Abstract
Aims
Overexpression and mutation of epidermal growth factor regulator (EGFR) are frequently found in the carcinogenesis of non‐small cell lung cancer (NSCLC). Because targeting of this receptor has proven therapeutic efficacy, studying EGFR has become a matter of particular scientific interest. The present study analysed the EGFR receptor, rate of EGFRvIII mutations, and rate of activated phosphorylated EGFR (pEGFR) by immunohistochemistry on cryostat sections.
Methods
Surgically obtained tumour specimens of a series of 78 NSCLC patients and 66 adjacent tumour free specimens were examined immunohistochemically using monoclonal antibodies to stain EGFR, pEGFR, and EGFRvIII.
Results
EGFRvIII and pEGFR expression was found in 42% and 26% of the tumours respectively and both were increased significantly compared with tumour free samples. EGFR, pEGFR, and EGFRvIII expression did not correlate with any of the previously tested markers (c‐erbB‐2, c‐erbB‐3, p53, ki‐67, and microvessel density). Similar distributions of immunohistochemical profiles were seen, regardless of histological subtype, age, or sex. In stage I patients, EGFR phosphorylation at tyrosine residue 845 proved to be an independent prognostic factor.
Conclusion
Because pEGFR correlated with poor prognosis, it can be speculated that it plays a crucial biological role in the pathogenesis of NSCLC.
Keywords: EGFR, EGFRvIII, phosphorylation, non‐small cell lung cancer, prognosis
Non‐small cell lung cancer (NSCLC) belongs to the group of most common malignant diseases with the highest mortality rate in industrial nations.1 Only 30% of patients can be treated surgically in a curative attempt, but for the majority of patients, traditional treatment options are of modest efficacy.2,3,4
Recently, new treatment strategies based on targeting molecular alterations have raised hopes of developing an effective and well tolerated medication against this fatal disease.3,4 Inhibition of the epidermal growth factor receptor (EGFR) is one of the most promising approaches.5,6,7 EGFR is a 170 kDa tyrosine kinase receptor consisting of an extracellular domain, a transmembrane domain, and a cytoplasmic domain comprised of the tyrosine kinase domain and the C‐terminus region with multiple tyrosine residues. Activation of the receptor occurs when a specific ligand binds and subsequently induces homodimerisation with another EGFR, or heterodimerisation with a member of the tyrosine kinase receptor family.4,8
Overexpression of EGFR is frequently found in NSCLC (32–81%).5 The EGFR signal regulates proliferation, apoptosis, angiogenesis, cell adhesion, and motility, and therefore the receptor has a great impact on tumour growth and progression.4 Nevertheless, the prognostic potential of EGFR overexpression in NSCLC is controversial.9 However, recent data on EGFR show that both mutations and the activation status, defined by phosphorylation, might have a strong impact on the clinical course.10,11,12 By far the most common mutation is EGFRvIII, found in 16%–39% of NSCLC.11,12,13,14 EGFRvIII lacks the extracellular ligand binding domain, because of deletion of exon 2–7. Although ligand binding is impossible, the tyrosine kinase is constitutively activated, which leads to various functional signalling differences from EGFR.15,16,17,18 The prognostic relevance of EGFRvIII has so far not been proven.12,19
The aim of the present study was to evaluate EGFR receptor status by analysing the rate of EGFRvIII mutations and the rate of EGFR phosphorylation on cryostat sections and to correlate these findings with clinical parameters, immunohistochemical expression of p53, ki‐67, c‐erbB‐2 and c‐erbB3, and microvessel density (MVD).
PATIENTS AND METHODS
Patients
In total, 155 lung tissue specimens from 78 patients and 11 postmortem normal lung tissue samples were analysed. Tumour and adjacent tumour free tissue samples were obtained surgically. From these, 78 tumour and 66 adjacent histological tumour free specimens were selected. One part of the sample was fixed in 10% formalin and routinely processed for paraffin embedding. The samples for immunohistochemical analysis were snap frozen immediately in liquid nitrogen and stored at −80°C until sectioning.
All surgical tumour specimens were classified histopathologically according to the World Health Organisation Classification.20 Histological diagnosis of the 78 NSCLC patients revealed 36 adenocarcinomas, 37 squamous cell carcinomas, 3 large cell carcinomas, and 2 carcinoid tumours. According to UICC recommendations, 51 patients were classified as stage I, 16 as stage II, 9 as stage III, and 2 as stage IV. All 78 patients were treated surgically between 1994 and 2001. Patients in advanced clinical stages were also treated with chemotherapy and/or radiotherapy. Median observation time was 29.3 months (range 0.6–127), and 29 patients died in this period of time. The median age of the patients at the time of surgery was 61 years (range 37–79), and the male to female ratio was 3.3:1 (60 men and 18 women).
Routine postmortem lung tissue samples of the 11 patients without a malignant disease served as the control group for immunohistochemical analysis.
Immunohistochemical staining
We examined fresh frozen lung tissue samples for the expression of EGFR, EGFR phosphorylation, and EGFRvIII with established immunoperoxidase staining methods as described previously.21
Frozen lung tissue specimens were cut with a cryostat at 3–5 μm and mounted on slides. The sections were air dried and fixed with acetone. Before incubation with primary antibodies, a permeabilisation step was performed. All antibodies were diluted with phosphate buffered saline (PBS) containing 1% bovine serum albumin (BSA). Primary antibodies were incubated for 45 minutes. The slides were washed three times in TPS for 5 minutes each, then incubated with the bridging antibody (Dako Z0259), followed by alkaline phosphatase complex (Dako D0651). For pEGFR staining, an additional antibody (Dako M0737) was used. Sections were slightly counterstained with Mayer's haemalum (Merck) and mounted with Aquatex (Merck).
The following primary antibodies were used: EGFR (clone EGFR 1; Pharmingen, USA), polyclonal rabbit phospho‐EGF receptor (Tyr845) antibody (Cell Signaling, Beverly, MA, USA; 1:200 dilution), and monoclonal mouse anti‐EGFRvIII (clone G100; Zymed, San Francisco, CA, USA; 1:100 dilution).
Immunohistochemical evaluation
The slides were evaluated by at least two of the authors (WH, SD, or BS) using a semiquantitative method on a Zeiss light microscope (Axioskop 2). The percentages of immunopositive cells in the representative areas of the sections were assessed, and the intensity of immunohistochemical staining divided into four categories: negative (–), low (+), moderate (2+), and high (3+). Only cells with moderate or high staining intensities were counted. Based on the results achieved from the tumour free specimens of the NSCLC patients (66 tumour free tissues), the cutoff levels were defined as follows (mean value ± 2SD): EGFR >0.3%, pEGFR >0.6% and EGFRvIII >0.2%. All cases with moderate or high staining intensity and percentages of immunopositive cells above the cutoff point were scored as “positive”.
Comparison with previous data
Previously we reported on the immunohistochemical expression of various markers in NSCLC.21 To evaluate the biological relevance of EGFR, pEGFR, and EGFRvIII, the results were compared retrospectively with the following parameters achieved from the same patients: c‐erbB‐2, c‐erbB‐3, p53, ki‐67, and MVD.
Statistical analysis
The Pearson χ2 test was used to evaluate the differences between the groups. Significance was determined using 95% confidence intervals. The log rank test and Cox proportional hazards model were applied to examine the relationship between cancer specific survival and the immunohistochemical markers. Cancer specific survival was defined as the time between surgery and death or last follow up. All statistical procedures were performed with SPSS statistical software (version 12.0, SPSS Inc, Chicago, IL, USA).
RESULTS
The analysis was based on three different groups of lung samples: (a) tumour specimens of NSCLC patients (tumour group), n = 78; (b) histologically tumour free specimens of the same NSCLC patients (tumour free group) to detect premalignant lesions and determine cutoff levels, n = 66; and (c) tumour free specimens of non‐tumour patients (control group) obtained by postmortem examination, n = 11.
Immunohistochemical expression of EGFR, pEGFR, and EGFRvIII
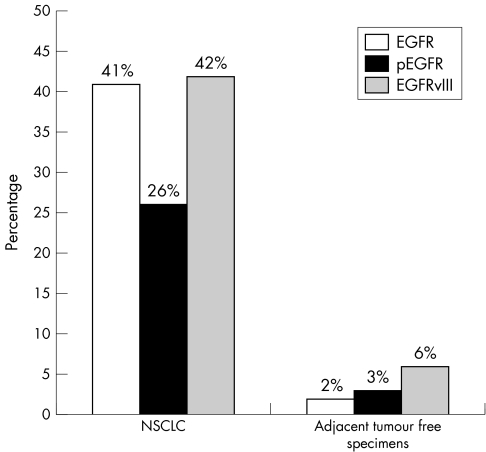
In the tumour group, EGFR expression was increased in 41%, pEGFR in 26%, and EGFRvIII in 42% of the cases (pEGFR staining, fig 1; EGFRvIII, fig 2). The tumour free cohort revealed a significantly decreased rate of expression of all three tested receptors: EGFR in 2%, pEGFR in 3%, and EGFRvIII in 6% of the samples (p = 0.000), and the normal lung tissues of the autopsy samples were negative for all three (fig 3). Increased pEGFR and EGFRvIII expression correlated significantly (p = 0.000) with phosphorylation or EGFRvIII mutation, whereas EGFR expression was independent of both (tables 1 and 2).
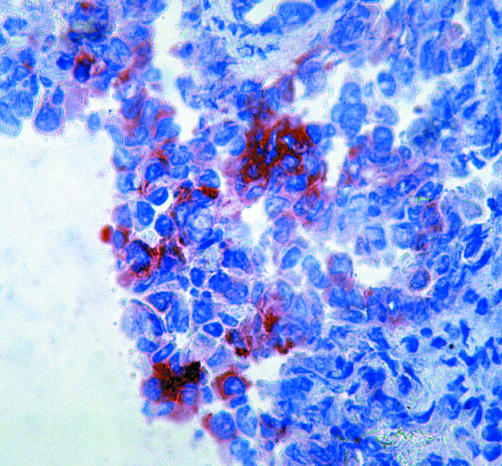
Figure 1 pEGFR positive cells in a NSCLC specimen, APAAP staining on cryostat sections.
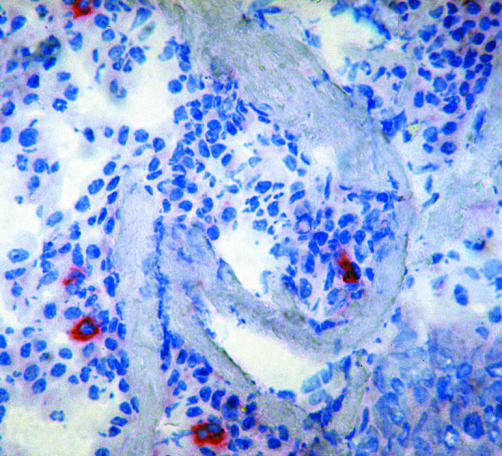
Figure 2 EGFRvIII positive cells in a NSCLC specimen, APAAP staining on cryostat sections.
Figure 3 Percentage of positive cases expressing EGFR, EGFRvIII, and pEGFR in the tumour group compared with the tumour free specimens.
Table 1 Comparison of immunohistochemical markers (number of positive cases in %).
| Parameter | Tumour specimens of NSCLC | Tumour free specimens of NSCLC | Tumour free specimens, controls | p* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Median (range) of positive cells, % | Positive | Median (range) of positive cells, % | Positive | Median (range) of positive cells, % | |||||||||
| pEGFR | 20/77 (26%) | 0 (0 to 11.9) | 2/66 (3%) | 0 (0 to 1.5) | 0/11 (0%) | 0 (0 to 0) | 0.000 | |||||||
| EGFRvIII | 33/78 (42%) | 0.1 (0 to 5.7) | 4/66 (6%) | 0 (0 to 0.5) | 0/11 (0%) | 0 (0 to 0) | 0.000 | |||||||
| EGFR | 32/79 (41%) | 3 (0 to 80) | 1/66 (2%) | 0 (0 to 1) | 0/11 (0%) | 0 (0 to 0) | 0.000 | |||||||
*Correlation of parameters with tumour (Pearson χ2). Cutoff values: pEGFR >0.6%, EGFRvIII >0.2%, EGFR ⩾0.3%. EGFR, epidermal growth factor receptor.
Table 2 Comparison of immunohistochemical markers in NSCLC with clinical parameters.
| Parameters | Total | pEGFR | EGFRvIII | EGFR | cerbB2 | cerbB3 | p53 | Ki‐67 | MVD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 79 | |||||||||||||||||
| Clinical parameters | ||||||||||||||||||
| AC | 37 | 6/36 | 14/36 | 11 | 16 | 17* | 13 | 13 | 11 | |||||||||
| SCC | 37 | 11 | 17 | 19 | 12 | 27* | 15 | 24 | 17 | |||||||||
| LCC | 3 | 2 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | |||||||||
| Carcinoid | 2 | 1/1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | |||||||||
| Death | 29 | 10 | 12 | 12 | 10 | 21 | 13 | 14 | 12 | |||||||||
| IHC | ||||||||||||||||||
| pEGFR | 20/77 | – | 15* | 9 | 8 | 14 | 10 | 8 | 7 | |||||||||
| EGFRvIII | 33/78 | 15* | – | 11 | 11 | 18 | 14 | 14 | 13 | |||||||||
| EGFR | 32 | 9 | 11 | – | 10 | 22 | 9 | 18 | 10 | |||||||||
| cerbB2 | 29 | 8/28 | 11/28 | 10 | – | 21 | 12 | 15 | 5* | |||||||||
| cerbB3 | 46 | 14 | 18 | 22 | 21 | – | 18 | 28* | 18 | |||||||||
| p53 | 29 | 10/27 | 14/27 | 9 | 12 | 18 | – | 18* | 12 | |||||||||
| Ki‐67 | 36 | 8 | 14 | 18 | 15 | 28* | 18* | – | 13 | |||||||||
| MVD | 29 | 7 | 13 | 10 | 5* | 18 | 12 | 13 | – |
*Significant correlation between two parameters (Pearson χ2 test, p<0.05). NSCLC, non‐small cell lung cancer; AC, adenocarcinoma; SCC, squamous cell carcinoma; LCC, large cell carcinoma; IHC, immunohistochemistry; EGFR, epidermal growth factor; MVD, microvessel density.
Comparison of EGFR, pEGFR and EGFRvIII with other immunohistochemical parameters
EGFR, pEGFR, and EGFRvIII expression did not correlate with any of the previously tested markers (c‐erbB‐2, c‐erbB‐3, p53, ki‐67, and microvessel density). Only for p53 expression was a statistical correlation with pEGFR expression detected (p = 0.096). All correlations are shown in table 2.
Comparison of EGFR, pEGFR and EGFRvIII with clinical characteristics
Similar distributions of immunohistochemical parameters were seen with regard to age and sex.
In the pEGFR positive group, large cell carcinomas (10% v 1.8%) and squamous cell carcinomas (SCC; 55% v 46%) were more commonly seen, whereas the number of adenocarcinomas (30% v 52.6%) was decreased compared with pEGFR negative patients. For EGFRvIII, the histological subtypes were distributed similarly, with a slightly increased rate of SCC (55% v 43%). However, none of these differences reached statistical significance.
No significant imbalance was found for the clinical stages of the patients, but there was a somewhat higher rate of stage I in the pEGFR and EGFRvIII positive cohorts compared with the negative cases.
Survival and EGFR, pEGFR, and EGFRvIII
Neither EGFR overexpression nor EGFRvIII expression was significantly correlated with survival. Only for pEGFR positive patients was a statistical trend to shorter survival observed (p = 0.091).
Subanalysis of stage I tumour patients
Because advanced clinical stages go together with poor prognosis, the predictive value of biological markers could be misinterpreted. Therefore 50 stage I patients were analysed separately.
Of 50 tissue slides, 23 (46%) were EGFRvIII positive. An inverse correlation between EGFRvIII and cerbB‐3 was found (p = 0.005). However, EGFRvIII revealed no prognostic relevance in log rank analysis.
Increased pEGFR expression was found in 15 of 49 (30.6%) examined patients, which did not correlate with any of the other immunohistochemical parameters. Furthermore, pEGFR expression was not influenced by the distribution of age, sex, or histology. Consequently, pEGFR seems to be an independent parameter. Interestingly, patients with positive pEGFR staining had a higher mortality (60% v 23,5%, p = 0.010) and the Kaplan‐Meier curve (fig 4) showed a significantly decreased survival probability (log rank p = 0.006).
Figure 4 Survival of 49 stage I NSCLC patients, Comparison of the survival probability between the pEGFR negative (A) and pEGFR positive (B) groups (log rank p = 0.0062).
DISCUSSION
EGFR represents a cornerstone in signal transduction and cell growth control. EGFR overexpression and mutation play an important role in the carcinogenesis of NSCLC and occur frequently in this disease.5,6,22,23 Because targeting of this receptor has proven therapeutic efficacy, studying EGFR has become a matter of particular scientific interest.24,25,26,27,28,29,30 Therefore, this study analysed the EGFR receptor by evaluating the rate of EGFRvIII mutations and the rate of activated phosphorylated EGFR by applying immunohistochemistry to cryostat sections.
We were able to demonstrate that (a) the rate of EGFR, pEGFR, and EGFRvIII expression in lung cancer specimens is significantly increased compared with tumour free specimens, and (b) pEGFR is a valuable prognostic factor, especially in stage I patients.
EGFR overexpression was observed in 41% of 79 NSCLC specimens,31 a result that lies in the range of those found in previous studies, which reported overexpression in 32–81% of the cases.32,33,34,35,36,37,38 In contrast to other solid tumours, EGFR expression in NSCLC is not associated with poor prognosis.4 In a previous study, our group found that neither EGFR‐PCR positivity nor EGFR‐immunohistochemical expression reached statistical significance in survival analysis.31 Similarly, in a recent meta‐analysis, 7 of 10 studies failed to prove the prognostic relevance of EGFR overexpression.9
Increasing knowledge about EGFR led to the presumption that EGFRvIII or EGFR phosphorylation are more likely to be of biological relevance in NSCLC.10,12,13,39,40 Our study showed that 42% of tumour samples were positive for EGFRvIII overexpression. Okamoto and colleagues published an EGFRvIII incidence of 39% using the same antibody and a comparable number of paraffin embedded NSCLC specimens.12 Interestingly, in stage I patients, an non‐significant inverse correlation with c‐erbB‐1 was seen. However, this was expected, as EGFRvIII represents the mutated variant of c‐erbB‐1.23,40 Furthermore a significantly decreased rate of c‐erbB‐3 was detected in cases with EGFRvIII expression, which indicates that transcription of the truncated form of c‐erbB‐341 is associated with EGFRvIII mutation.
Another investigation in our study analysed EGFR phosphorylation in NSCLC specimens. We used an antibody that detects epitopes that are exposed only after phosphorylation of Tyr845. This tyrosine residue is located in the activation loop of the receptor kinase domain and seems to be important for maintaining tyrosine kinase in an active state.42 Tyr845 and Tyr1101 have been identified as c‐src dependent sites of phosphorylation.43 In this study, 26% of the tumour specimens showed increased Tyr845 phosphorylation.
Furthermore, a strong association between EGFRvIII and pEGFR (p = 0.000) was seen. Association between EGFRvIII and pEGFR is of special interest because the oncogenic potential of EGFRvIII is caused by constitutive activation of the tyrosine kinase domain, which leads to autophosphorylation as well as phosphorylation of substrates such as Shc.17,18,44 Analysis of individual phosphorylation sites showed that EGFRvIII uses the same spectrum of autophosphorylation sites as normal EGFR.15,45
So far, five autophosphorylation sites have been identified in EGFR: three major (Tyr1068, Tyr1148, and Tyr1173) and two minor (Tyr992 and Tyr1086) sites.43 The analysis of the Tyr845 site in our study showed that not all EGFRvIII positive cases (33 tumours) has positive staining with the p845 antibody (17 tumours were p845 negative).
Okamoto et al investigated the major autophosphorylation site Tyr1173, and found that all EGFRvIII expressing tumours (7 cases) were p1173 positive but neither parameter was of prognostic relevance.12 In contrast, we found that Tyr p845 positive expression correlated marginally with the prognosis for the whole group (n = 77). This finding agrees with the assumption that c‐Src plays a significant role in the regulation of growth factor receptor function and signal transduction.43
Eliminating the prognostic bias of advanced clinical stages by analysing stage I patients separately, Tyr845 phosphorylation was highly predictive for poor survival. In accordance with these results, a previous smaller study (n = 36) revealed pEGFR expression in 44% of the cases, and a correlation with significant survival disadvantage was seen, independent of stage.10 The authors used the same antibody as us, but on paraffin embedded material.
Theoretically, an increased rate of pEGFR or EGFRvIII should lead to an increased rate of proliferating cells, marked by an increased rate of ki‐67, p53, or MVD, which signifies biologically more aggressive tumours.46 Surprisingly, no correlations were seen for ki‐67 or MVD, but in stage I patients a marginal correlation was detected between pEGFR and p53 expression.
The microscopically tumour free samples of lung cancer patients revealed a very low rate of positive cases (⩽6%) for all three EGFR tested parameters. With regard to the significantly higher rates in the tumour samples, overexpression seems to be a late event in lung carcinogenesis. It is well known that EGFR expression can revert to normal after smoking cessation.35 To what extent EGFRvIII or pEGFR expression are reversible is unclear. The theoretical method of EGFR receptor regulation by endocytosis, internalisation, and recycling47 cannot be directly applied to EGFRvIII, because Huang et al45 found that defective endocytosis and receptor downregulation lead to prolonged signalling of EGFRvIII.
TAKE HOME MESSAGES
EGFRvIII and pEGFR expression was found in 42% and 26% of the tumours respectively, and both were at significantly higher levels than in tumour free samples.
EGFR, pEGFR, and EGFRvIII expression did not correlate with any of the previously tested markers (c‐erbB‐2, c‐erbB‐3, p53, ki‐67, and microvessel density).
In stage I patients, EGFR phosphorylation at tyrosine residue 845 proved to be an independent prognostic factor.
Correlation of pEGFR with poor prognosis indicates that it plays a crucial biological role in the pathogenesis of NSCLC.
As phosphorylation of tyrosine 845 has proven to be an independent prognostic factor in early tumour stages, it can be speculated that it plays a crucial role in EGFR signalling and thus the pathogenesis of NSCLC. Whether or not pEGFR could function as a predictor for anti‐EGFR therapies needs to be answered in further studies.
Abbreviations
EGFR - epidermal growth factor regulator
MVD - microvessel density
NSCLC - non‐small cell lung cancer
pEGFR - phosphorylated epidermal growth factor regulator
SCC - squamous cell carcinoma
References
- 1.Parkin D M. Global cancer statistics in the year 2000. Lancet Oncol 20012533–543. [DOI] [PubMed] [Google Scholar]
- 2.Dy G K, Adjei A A. Novel targets for lung cancer therapy: part I. J Clin Oncol 2002202881–2894. [DOI] [PubMed] [Google Scholar]
- 3.Bunn P A, Jr, Shepherd F A, Sandler A.et al Ongoing and future trials of biologic therapies in lung cancer. Lung Cancer 200341175–186. [DOI] [PubMed] [Google Scholar]
- 4.Kris M G, Azzoli C G, Miller V A. Epidermal growth factor receptor blockade: targeted therapy for non‐small‐cell lung cancer. In: ASCO Educational Book. Alexandria, USA: ASCO, 2002693–699.
- 5.Selvaggi G, Novello S, Torri V.et al Epidermal growth factor receptor overexpression correlates with a poor prognosis in completely resected non‐small‐cell lung cancer. Ann Oncol 20041528–32. [DOI] [PubMed] [Google Scholar]
- 6.Hong W K, Ullrich A. The role of EGFR in solid tumours and implications for therapy. Oncol Biother 200011–35. [Google Scholar]
- 7.Onn A, Correa A M, Gilcrease M.et al Synchronous overexpression of epidermal growth factor receptor and HER2‐neu protein is a predictor of poor outcome in patients with stage I non‐small cell lung cancer. Clin Cancer Res 200410136–143. [DOI] [PubMed] [Google Scholar]
- 8.Arteaga C L. Epidermal growth factor receptors and erbB‐2 in human lung cancer. In: Pass HI, ed. Lung cancer: principles and practice. MA: Lippincott‐Raven, 199699–106.
- 9.Nicholson R I, Gee J M W, Harper M E. EGFR and cancer prognosis. Eur J Cancer 2001379–15. [DOI] [PubMed] [Google Scholar]
- 10.Kanematsu T, Yano S, Uehara H.et al Phosphorylation, But not overexpression, of epidermal growth factor receptor is associated with poor prognosis of non‐small cell lung cancer patients. Oncology Res 200313289–298. [DOI] [PubMed] [Google Scholar]
- 11.Moscatello D K, Holgado‐Madruga M, Godwin A K.et al Frequent expression of a mutant epidermal growth factor receptor in multiple human tumours. Cancer Res 1995555536–5539. [PubMed] [Google Scholar]
- 12.Okamoto I, Kenyon L C, Emlet D R.et al Expression of constitutively activated EGFRvIII in non‐small cell lung cancer. Cancer Sci 20039450–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorimer I A J. Mutant epidermal growth factor receptors as targets for cancer therapy. Curr Cancer Drug Targets 2002291–102. [DOI] [PubMed] [Google Scholar]
- 14.Garcia de Palazzo I E, Adams G P, Sundareshan P.et al Expression of mutated epidermal growth factor receptor by non‐small cell lung carcinomas. Cancer Res 1993533217–3220. [PubMed] [Google Scholar]
- 15.Fernandes H, Cohen S, Bishayee S. Glycosylation‐induced conformational modification positively regulates receptor‐receptor association: a study with an aberrant epidermal growth factor receptor (EGFRvIII/DeltaEGFR) expressed in cancer cells. J Biol Chem 20012765375–5383. [DOI] [PubMed] [Google Scholar]
- 16.Chu C T, Everiss K D, Wikstrand C J.et al Receptor dimerization is not a factor in the signalling activity of a transforming variant epidermal growth factor receptor (EGFRvIII). Biochem J 1997324855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moscatello D K, Holgado‐Madruga M, Emlet D R.et al Constitutive activation of phosphatidylinositol 3‐kinase by a naturally occurring mutant epidermal growth factor receptor. J Biol Chem 1998273200–206. [DOI] [PubMed] [Google Scholar]
- 18.Moscatello D K, Montgomery R B, Sundareshan P.et al Transformational and altered signal transduction by a natural occuring mutant EGF receptor. Oncogene 19961385–96. [PubMed] [Google Scholar]
- 19.Feldkamp M M, Lala P, Lau N.et al Expression of activated epidermal growth factor receptors, Ras‐guanosine triphosphate, and mitogen‐activated protein kinase in human glioblastoma multiforme specimens. Neurosurgery 199945144253. [DOI] [PubMed] [Google Scholar]
- 20.Brambilla E, Travis W D, Colby T V.et al The new World Health Organization classification of lung tumours. Eur Respir J 2001181059–1068. [DOI] [PubMed] [Google Scholar]
- 21.Hilbe W, Dirnhofer S, Oberwasserlechner F.et al Immunohistochemical typing of non‐small cell lung cancer on cryostat sections: correlation with clinical parameters and prognosis. J Clin Pathol 200356736–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voldborg B R, Damstrup L, Spang‐Thomsen M.et al Epidermal growth factor receptor (EGFR) and EGFR mutations, function and possible role in clinical trials. Ann Oncol 199781197–1206. [DOI] [PubMed] [Google Scholar]
- 23.Ekstrand A J, Longo N, Hamid M L.et al Functional characterization of an EGF receptor with a truncated extracellular domain expressed in glioblastomas with EGFR gene amplification. Oncogene 199492313–2320. [PubMed] [Google Scholar]
- 24.Milas L, Mason K, Hunter N.et al In vivo enhancement of tumour radioresponse by C225 antiepidermal growth factor receptor antibody. Clin Cancer Res 20006701–708. [PubMed] [Google Scholar]
- 25.Yang X D, Jia X C, Corvalan J R.et al Development of ABX‐EGF, a fully human anti‐EGF receptor monoclonal antibody, for cancer therapy. Crit Rev Oncol Hematol 20013817–23. [DOI] [PubMed] [Google Scholar]
- 26.Kris M G, Natale R B, Herbst R S.et al Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non‐small cell lung cancer: a randomized trial. JAMA 20032902149–2158. [DOI] [PubMed] [Google Scholar]
- 27.Fukuoka M, Yano S, Giaccone G.et al Multi‐institutional randomized phase II trial of gefitinib for previously treated patients with advanced non‐small‐cell lung cancer. J Clin Oncol 2003212237–2246. [DOI] [PubMed] [Google Scholar]
- 28.Shepherd F A, Pereira J, Ciuleanu T E.et al A randomized placebo‐controlled trial of erlotinib in patients with advanced non‐small cell lung cancer (NSCLC) following failure of first line or second line chemotherapy [abstract]. Proc Annu Meet Am Assoc Cancer Res 200422P7022 [Google Scholar]
- 29.Shin D M, Nemunaitis J, Zinner R G.et al A phase I clinical and biomarker study of ci‐1033, a novel pan‐erbb tyrosine kinase inhibitor in patients with solid tumours [abstract]. Proc Annu Meet Am Assoc Cancer Res 200120P324 [Google Scholar]
- 30.Mishima K, Johns T G, Luwor R B.et al Growth suppression of intracranial xenografted glioblastomas overexpressing mutant epidermal growth factor receptors by systemic administration of monoclonal antibody (mAb) 806, a novel monoclonal antibody directed to the receptor. Cancer Res 2001615349–5354. [PubMed] [Google Scholar]
- 31.Hilbe W, Dlaska M, Dirnhofer S.et al Characterisation and predictive value of epidermal growth factor receptor status using quantitative real‐time PCR combined with immunohistochemistry on non‐small cell lung cancer specimens. Int J Oncol 200323893–899. [PubMed] [Google Scholar]
- 32.Fontanini G, Vignati S, Bigini D.et al Epidermal growth factor receptor (EGFr) expression in non‐small cell lung carcinomas correlates with metastatic involvement of hilar and mediastinal lymph nodes in the squamous subtype. Eur J Cancer 199531178–183. [DOI] [PubMed] [Google Scholar]
- 33.Fujino S, Enokibori T, Tezuka N.et al A comparison of epidermal growth factor receptor levels and other prognostic parameters in non‐small cell lung cancer. Eur J Cancer 1996322070–2074. [DOI] [PubMed] [Google Scholar]
- 34.Pastorino U, Andreola S, Tagliabue E.et al Immunocytochemical markers in stage I lung cancer: relevance to prognosis. J Clin Oncol 1997152858–2865. [DOI] [PubMed] [Google Scholar]
- 35.Kurie J M, Shin H J, Lee J S.et al Increased epidermal growth factor receptor expression in metaplastic bronchial epithelium. Clin Cancer Res 199621787–1793. [PubMed] [Google Scholar]
- 36.Rusch V, Klimstra D, Venkatraman E.et al Overexpression of the epidermal growth factor receptor and its ligand transforming growth factor alpha is frequent in resectable non‐small cell lung cancer but does not predict tumour progression. Clin Cancer Res 19973515–522. [PubMed] [Google Scholar]
- 37.Pfeiffer P, Nexø E, Bentzen S M.et al Enzyme‐linked immunosorbent assay of epidermal growth factor receptor in lung cancer: comparison with immunohistochemistry, clinicopathological features and prognosis. Br J Cancer 19987896–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brabender J, Danenberg K D, Metzger R.et al Epidermal growth factor receptor and HER2‐neu mRNA expression in non‐small cell lung cancer is correlated with survival. Clin Cancer Res 200171850–1855. [PubMed] [Google Scholar]
- 39.Wikstrand C J, Hale L P, Batra S K.et al Monoclonal antibodies against EGFRvIII are tumour specific and react with breast and lung carcinomas and malignant gliomas. Cancer Res 1995553140–3148. [PubMed] [Google Scholar]
- 40.Pedersen M W, Meltorn M, Damstrup L.et al The type III epidermal growth factor receptor mutation. Biological significance and potential target for anti‐cancer therapy. Ann Oncol 200112745–760. [DOI] [PubMed] [Google Scholar]
- 41.Srinivasan R, Leverton K E, Sheldon H.et al Intracellular expression of the truncated extracellular domain of c‐erbB‐3/HER3. Cell Signal 200113321–330. [DOI] [PubMed] [Google Scholar]
- 42.Hubbard S R, Wei L, Ellis L.et al Crystal structure of the tyrosine kinase domain of the human insulin receptor. Nature 1994372746–754. [DOI] [PubMed] [Google Scholar]
- 43.Biscardi J S, Maa M C, Tice D A.et al c‐Src‐mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem 19992748335–8343. [DOI] [PubMed] [Google Scholar]
- 44.Prigent S A, Nagane M, Lin H.et al Enhanced tumorigenic behavior of glioblastoma cells expressing a truncated epidermal growth factor receptor is mediated through the Ras‐Shc‐Grb2 pathway. J Biol Chem 199627125639–25645. [DOI] [PubMed] [Google Scholar]
- 45.Huang H‐J S, Nagane M, Klingbeil C K.et al The enhanced tumourigenic activity of a mutant epidermal growth factor receptor common in human cancer is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J Biol Chem 19972722927–2935. [DOI] [PubMed] [Google Scholar]
- 46.Harpole D H, Jr, Herndon J E, Wolfe W G.et al A prognostic model of recurrence and death in stage I non‐small cell lung cancer utilizing presentation, histopathology, and oncoprotein expression. Cancer Res 19955551–56. [PubMed] [Google Scholar]
- 47.Herbst R S, Opresko L K, Walsh B J.et al Regulation of postendocytic trafficking of the epidermal growth factor receptor through endosomal retention. J Biol Chem 199426912865–12873. [PubMed] [Google Scholar]