) | (0.6) | (0.1) | (0.1) | |||||||||
| BN | 29.6 | 36.3 | 51.3 | 15 | 5.8 | 34 | 5 | 7.1 | 12.5 | 2.3 | 0 | 7.1 |
| (2.8) | (2.3) | (1.1) | (3.5) | (3.1) | (1.5) | (1.5) | (1.9) | (1.3) | (1.2) | (1.1) | ||
| DN | 43 | 55.3 | 73 | 26 | 6.3 | 51.4 | 6.7 | 10 | 14.2 | 5.8 | 0 | 11.1 |
| (7.7) | (2.3) | (1.7) | (7.9) | (0.2) | (3.2) | (3) | (3.3) | (3.4) | (2.8) | (3.1) | ||
| CMM* | 78.6 | 84.4 | 91.5 | 72.3 | 79 | 83 | 11.8 | 13.7 | 16.8 | 13.3 | 0 | 14.6 |
| (3.6) | (3.6) | (1.5) | (3.8) | (0.8) | (2.4) | (2.6) | (2.6) | (2.5) | (2.5) | (2.2) | ||
| RGP‐ CMM | 63.5 | 65.6 | 81.2 | 52 | – | 67.8 | 9.6 | 12.3 | 17 | 9.2 | – | 12.6 |
| (5.9) | (8.1) | (1.1) | (7.5) | (3.6) | (3.5) | (6.6) | (4.8) | (3.2) | (4) | |||
| VGP‐ CMM | 86.2 | 93.8 | 97 | 80 | 79 | 90.6 | 12.9 | 14 | 17 | 15 | 0 | 15.5 |
| (3.8) | (1.7) | (1.3) | (4) | (0.8) | (1.8) | (3.4) | (3.2) | (3) | (.3.4) | (2.6) | ||
| Met MM | 41.4 | – | – | – | – | – | 11.4 | – | – | – | – | – |
| (4.1) | (3.2) | |||||||||||
BN, benign naevi; MDN, dysplastic naevi; CMM*; cutaneous malignant melanoma referred to total count of RGP‐CMM and VGP‐CMM; RGP‐CMM, radial growth phase cutaneous malignant melanoma; VGP‐CMM, vertical growth phase cutaneous malignant melanoma; Met MM, metastatic malignant melanoma. P, parenchyma; S, stroma; B, base; V, perivascular; E, epidermis; SIL, skin infiltrating lymphocytes (total TIL). The differences among the TIL values in normal and lesional skin reached statistical significance (p<0.01)
Table 4 Immunohistochemical evaluation of percentage of CD3+ and TIA‐1+ cells in the melanocytic skin lesions.
| Material | CD3 | TIA‐1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | S | B | V | E | SILs | P | S | B | V | E | SILs | |
| Normal skin | – | 3.0 (0.2) | – | – | – | 3.0 (0.2) | – | 3.0 (0.2) | – | – | – | 3.0 (0.2) |
| BN | 24.7 (2.6) | 28.5 (1.3) | 38 (0.9) | 12 (2.7) | 5.2 (2.8) | 26.3 (1) | 13.2 (0.2) | 17.9 (1.6) | 26.3 (1.4) | 6.7 (1.5) | 2.5 (1.3) | 16.4 (0.7) |
| DN | 36.3 (6.5) | 45 (1.4) | 58 (2.9) | 20 ( 6.4) | 5 (5) | 40 (1.3) | 23.4 (4.7) | 25.5 (2.7) | 40 (2.9) | 14.5 (4.3) | 4 (4) | 2.8 (1.7) |
| CMM* | 65.7 (3.6) | 71.3 (3.2) | 75.3 (1.9) | 57.7 (4.2) | 79 (1) | 68.2 (2.5) | 59.3 (3.3) | 64.2 (3) | 68.9 (1.6) | 49.3 (4.1) | 70 (0) | 60.8 (2.4) |
| RGP | 52.8 (5.2) | 53.7 (7.2) | 64 (3) | 43 (7) | – | 54 (4) | 48.3 (5.2) | 48 (7.1) | 58.3 (2.8) | 31.7 (6.3) | – | 45.8 (3.7) |
| VGP | 72 (4.4) | 80.2 (1.5) | 81 (1.4) | 65 (4.8) | 79 (1) | 75 (1.9) | 64.3 (3.9) | 72 (1.3) | 73.8 (1) | 57.4 (4.4) | 70 (0) | 67.7 (1.8) |
| Met MM | 29.5 (1.6) | – | – | – | – | – | 20.6 (1.5) | – | – | – | – | – |
BN, benign naevi; MDN, dysplastic naevi; CMM*; cutaneous malignant melanoma referred to total count of RGP‐CMM and VGP‐CMM; RGP‐CMM, radial growth phase cutaneous malignant melanoma; VGP‐CMM, vertical growth phase cutaneous malignant melanoma; Met MM, metastatic malignant melanoma. P, parenchyma; S, stroma; B, base; V, perivascular; E, epidermis; SIL (total TIL), skin infiltrating lymphocytes. The differences among the TIL values in normal and lesional skin reached statistical significance (p<0.01)
There was a gradual increase in the staining intensity with the transition from normal skin to BN to MDN to RGP‐CMM to VGP‐CMM. At the parenchyma, the staining intensity staining values (in these transitions, respectively) were: 0.0 (0.0) v 0.9 (0.1) v 1.6 (0.3) v 2.4 (0.3) v 2.9 (0.1) for LCA+ cells; 0.6 (0.1) v 0.5 (0.2) v 0.7 (0.3) v 1 (0.4) v 1.3 (0.3) for CD20+ cells; 0.0 (0.0) v 0.9 (0.1) v 1.7 (0.3) v 2.3 (0.2) v 2.8 (0.2) for CD3+ cells, and 0.0 (0.0) v 0.8 (0.1) v 1.7 (0.3) v 2.2 (0.2) v 2.8 (0.2) for TIA‐1+ cells. In addition, when the staining intensity of SIL was examined, there was a gradual statistically significant increase in the staining values (tables 3, 4; figs 1,2).
A gradual increase in the percentage of positive cells also occurred with the transition from normal skin to BN to MDN to RGP‐CMM to VGP‐CMM. Compared with normal skin, the staining values for the percentage of positive cells at the parenchyma statistically significantly increased for LCA+, CD20+ , CD3+, and TIA‐1+ cells, as were the, the staining values for SIL were statistically significantly increased (tables 2–4; figs 1, 2).
There was a gradual increase in the immunoreactivity score with the transition from normal skin to BN to MDN to RGP‐CMM to VGP‐CMM. Compared with normal skin, at the parenchyma, IRS values were statistically significantly increased (p<0.01) for LCA+, CD20+, CD3+, and TIA‐1+ cell, as were the IRS staining values for stromal TIL (table 5; figs 1, 2).
Table 5 Immunohistochemical evaluation of tumour infiltrating lymphocytes (total counts) in the melanocytic skin lesions.
| Material | LCA | CD20 | CD3 | TIA‐1 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SI | PP | IRS | SI | PP | IRS | SI | PP | IRS | SI | PP | IRS | |||||||||||||
| Normal skin | 0.6 (0.01) | 1.0 (0.1) | 1.0 (0.01) | 0.6 (0.1) | 0.2 (0.1) | 1.0 (0.01) | 0.8 (0.02) | 0.8 (0.2) | 0.8 (0.02) | 0.8 (0.2) | 0.8 (0.2) | 0.8 (0.2) | ||||||||||||
| BN | 0.8 (0.03) | 1.6 (0.1) | 1.6 (0.1) | 0.6 (0.1) | 0.5 (0.1) | 0.5 (0.1) | 0.8 (0.02) | 1.2 (0.1) | 1.3 (0.1) | 0.7 (0.1) | 1.0 (0.1) | 1.0 (0.1) | ||||||||||||
| DN | 1.5 (0.2) | 2.1 (0.2) | 4.3 (0.6) | 0.7 (0.2) | 0.7 (0.2) | 1.3 (0.4) | 1.8 (0.2) | 1.8 (0.2) | 3.8 (0.5) | 1.3 (0.2) | 1.4 (0.1) | 3 .0 (0.4) | ||||||||||||
| CMM* | 2.7 (0.1) | 3.5 (0.1) | 10.1 (0.4) | 1.3 (0.2) | 1.3 (0.2) | 2.9 (0.5) | 2.6 (0.1) | 3.3 (0.1) | 9.4 (0.4) | 2.6 (0.1) | 2.9 (0.1) | 8.0 (0.4) | ||||||||||||
| RGP | 2.3 (0.2) | 3.1 (0.2) | 8.0 (0.8) | 1.0 (0.4) | 0.9 (0.3) | 2.2 (0.8) | 2.2 (0.2) | 2.8 (0.2) | 6.8 (0.6) | 2.1 (0.2) | 2.3 (0.2) | 5.4 (0.6) | ||||||||||||
| VGP | 2.9 (0.03) | 3.7 (0.1) | 11.1 (0.3) | 1.4 (0.2) | 1.1 (0.2) | 3.3 (0.6) | 2.9 (0.1) | 3.6 (0.1) | 10.7 (0.3) | 2.8 (0.1) | 3.1 (0.1) | 9.2 (0.3) | ||||||||||||
| Met MM | 1 (0.0) | 2.5 (0.2) | 2.5 (0.2) | 0.6 (0.2) | 1.0 (0.3) | 1.0 (0.3) | 1.0 (0.0) | 1.9 (0.1) | 1.9 (0.1) | 1.0 (0.0) | 1.4 (0.2) | 1.4 (0.2) | ||||||||||||
BN, benign naevi; MDN, dysplastic naevi; CMM*; cutaneous malignant melanoma referred to total count of RGP‐CMM and VGP‐CMM; RGP‐CMM, radial growth phase cutaneous malignant melanoma; VGP‐CMM, vertical growth phase cutaneous malignant melanoma; Met MM, metastatic malignant 22 melanoma; SI, score intensity; PP, percentage of positive cells; IRS, immuno‐reactivity score; P, parenchyma; S, stroma; B, base; V, perivascular; E, epidermis; SIL , skin infiltrating lymphocytes. The differences among the TIL values in normal and lesional skin reached statistical significance (p<0.01)
Most of the tumour infiltrating lymphocytes were of T rather than B cell lineage. Immunohistochemical analysis of the percentage of positive cells revealed that TIL were predominantly CD3+ (T cell lineage) with few CD20+ cells (B cell lineage):
21.8 (1.1) v 5.6 (0.9), 33 (2.5) v 7.3 (2.2), 52.7 (4.2) v 12 (3.8), 75 (1.7) v 14 (2.5), and 29.5 (1.6) v 11.4 (3.2 for BN, MDN, RGP‐CMM, VGP‐CMM, and Met‐MM, respectively. In addition, when the percentage of positive cells was examined, there were statistically significant differences between B and T cells (tables 2–4; figs 1, 2).
TIL were denser at the stroma than in the parenchyma. Compared with TIL at the parenchyma, stromal TIL (the sum of TIL at the stroma, base, margin, and perivascular areas) were denser. In normal skin, BN, MDN, RGP‐CMM, and VGP‐CMM, these values were: 0.0 (0.0) v 3.9 (0.6), 29.6 (2.8) v 34 (1.5), 43 (7.7) v 51.4 (3.2), 63.5 (5.9) v 67.8 (3.6), and 86.2 (3.8) v 91.6 (1.8) for LCA (table 6; figs 1, 2).
Table 6 The mean values of the tumour infiltrating lymphocytes at the parenchyma and stroma in the melanocytic skin lesions The table size for tables 3, 4, and 5 isn't quite large enough to allow each of the SD figures to align on the same line as the mean, so I have adjusted it so that they're all on the second line, rather than a mixture, which looked quite confusing; is this OK?
| Material | LCA | CD20 | CD3 | TIA‐1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | S | P | S | P | S | P | S | |||||||||
| Normal skin | 0.0 (0.0) | 3.9(0.6) | 0.0 (0.0) | 0.8 (0.1) | 0.0 (0.0) | 3 (0.2) | 0.0 (0.0) | 3.0 (0.2) | ||||||||
| BN | 29.6 (2.8) | 34 (1.5) | 5.0 (1.5) | 7.1 (1.1) | 24.7 (2.6) | 26.3 (1.0) | 13.2 (0.2) | 16.4 (0.7) | ||||||||
| DN | 43 (7.7) | 51.4 (3.2) | 6.7 (3.0) | 11.1 (3.1) | 36.3 (6.5) | 40 (1.3) | 23.4 (4.7) | 28 (1.7) | ||||||||
| RGP‐CMM | 63.5 (5.9) | 67.8 (3.6) | 9.6 (3.5) | 12.6 (4.0) | 52.8 (5.2) | 54 (4.0) | 48.3 (5.2) | 45.8 (3.7) | ||||||||
| VGP‐CMM | 86.2 (3.8) | 91.6 (1.8) | 12.9 (3.4) | 15.5 (2.6) | 72 (4.4) | 75 (1.9) | 64.3 (3.9) | 70.1 (2.3) | ||||||||
| Met‐MM | 41.4 (4.1) | – | 11.4 (3.2) | – | 29.5 (1.6) | – | 20.6 (1.5) | – | ||||||||
BN, benign naevi; MDN, dysplastic naevi; CMM*; cutaneous malignant melanoma referred to total count of RGP‐CMM and VGP‐CMM; RGP‐CMM, radial growth phase cutaneous malignant melanoma; VGP‐CMM, vertical growth phase cutaneous malignant melanoma; Met MM, metastatic malignant melanoma; P, parenchyma and SIL , skin infiltrating lymphocytes. The differences among the TIL values in normal and lesional skin reached statistical significance (p<0.01)
The majority of CD3+ cells were TIA‐1+ with cytotoxic potential. When the percentages of CD3+ and TIA‐1+ positive cells were compared, the majority of CD3+ cells were TIA‐1+ cells. The TIA‐1+ represented 48%, 53.3%, 70%, and 75.6% of total TIL in BN, MDN, RGP‐CMM, and VGP‐CMM, respectively (tables 2–4, figs 1, 2).
Compared with primary melanomas, there was a decrease in the tumour infiltrating lymphocytes in the metastatic melanomas. In met‐CMM, TIL were evaluated only at the parenchyma. When TIL values were compared between primary melanomas (both radial and vertical growth phases), and Met‐CMM; there was a statistically significant decrease in TIL in the latter (81.1 (2.5) v 39.6 (3.7) and 78.4 (3.5) v 39.6 (3.7 for total and parenchymal TIL, respectively (tables 1–6, figs 1, 2).
The association between TIL and selected clinicopathological features (metastasis, recurrence, size, location, and histological type) of melanomas was examined and tested for statistical significance. There were no significant correlations between these features and TIL.
DISCUSSION
Malignant melanoma, the deadliest skin cancer, is often infiltrated by TIL. In vivo, the latter can localise in tumorous tissues and mediate tumour regression. In vitro, TIL from melanoma can recognise and kill autologous tumour cells in an HLA restricted manner. Thus melanoma is an example of a tumour in which the presence of TIL not only seems to be a favourable prognostic factor but also underlines the concept of tumour immunosurveillance and immunotherapeutic strategies. In this investigation, we hypothesised that the transition from normal skin to BN to MDN to RGP‐CMM to VGP‐CMM to Met‐CMM is associated with alterations in TIL in response to altered antigenicity of the tumour cells. In addition, the immunophenotypic characterisation of TIL is still incomplete. To explore our hypothesis and to fill this existing gap in the literature, we examined TIL in 74 melanocytic lesions. Our data clearly demonstrated five observations: (a) a gradual increase in the number of TIL occurred with transitions from normal skin to BN to MDN to RGP‐CMM to VGP‐CMM, (b) most TIL were of T rather than B cell lineage, (c) TIL were denser at the stroma than in the parenchyma, (d) the majority of CD3+ cells were TIA‐1+ T cells with cytotoxic potential, and (e) compared with primary melanomas, there was a decrease in TIL in Met‐CMM.
In our series, the presence of TIL in melanoma concurs with previous reports.20,29 This infiltrate reflects ongoing immune responses against the tumour cells. It also suggests that the lesional cells have sufficient immunogenicity to trigger a host immune response with concomitant recruitment of immune cells. The presence of TIL both in the parenchyma and stroma agrees with similar findings in the renal, colorectal, and oesophageal carcinomas. In these tumours, this positioning of TIL is associated with much better survival.30
The gradual increase in TIL with the transition from normal skin to BN to MDN to RGP‐CMM to VGP‐CMM—that is, with increased cellular growth rate and atypism, may be explained by three possibilities. Firstly, these transitions are associated with an increased immunogenicity of the lesional cells, which may induce local activation and proliferation of immune cells. The immunogenicity of the melanomatous cells is attributed to the existence of several self antigens called melanoma antigens ( MART‐1, gp100, gp75, and tyrosinase). The level of these antigens has an important impact on TIL (CTL) activation status. The MART‐1 (melanoma antigen recognised by T cells‐1), gp100, and tyrosinase related protein‐1 (gp75), but not tyrosinase, are recognised by HLA‐A2 restricted melanoma specific CTLs31 The gp100 antigen, widely expressed in melanocytic cells, acts as a target for antimelanoma CTL. Thus it constitutes a useful target for specific immunotherapy against melanoma.31 Secondly, it is possible that this increase in TIL during melanomagenesis may be due to increase in the load of associated antigens on tumour cells. These antigens possibly include cancer testis antigens, and mutated genes such as p53 (tumour suppressor) and Ras (oncogene). These antigens can induce local activation and proliferation of the immunocytes.32,33,34
Interestingly, melanomas express the seven major cancer testis antigens (MAGE‐1, MAGE‐3, MAGE‐4, MAGE‐10, NY‐ESO‐1, SSX‐2, and SCP‐1), as well as some viral antigens (herpes C virus antigens).33 In addition, mutant p53 can induce specific CTL responses that mediate lysis of the transformed cells in animal models.35,36 In many human cancers, the accumulated p53 protein in the cytosol can be effectively presented by major histocompatibility complex class I molecules to elicit specific CTL responses. Ras mutations involve single amino acid substitutions, mostly at positions 12 and 61. CD4+ and CD8+ T cell responses mediating tumour cell lysis can be induced by mutant Ras peptides in animal models.[371] In metastatic pancreatic cancer, Ras specific proliferative T cell responses were found after immunisation with MHC class I restricted Ras peptides.38 It is still possible that the genetically damaged melanocytes, but not the normal ones, express addressins molecules that recruit more MICs to the damaged melanocytes. Thirdly, the increase in TIL with these transitions may reflect enhanced cytokine production by the tumour cells—that is, the dysplastic naevus and melanomatous cells may produce more cytokines and chemokines than the naevus cells. The melanomatous cells recruit's more immune cells.39
In our series, the findings of higher counts of TIL in MDN, compared with BN, agree with previous studies.29,40,41,42,43,44 These findings are: (a) increased antigenic load on the dysplastic naevus cells, (b) local proliferation of the lymphocytes in MDN, and (c) enhanced recruitment of the lymphocytes in MDN by soluble factors or adhesion molecules. Van Duinen et al have examined the expression of intercellular adhesion molecule‐1, inducible cell adhesion molecule‐110, vascular cell adhesion molecule‐1, E‐selectin, lymphocyte function associated antigen‐1, and the integrins in 20 BN, 35 MDN, 6 melanomas in situ, and 20 malignant melanomas, using both indirect immunoperoxidase and immunofluorescence staining methods. They found that the transition from benign to malignant neoplasms was associated with altered expression of these molecules.45 To date, a heated controversy still continues over the criteria used for the diagnosis of MDN and the distinction between them, and BN is not accepted by all the scientific community. Our findings suggest the possible use of lymphocytic infiltration as a diagnostic tool in separating MDN from BN. Nevertheless, TIL phenotype in these lesions might prove to be of diagnostic utility if substantiated by larger studies in the future studies.
Our study revealed a variable positioning of TIL in the melanocytic lesions with a significantly increased density of TIL in the stroma of these lesions—that is, site dedicated trafficking and retention of TIL in the melanocytic lesions. Such site dependent density partitioning may increase the likelihood that TIL bearing specific antigenic specificities will encounter the corresponding antigen. Furthermore, this site targeted recruitment and localisation may be achieved by regulated expression of particular homing receptors on TIL (including adhesion and chemokine receptors), and by regulation of their counter receptors expressed by endothelial and lesional cells. Accordingly, we speculate that the increased stromal TIL in our series may reflect an increase in these homing receptors and counter receptors.46,47.
The presence of both B and T lymphocytes in the melanocytic lesions not only agrees with previous reports,48,49,50 but also suggests the involvement of both cell mediated and humoral immunity in melanomagenesis. The majority of TIL were CD3+ T cells, which coincides with findings of others,48,49,50,51,52 and reflects increased T cell proliferation. The CD3 monoclonal antibody used in this investigation is considered a pan T cell marker. The majority of lymphocytes were CD3+ (T cell lineage). These findings coincide with those reported in the inflammatory infiltrate associated with melanomas in Sinclair pigs. In addition, the presence of high numbers of CD3+ T lymphocytes in areas where tumour cells were sparse, but low numbers of CD3+ T lymphocytes in areas where tumour cells were abundant, concurs with the results reported in regressing melanoma in Sinclair pigs and in humans.48,53 Taken together, these results suggest that T lymphocytes play an important role in immune defence against melanoma.
The CD20 monoclonal antibody used in this report was a pan B cell marker. The low number of CD20 B cells located in the melanocytic lesions of our series is consistent with similar findings in the animal models.49 This finding suggests that the local antibody response does not play as much a role in the immune defence against melanoma.
The CD3+ CD4+ T cells play a critical role in generating and maintaining strong immune responses against the recognised melanoma associated antigens. Several melanoma expressed antigens contain determinants that are recognised by CD4+ and CD8+ T cells. The integration of these antigens and their derivative peptides in vaccine trials allows efficacious augmentation of anti‐melanoma specific CD4+ and CD8+ T cell mediated immune response in a therapeutic setting. Several studies have shown that CD4+ T cells are associated with regression in primary melanoma and rejection of tumours in adoptive transfer models. The mechanism by which CD4+ mediate their antitumour effects involves Fas ligand/Fas interactions and secretion of several cytokines such as granulocyte macrophage colony stimulating factor, tumour necrosis factor‐αand interferon‐γ.54,55. On the other hand, cytotoxic T cells have been implicated in the control of the progression of human melanoma. The CD3+ CD8+ cytotoxic T lymphocyte is an important effector cell against melanoma cells.56,57 Further investigations are warranted to examine the positioning of CD4+ and CD8+ T cells in the entire spectrum of the melanocytic skin lesions.
A relatively large number of T lymphocytes (CD3+ cells) had cytotoxic potential (TIA‐1+). CTLs are able to recognise and destroy tumour cells through the release of cytoplasmic granules, which contain TIA‐1. TIA‐1 is an intracellular, 15 kDa, membrane associated protein that can induce apoptotic cell death via RNA binding. TIA‐1 can also act as a regulator of alternative pre‐mRNA splicing pathway. The significantly higher density of TIA‐1+ cells in CMM compared withBN and MDNs not only agrees with other reports,29 but also suggests a possible pathogenetic and prognostic role for TIA‐1+ cells. Monoclonal antibodies against TIA‐1 define a subpopulation of CD8+ T cells and natural killer (NK) cells that have cytotoxic activity.58 Therefore, it is possible that increased numbers of TIA‐1+ cells reflect a sum of both cytotoxic T cells and NK cells. Further investigations using antibodies against NK cells (CD56) are essential to examine this possibility. Interestingly, the NK cells have much more potent killing effects than CD3+ TIL on the melanoma cell lines. In support, previous reports indicated that most of the killing of the melanoma cells in short term TIL cultures is mediated by CD3‐ NK cells, whereas CD3+ T cells had weak anti‐tumour effects.52
Compared with primary melanomas, there was a decrease in TIL in metastatic melanomas. The presence of TIL in the parenchyma of metastatic melanomatous deposits in the lymph nodes agree with previous reports.50 The presence of these tumour deposits may reflect breakdown of the immune defence with subsequent escape of the tumour cells from the immune defence. The predominance of CD3 together with paucity of CD20 positive cells in this infiltrate concur with other studies.48,50,59 The low numbers of TIL in Met19 CMM may have several explanations: (a) decreased density of the dendritic cells in the affected lymph nodes, (b) downregulation of the early lymphocyte activation markers interleukin‐2 receptor (IL‐2R) ‐α and CD69, and (c) ownregulation or loss of expression of interleukin‐2 (IL2) by TIL. In turn, this loss may lead to impairment of the usual pathways of lymphocyte activation via IL2 within the lymph node metastases.50,52 Interestingly, in our series, the development of these metastatic malignancy in the face of intense immune cells in the lymph nodes might be explained by several possibilities: 1) although immune cells are gathered through diverse signals in the nodal tissues, they are anergic or senescent and therefore can not lyse the tumour cells, 2) immune cells surrounding these deposits might have diverse functions in the lymph node microenviroment, in a way that allows tumour growth, 3) metastatic melanoma cells might loose their HLA molecules, 4) T cell unresponsiveness due to downregulation of ζ chain T‐cell receptor (TCR/CD3) complex, and 5) melanoma cells may secrete cytokines (IL‐10) that suppress the immune response.60 In future studies, it would be of interest to look at metastatic melanoma to sites other than lymph nodes where the full range of TIL analysis could be performed and compared withthe progression series.
The presence of TIL has been shown to carry prognostic significance in several malignant tumours.5,6,61,62,63. In melanoma, the intensity of TIL may be categorised as brisk, non‐brisk, and absent. Studies that examined the prognostic ramifications of TIL in melanomas reported contrasting results. Several previous studies have reported that the intensity of TIL is a strong prognostic factor in melanoma. In support, the 5 and 10 year rates for VGP‐CMM with a brisk infiltrate were 77% and 55%, respectively; 53% and 45%, respectively for tumours with a non‐brisk TIL and 37% and 27%, respectively, for tumours with absent TIL.20. In our study, the impact of TIL on the melanocytic lesions was examined by assessing the association between TIL and the clinicopathological variables. In agreement with similar findings for breast cancer, we found a lack of any significant correlation between TIL and the clinicopathological features in these lesions.24 Our findings raise the notion that TIL may be anergic or senescent. It is also possible that TIL in these lesions are reprogrammed to loose their antitumour effects, or that their interactions with the antigen presenting cells, such as dendritic cells, are impaired.64
To summarise, melanomagenesis is associated with alteration of TIL. The gradual increase in the counts of TIL occurred with transitions from BN to MDN to melanomas suggests increased tumour antigenicity. Although both CD20 (humoral immunity) and CD3 (cell mediated immunity) were present during melanomagenesis, the latter was more prevalent. In addition, relatively large numbers of T lymphocytes (CD3 positive cells) had cytotoxic potential (TIA‐1 positive). The possible pathogenetic and prognostic ramifications of our findings warrant further investigation.
TAKE HOME MESSAGES
The presence of tumour infiltrating lymphocytes (TIL) is a constant feature in melanomas.
We found that there was a gradual increase in TIL as the melanocytic lesions moved towards various stages, from benign naevi (BN) to melanocytic dysplastic naevi (MDN) to radial growth phase cutaneous malignant melanoma (RGP‐CMM) to vertical growth phase cutaneous malignant melanoma (VGP‐CMM).
The numbers of TIL were higher at the stroma than at the parenchyma.
The gradual increase in TIL during melanoma tumorigenesis may reflect increased antigenicity of the tumour cells.
Abbreviations
BN - benign naevi
CTL - cytotoxic T cell
IL‐2R - interleukin‐2 receptor
IRS - immunoreactivity score
MDN - melanocytic dysplastic naevi
NK - natural killer
RGP‐CMM - radial growth phase cutaneous malignant melanoma
TIL - tumour infiltrating lymphocytes
VGP‐CMM - vertical growth phase melanoma
References
- 1.Hussein M R, Sun M, Roggero E.et al Loss of heterozygosity, microsatellite instability, and mismatch repair protein alterations in the radial growth phase of cutaneous malignant melanomas. Mol Carcinog 20023435–44. [DOI] [PubMed] [Google Scholar]
- 2.Hussein M R, Roggero E, Tuthill R J.et al Identification of novel deletion loci at 1p36 and 9p22–21 in melanocytic dysplastic nevi and cutaneous malignant melanomas. Arch Dermatol 2003139816–817. [DOI] [PubMed] [Google Scholar]
- 3.Hussein M R. Genetic pathways to melanoma tumorigenesis. J Clin Pathol 200457797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbott J J, Amirkhan R H, Hoang M P. Malignant melanoma with a rhabdoid phenotype: histologic, immunohistochemical, and ultrastructural study of a case and review of the literature. Arch Pathol Lab Med 2004128686–688. [DOI] [PubMed] [Google Scholar]
- 5.Wiznerowicz M, Fong A Z, Mackiewicz A.et al Double‐copy bicistronic retroviral vector platform for gene therapy and tissue engineering: application to melanoma vaccine development. Gene Ther 199741061–1068. [DOI] [PubMed] [Google Scholar]
- 6.Aruga A, Shu S, Chang A E. Tumor‐specific granulocyte/macrophage colony‐stimulating factor and interferon gamma secretion is associated with in vivo therapeutic efficacy of activated tumordraining lymph node cells. Cancer Immunol Immunother 199541317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole D J, Taubenberger J K, Pockaj B A.et al Histopathological analysis of metastatic melanoma deposits in patients receiving adoptive immunotherapy with tumor‐infiltrating lymphocytes. Cancer Immunol Immunother 199438299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang X, Kawakami Y, el‐Gamil M.et al Identification of a tyrosinase epitope recognized by HLA‐A24‐restricted, tumor‐infiltrating lymphocytes. J Immunol 19951551343–1348. [PubMed] [Google Scholar]
- 9.Keller J M, Maize J C. The clinical and histological differential diagnosis of malignant melanoma. Semin Oncol 199623693–702. [PubMed] [Google Scholar]
- 10.Metcalf J S. Melanoma: criteria for histological diagnosis and its reporting. Semin Oncol 199623688–692. [PubMed] [Google Scholar]
- 11.Wallon‐Dumon G, Dreno B. [The clinical diagnosis of melanoma]. Presse Med 20033233–38. [PubMed] [Google Scholar]
- 12.Giard R W, Neumann H A. [Diagnosis of pigmented skin lesions: how to recognize a malignant melanoma]. Ned Tijdschr Geneeskd 20041482261–2267. [PubMed] [Google Scholar]
- 13.Clemente C, Cochran A J, Elder D E.et al Histopathologic diagnosis of dysplastic nevi: concordance among pathologists convened by the World Health Organization Melanoma Programme. Hum Pathol 199122313–319. [DOI] [PubMed] [Google Scholar]
- 14.Wennberg A M. Basal cell carcinoma–new aspects of diagnosis and treatment. Acta Derm Venereol Supp l (Stockh) 20002095–25. [PubMed] [Google Scholar]
- 15.Brodland D G. Diagnosis of nonmelanoma skin cancer. Clin Dermatol 199513551–557. [DOI] [PubMed] [Google Scholar]
- 16.Balch C M, Buzaid A C, Atkins M B.et al A new American Joint Committee on Cancer staging system for cutaneous melanoma. Cancer 2000881484–1491. [DOI] [PubMed] [Google Scholar]
- 17.Hussein M R, Rashad U M. Rhinosporidiosis in Egypt: A case report and review of literature. Mycopathologia 2005159205–207. [DOI] [PubMed] [Google Scholar]
- 18.Clark W H, Jr, Elder D E, Guerry Dt.et al Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst 1989811893–1904. [DOI] [PubMed] [Google Scholar]
- 19.Slominski A, Ross J, Mihm M C. Cutaneous melanoma: pathology, relevant prognostic indicators and progression. Br Med Bull 199551548–569. [DOI] [PubMed] [Google Scholar]
- 20.Clemente C G, Mihm M C, Jr, Bufalino R.et al Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 1996771303–1310. [DOI] [PubMed] [Google Scholar]
- 21.Davidorf F H, Lang J R. Lymphocytic infiltration in choroidal melanoma and its prognostic significance. Trans Ophthalmol Soc U K 197797394–401. [PubMed] [Google Scholar]
- 22.Saito T, Tanaka R, Kouno M.et al [Immunohistological analysis of infiltrating lymphocyte subpopulations in gliomas and metastatic brain tumors]. No To Shinkei 198739339–345. [PubMed] [Google Scholar]
- 23.Becker I, Roggendorf W. Immunohistological investigation of mononuclear cell infiltrates in meningiomas. Acta Neuropathol (Berl) 198979211–216. [DOI] [PubMed] [Google Scholar]
- 24.Marsigliante S, Biscozzo L, Marra A.et al Computerised counting of tumour infiltrating lymphocytes in 90 breast cancer specimens. Cancer Lett 199913933–41. [DOI] [PubMed] [Google Scholar]
- 25.Hussein M R. The relationships between p53 protein expression and the clinicopathological features in the uveal melanomas. Cancer Biol Ther 20053983–988. [DOI] [PubMed] [Google Scholar]
- 26.Hussein M R, Ismael H H. Alterations of p53, Bcl‐2, and hMSH2 protein expression in the normal breast, benign proliferative breast disease, in situ and infiltrating ductal breast carcinomas in the Upper Egypt. Cancer Biol Ther 20043 [DOI] [PubMed] [Google Scholar]
- 27.Chan W Y, Cheung K K, Schorge J O.et al Bcl‐2 and p53 protein expression, apoptosis, and p53 mutation in human epithelial ovarian cancers. Am J Pathol 2000156409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hussein M R, Al‐Badaiwy Z H, Guirguis M N. Analysis of p53 and bcl‐2 protein expression in the non‐tumorigenic, pretumorigenic, and tumorigenic keratinocytic hyperproliferative lesions. J Cutan Pathol 200431643–651. [DOI] [PubMed] [Google Scholar]
- 29.Lyle S, Salhany K E, Elder D E. TIA‐1 positive tumor‐infiltrating lymphocytes in nevi and melanomas. Mod Pathol 20001352–55. [DOI] [PubMed] [Google Scholar]
- 30.Wakabayashi O, Yamazaki K, Oizumi S.et al CD4+ T cells in cancer stroma, not CD8+ T cells in cancer cell nests, are associated with favorable prognosis in human non‐small cell lung cancers. Cancer Sci 2003941003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robbins P F, El‐Gamil M, Li Y F.et al The intronic region of an incompletely spliced gp100 gene transcript encodes an epitope recognized by melanoma‐reactive tumor‐infiltrating lymphocytes. J Immunol 1997159303–308. [PubMed] [Google Scholar]
- 32.Luftl M, Schuler G, Jungbluth A A. Melanoma or not? Cancer testis antigens may help. Br J Dermatol 20041511213–1218. [DOI] [PubMed] [Google Scholar]
- 33.Prasad M L, Jungbluth A A, Patel S G.et al Expression and significance of cancer testis antigens in primary mucosal melanoma of the head and neck. Head Neck 2004261053–1057. [DOI] [PubMed] [Google Scholar]
- 34.dos Santos N R, Torensma R, de Vries T J.et al Heterogeneous expression of the SSX cancer/testis antigens in human melanoma lesions and cell lines. Cancer Res 2000601654–1662. [PubMed] [Google Scholar]
- 35.Tilkin A F, Lubin R, Soussi T.et al Primary proliferative T cell response to wild‐type p53 protein in patients with breast cancer. Eur J Immunol 1995251765–1769. [DOI] [PubMed] [Google Scholar]
- 36.Noguchi Y, Richards E C, Chen Y T.et al Influence of interleukin 12 on p53 peptide vaccination against established Meth A sarcoma. Proc Natl Acad Sci USA 1995922219–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fenton R G, Keller C J, Hanna N.et al Induction of T‐cell immunity against Ras oncoproteins by soluble protein or Ras‐expressing Escherichia coli. J Natl Cancer Inst 1995871853–1861. [DOI] [PubMed] [Google Scholar]
- 38.Gjertsen M K, Bakka A, Breivik J.et al Vaccination with mutant ras peptides and induction of Tcell responsiveness in pancreatic carcinoma patients carrying the corresponding RAS mutation. Lancet 19953461399–1400. [DOI] [PubMed] [Google Scholar]
- 39.Vicari A P, Caux C. Chemokines in cancer. Cytokine Growth Factor Rev 200213143–154. [DOI] [PubMed] [Google Scholar]
- 40.Kornstein M J, Brooks J S, Elder D E. Immunoperoxidase localization of lymphocyte subsets in the host response to melanoma and nevi. Cancer Res 1983432749–2753. [PubMed] [Google Scholar]
- 41.Niebauer G, Kokoschka E M. [Dysplastic nevus syndrome]. Wien Klin Wochenschr 198698673–678. [PubMed] [Google Scholar]
- 42.Flageul B, Bachelez H, Boumsell L.et al Infiltrating lymphocytes in benign and malignant naevomelanocytic lesions. Nouv Rev Fr Hematol 1990329–11. [PubMed] [Google Scholar]
- 43.Roush G C, Barnhill R L. Correlation of clinical pigmentary characteristics with histopathologically‐confirmed dysplastic nevi in nonfamilial melanoma patients. Studies of melanocytic nevi IX. Br J Cancer 199164943–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salopek T G, Jimbow K. Dysplastic melanocytic nevus. Immunohistochemical heterogeneity by melanosomal markers and S‐100. Am J Dermatopathol 199113130–136. [DOI] [PubMed] [Google Scholar]
- 45.van Duinen C M, van den Broek L J, Vermeer B J.et al The distribution of cellular adhesion molecules in pigmented skin lesions. Cancer 1994732131–2139. [DOI] [PubMed] [Google Scholar]
- 46.Butcher E C, Williams M, Youngman K.et al Lymphocyte trafficking and regional immunity. Adv Immunol 199972209–253. [DOI] [PubMed] [Google Scholar]
- 47.Campbell J J, Butcher E C. Chemokines in tissue‐specific and microenvironment‐specific lymphocyte homing. Curr Opin Immunol 200012336–341. [DOI] [PubMed] [Google Scholar]
- 48.Fritsch M, Rosenberg S A, Duray P H. Immunohistologic responses within dermal metastatic melanoma lesions of patients treated with a synthetic peptide vaccine. J Immunother 200023557–569. [DOI] [PubMed] [Google Scholar]
- 49.Perez J, Garcia P M, Bautista M J.et al Immunohistochemical characterization of tumor cells and inflammatory infiltrate associated with cutaneous melanocytic tumors of Duroc and Iberian swine. Vet Pathol 200239445–451. [DOI] [PubMed] [Google Scholar]
- 50.Barbour A H, Coventry B J. Dendritic cell density and activation status of tumour‐infiltrating lymphocytes in metastatic human melanoma: possible implications for sentinel node metastases. Melanoma Res 200313263–269. [DOI] [PubMed] [Google Scholar]
- 51.Balch C M, Riley L B, Bae Y J.et al Patterns of human tumor‐infiltrating lymphocytes in 120 human cancers. Arch Surg 1990125200–205. [DOI] [PubMed] [Google Scholar]
- 52.Azogui O, Avril M F, Margulis A.et al Tumor‐infiltrating CD3‐ NK cells are more effective than CD3+ T cells in killing autologous melanoma cells. J Invest Dermatol 199197425–429. [DOI] [PubMed] [Google Scholar]
- 53.Tefany F J, Barnetson R S, Halliday G M.et al Immunocytochemical analysis of the cellular infiltrate in primary regressing and non‐regressing malignant melanoma. J Invest Dermatol 199197197–202. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi T, Chapman P B, Yang S Y.et al Reactivity of autologous CD4+ T lymphocytes against human melanoma. Evidence for a shared melanoma antigen presented by HLA‐DR15. J Immunol 1995154772–779. [PubMed] [Google Scholar]
- 55.Thomas W D, Hersey P. CD4 T cells kill melanoma cells by mechanisms that are independent of Fas (CD95). Int J Cancer 199875384–390. [DOI] [PubMed] [Google Scholar]
- 56.Markus N R, Rosenberg S A, Topalian S L. Analysis of cytokine secretion by melanoma‐specific CD4+ T lymphocytes. J Interferon Cytokine Res 199515739–746. [DOI] [PubMed] [Google Scholar]
- 57.Morisaki T, Morton D L, Uchiyama A.et al Characterization and augmentation of CD4+ cytotoxic T cell lines against melanoma. Cancer Immunol Immunother 199439172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anderson P. TIA‐1: structural and functional studies on a new class of cytolytic effector molecule. Curr Top Microbiol Immunol 1995198131–143. [DOI] [PubMed] [Google Scholar]
- 59.Hernberg M, Turunen J P, Muhonen T.et al Tumor‐infiltrating lymphocytes in patients with metastatic melanoma receiving chemoimmunotherapy. J Immunother 199720488–495. [DOI] [PubMed] [Google Scholar]
- 60.Matsuda M, Petersson M, Lenkei R.et al Alterations in the signal‐transducing molecules of T cells and NK cells in colorectal tumor‐infiltrating, gut mucosal and peripheral lymphocytes: correlation with the stage of the disease. Int J Cancer 199561765–772. [DOI] [PubMed] [Google Scholar]
- 61.Kawata A, Une Y, Hosokawa M.et al Tumor‐infiltrating lymphocytes and prognosis of hepatocellular carcinoma. Jpn J Clin Oncol 199222256–263. [PubMed] [Google Scholar]
- 62.Kolbeck P C, Kaveggia F F, Johansson S L.et al The relationships among tumor‐infiltrating lymphocytes, histopathologic findings, and long‐term clinical follow‐up in renal cell carcinoma. Mod Pathol 19925420–425. [PubMed] [Google Scholar]
- 63.Eerola A K, Soini Y, Paakko P. A high number of tumor‐infiltrating lymphocytes are associated with a small tumor size, low tumor stage, and a favorable prognosis in operated small cell lung carcinoma. Clin Cancer Res 200061875–1881. [PubMed] [Google Scholar]
- 64.Hussein M R. Dendritic cells and melanoma tumorigenesis: an insight. Cancer Biol Ther 20054 [DOI] [PubMed] [Google Scholar]