Abstract
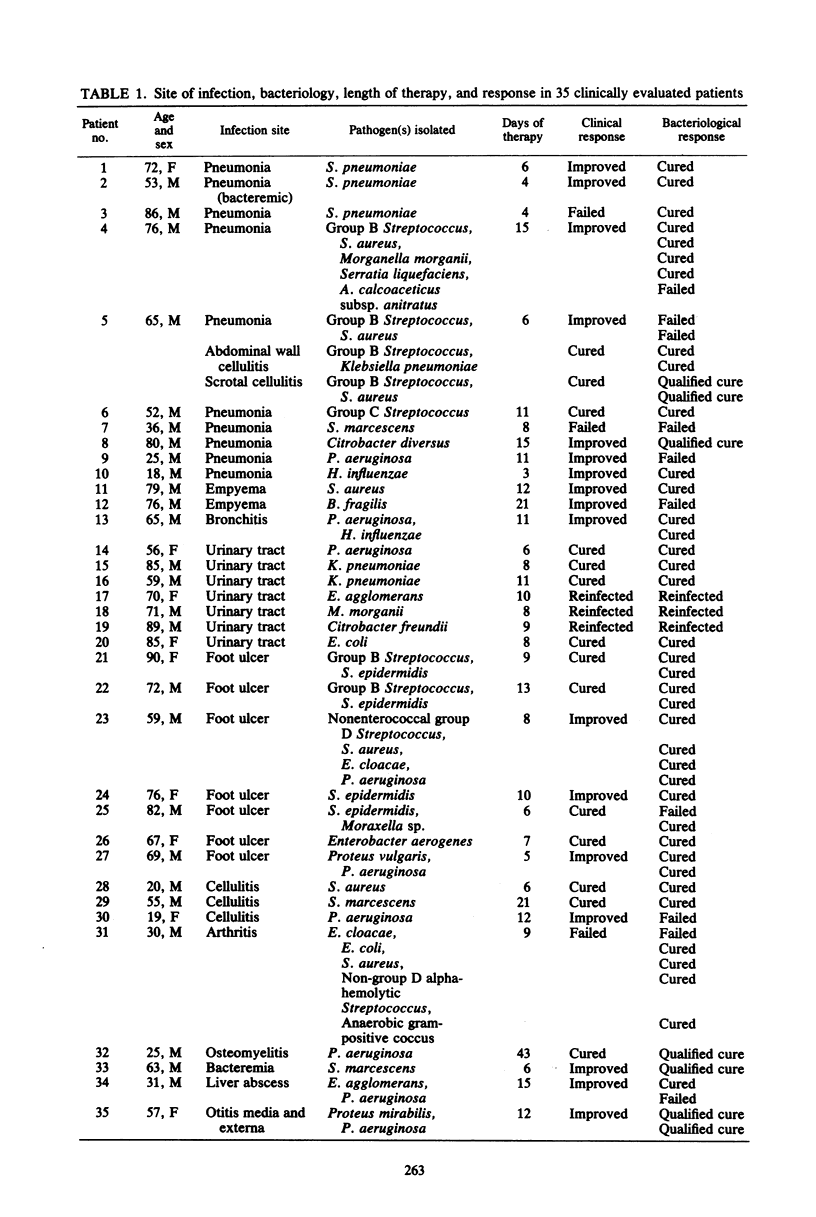
We evaluated the efficacy and safety of ceftriaxone in 50 adults with serious infections, usually giving 1 g every 12 h. Of the 35 patients who could be evaluated for clinical efficacy, 15 had failed on previous therapy, 15 had nosocomial infections, and all but 1 had underlying diseases. One patient had three sites of infection. Favorable responses were seen in 34 of 37 infections, including 11 of 13 respiratory tract infections, all 7 urinary tract infections, all 12 skin and soft tissue infections, 1 of 2 bone and joint infections, a catheter-related septicemia, a liver abscess, and an otitis media and externa. Favorable bacteriological responses were seen for 48 of 58 organisms. This included 6 of 7 Staphylococcus aureus strains, 14 of 16 other aerobic gram-positive cocci, 18 of 20 Enterobacteriaceae, 6 of 9 Pseudomonas aeruginosa, and 1 of 2 anaerobes. Peak plasma ceftriaxone levels on day 1 were 152 micrograms/ml by bioassay and 78 micrograms/ml by high-pressure liquid chromatography. Four of the 31 initial isolates of aerobic gram-negative rods developed resistance to ceftriaxone on disk diffusion testing. Diarrhea occurred in 3 of 50 patients. All three had received a higher than usual dose. Drug administration was stopped twice, once for a thrombocytopenia and once for a thrombocytopenia with leukopenia. Neither problem could be attributed exclusively to ceftriaxone. Other adverse reactions were eosinophilia, abdominal pain, inguinal candidiasis, and nonsuppurative phlebitis. Even among debilitated adults, ceftriaxone was safe and effective in a twice daily regimen.
Full text
PDF

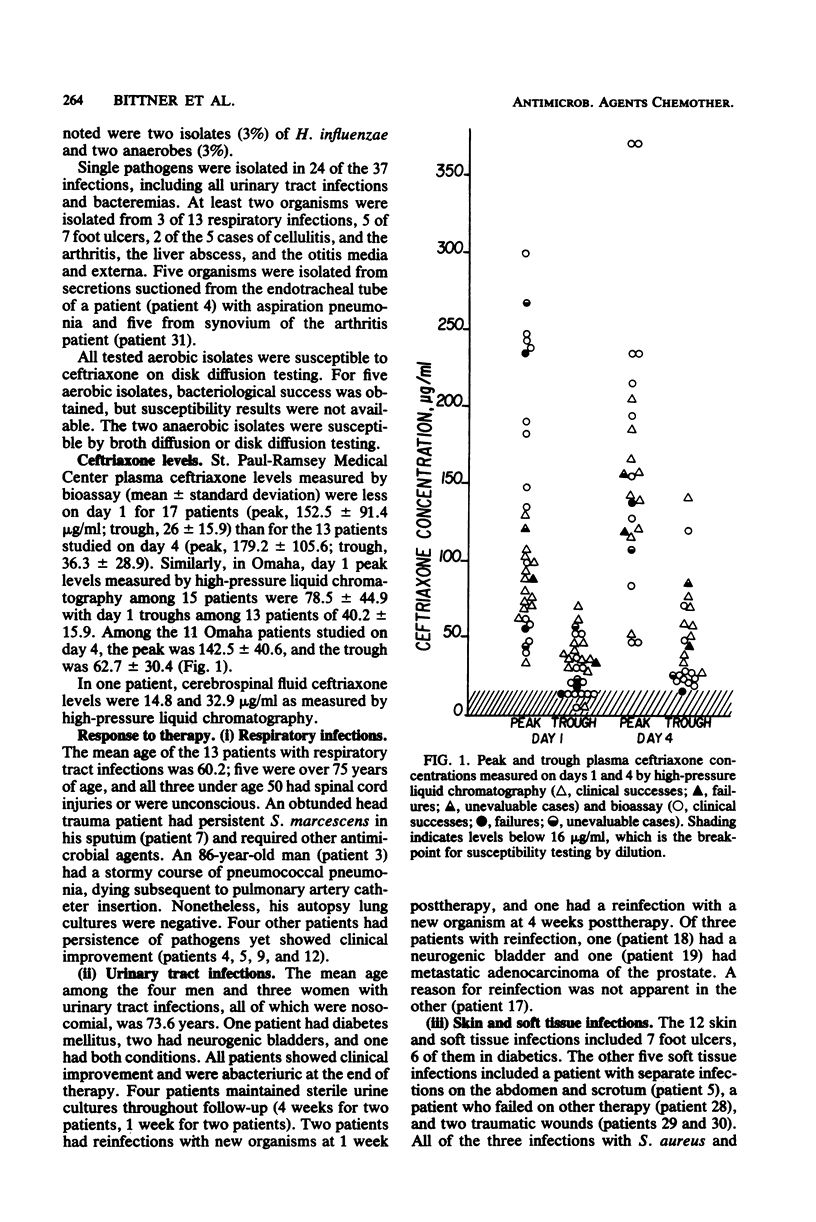


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angehrn P., Probst P. J., Reiner R., Then R. L. Ro 13-9904, a long-acting broad-spectrum cephalosporin: in vitro and in vivo studies. Antimicrob Agents Chemother. 1980 Dec;18(6):913–921. doi: 10.1128/aac.18.6.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J. S., Hasselquist S. M., Simon G. L. Efficacy of ceftriaxone in serious bacterial infections. Antimicrob Agents Chemother. 1982 Mar;21(3):402–406. doi: 10.1128/aac.21.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. J., Westmacott D., Wong-Kai-In P. Comparative in-vitro activity and mode of action of ceftriaxone (Ro 13-9904), a new highly potent cephalosporin. J Antimicrob Chemother. 1981 Sep;8(3):193–203. doi: 10.1093/jac/8.3.193. [DOI] [PubMed] [Google Scholar]
- Kirby W. M., Craig W. A. Theory and applications of pulse dosing: a summary of the symposium. Rev Infect Dis. 1981 Jan-Feb;3(1):1–3. doi: 10.1093/clinids/3.1.1. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Meropol N. J., Fu K. P. Antibacterial activity of ceftriaxone (Ro 13-9904), a beta-lactamase-stable cephalosporin. Antimicrob Agents Chemother. 1981 Mar;19(3):414–423. doi: 10.1128/aac.19.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel I. H., Chen S., Parsonnet M., Hackman M. R., Brooks M. A., Konikoff J., Kaplan S. A. Pharmacokinetics of ceftriaxone in humans. Antimicrob Agents Chemother. 1981 Nov;20(5):634–641. doi: 10.1128/aac.20.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickup M. E., Bird H. A., Lowe J. R., Lees L., Wright V. A pharmacokinetic and tolerance study of Ro13-9904, a new cephalosporin antibiotic. Br J Clin Pharmacol. 1981 Aug;12(2):111–115. doi: 10.1111/j.1365-2125.1981.tb01188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Moellering R. C., Jr, Martin R. R., Perkins R. L., Strike D. G., Gootz T. D., Sanders W. E., Jr Resistance to cefamandole: a collaborative study of emerging clinical problems. J Infect Dis. 1982 Jan;145(1):118–125. doi: 10.1093/infdis/145.1.118. [DOI] [PubMed] [Google Scholar]
- Stoeckel K., McNamara P. J., Brandt R., Plozza-Nottebrock H., Ziegler W. H. Effects of concentration-dependent plasma protein binding on ceftriaxone kinetics. Clin Pharmacol Ther. 1981 May;29(5):650–657. doi: 10.1038/clpt.1981.90. [DOI] [PubMed] [Google Scholar]
- Yu V. L. Enterococcal superinfection and colonization after therapy with moxalactam, a new broad-spectrum antibiotic. Ann Intern Med. 1981 Jun;94(6):784–785. doi: 10.7326/0003-4819-94-6-784. [DOI] [PubMed] [Google Scholar]


