Abstract
Aims
Whereas focal accumulations of reactive lymphocytes around mast cell (MC) infiltrates are often seen in indolent systemic mastocytosis (ISM) involving the bone marrow, an association of systemic mastocytosis (SM) with malignant lymphoma/lymphatic leukaemia is very rare. This report contributes to the differential diagnosis of ISM by demonstrating that such lymphocytic aggregates may be neoplastic.
Methods
Biopsy specimens (bone marrow and gastrointestinal mucosa) of a 69 year old woman with mild blood lymphocytosis and a history of urticaria pigmentosa‐like skin lesions that had disappeared a few years earlier, were investigated immunohistochemically using antibodies against CD3, CD5, CD20, CD23, CD25, CD34, CD117, chymase, and tryptase. Rearrangements of the IgH and TCRy genes were studied by seminested PCR. Mutation analysis of c‐kit was performed by melting point analysis of nested PCR using amplified DNA from pooled microdissected single cells (MC and B cells) of both sites.
Results
The histomorphological features of the bone marrow corresponded to that of ISM with multifocal accumulations of MC surrounded by clusters of lymphocytes of mature appearance. However, these lymphocytes revealed an aberrant immunophenotype with coexpression of CD5, CD20, and CD23, thus enabling the final diagnosis of SM with an associated clonal haematological non‐MC lineage disease, in particular SM with associated B cell chronic lymphocytic leukaemia (SM‐CLL). Monoclonality for both ISM and B‐CLL could be confirmed by demonstrating the typical activating c‐kit point mutation D816V in bone marrow MC, and a monoclonal IgH rearrangement in bone marrow B cells.
Conclusions
Usually, focal accumulations of lymphocytes around MC infiltrates in the bone marrow of patients with SM are reactive in nature (lymphocytosis). However, a low grade malignant lymphoma should also be included in the differential diagnosis. We describe here the first case, to our knowledge, with synchronous diagnosis of SM and associated B‐CLL. This diagnosis could only be established by application of appropriate immunohistochemical and molecular techniques, as the bone marrow histology on first investigation resembled that of typical ISM.
Keywords: mast cell, systemic mastocytosis, chronic lymphocytic leukaemia
It has been recognised for some time that systemic mastocytosis (SM) may be associated with other haematological neoplasias.1,2,3 This finding has been acknowledged in the recent WHO classification of mastocytosis by defining a special subtype of SM, namely “SM associated with another clonal haematological non‐mast cell lineage disease” (SM‐AHNMD).4 We report here on a case of SM associated with chronic lymphocytic leukaemia (SM‐CLL, which come within the category of SM‐AHNMD) exhibiting remarkable and puzzling histomorphological features. Among these, the most impressive was the bone marrow histology, which initially resembled that of indolent SM (ISM) with multifocal mixed infiltrates consisting of neoplastic mast cells (MC) and adjacent clusters of lymphocytes. The close topographic association of MC and lymphocytic infiltrates is a well recognised feature of many cases of ISM. In our own cases of ISM, these lymphocyte clusters proved to be polyclonal as assessed by both immunophenotyping and by IgH gene rearrangement analysis.5 Interestingly, recently published data suggest that lesional lymphocytes in ISM may also carry the activating c‐kit point mutation D816V, which is typically found in neoplastic MC in cases of SM.6,7
CASE REPORT
A 69 year old woman presented with a history of long standing (>25 years) urticaria pigmentosa‐like skin lesions, which had resolved spontaneously 5 years before admission. At presentation, she suffered from itchy, irritable skin, epigastric pain, and abdominal cramping. Laboratory tests showed no signs of coeliac disease, and levels of gliadin and tissue transglutaminase were not increased. Serum tryptase level was found to be markedly elevated (130 μg/l; normal range 1–15). Lymphadenopathy and hepatosplenomegaly were absent, but mild blood leucocytosis with lymphocytosis (6400 lymphocytes/μl) was detected (phenotyping of blood lymphocytes was not performed). Gastroduodenoscopy revealed diffuse oedema of gastric and duodenal mucosa, and mild villous atrophy of duodenal mucosa. Focal involvement of the duodenal mucosa by mastocytosis was detected histologically, while gastric mucosa showed diffuse MC hyperplasia. A trephine biopsy specimen of the iliac crest was obtained for staging of suspected SM and lymphocytic leukaemia. Based on extensive immunohistological and molecular studies, a final diagnosis of SM involving the bone marrow and duodenal mucosa associated with B cell chronic lymphocytic leukaemia was established.
METHODS
Biopsy specimens from the iliac crest and gastroduodenal mucosa were routinely processed and fixed in buffered 5% formalin, the bone marrow trephine being mildly decalcified in edetic acid. All tissue samples were embedded in paraffin wax. In addition to conventional stains such as haematoxylin and eosin, Giemsa, and naphthol AS‐D chloroacetate esterase, the sections were immunostained using the avidin biotin complex method with antibodies against various lymphocyte and MC related antigens (table 1).8 Molecular studies were performed using a seminested PCR technique with primers specific for the IgH rearrangement according to previously described protocols, after extraction of the DNA with phenol/chloroform/isoamyl alcohol and proteinase K digestion.9 Screening for the c‐kit mutation D816V in lesional and non‐lesional MC of the bone marrow and gastroduodenal mucosa and in lesional neoplastic CD23 positive bone marrow lymphocytes was performed using melting point analysis of nested PCR products amplified from laser dissected single cells pooled to a total of 50 cells per PCR tube. The techniques have been described in detail elsewhere.10
Table 1 SM‐CLL: Immunophenotypical characteristics of mast cells and B lymphocytes in the bone marrow.
| Antigen | MC | BL | ||
|---|---|---|---|---|
| Tryptase | + | − | ||
| Chymase | + | − | ||
| KIT (CD117) | + | − | ||
| CD25 | +* | − | ||
| CD2 | +* | − | ||
| CD20 | − | + | ||
| CD23 | − | +* | ||
| CD5 | − | +* | ||
| CD3 | − | − | ||
| CD34 | − | − |
*Aberrant immunophenotype of the cell. MC, mast cells; BL, B lymphocytes.
Histological and immunophenotypical studies
Duodenal mucosa
A slight atrophy of villi with focal slight increase of intraepithelial T cells expressing CD3 was found. However, the picture was dominated by a focal compact infiltrate consisting predominantly of medium sized round MC with aberrant expression of CD2 and CD25 within the deeper layers of the lamina propria. The diagnosis was systemic mastocytosis with intestinal (duodenal) involvement.
Gastric mucosa
There was a diffuse increase of loosely scattered, mostly round, MC found within the lamina propria without both focal infiltrates and aberrant immunophenotype. The diagnosis was gastric mucosa with MC hyperplasia.
Bone marrow
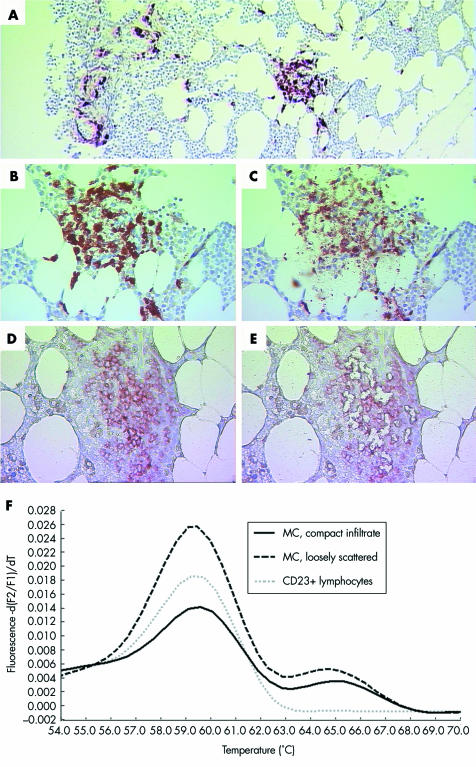
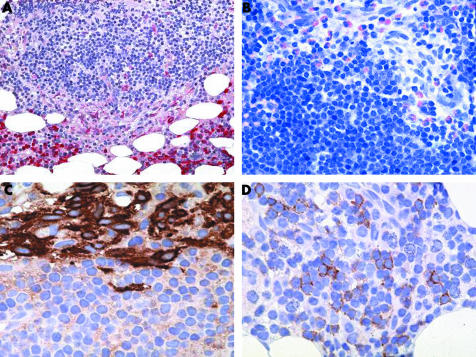
The slightly hypocellular marrow contained multifocal mixed infiltrates consisting of both spindle shaped hypogranulated MC and small mature appearing lymphocytes with inconspicuous nucleoli (figs 1 and 2). Large lymphocytic aggregates were always located in the vicinity of compact MC infiltrates, thus representing the typical histomorphological features of ISM. Immunohistochemically, the MC exhibited an aberrant immunophenotype with coexpression of CD2 and CD25. Additionally, there was also a slight diffuse increase of spindle shaped, loosely scattered MC, which also partly expressed CD2 and CD25. The lymphocytes also showed an atypical immunophenotype with coexpression of CD5, CD20, and CD23. However, there was no significant diffuse increase in neoplastic B cells in the bone marrow sections analysed. In the non‐affected areas, haematopoiesis was found to be completely intact. Altogether, the degree of infiltration was estimated as 5–10% of the section area for SM but only 5% for B‐CLL. The diagnosis was SM with an associated B‐CLL.
Figure 1 SM‐AHNMD (SM‐CLL) involving the bone marrow. (A) Anti‐MC tryptase (MCT) stained bone marrow section with a compact infiltrate (window) and loosely scattered MC (left). The compact MC infiltrate (B) before and (C) after laser microdissection of single MC. CLL infiltrate consisting of CD23 positive neoplastic lymphocytes (D) before and (E) after laser microdissection of single lymphocytes. (F) Melting point analysis of representative nested PCR products amplified from MC of compact (solid line) and loosely scattered (dashed black line) infiltrates, and from CD23 positive neoplastic lymphocytes of CLL infiltrates (dotted grey line). Wild type specific melting curves peak at ∼59.5°C, while D816V specific melting curves peak at ∼65°C. MC from both compact and loosely scattered infiltrates presented the D816V mutation heterozygously, while CD23 positive neoplastic lymphocytes in numerous experiments (n = 10) only showed the wild type. Original magnification: (A)×25; (B–E))×100.
Figure 2 SM‐AHNMD (SM‐CLL) involving the bone marrow. (A) The naphthol AS‐D chloroacetate esterase (CAE) stain shows a mixed compact infiltrate consisting predominantly of small (CAE negative) lymphocytes with perifocally aggregated spindle shaped (moderately CAE positive) mast cells. Note the intact haematopoiesis in the lower part of the picture with prominent (strongly CAE positive) neutrophilic granulocytopoiesis. (B) Giemsa stain again demonstrates a relatively monomorphic lympoid infiltrate with an abundance of small lymphocytes. These cells contain only small amounts of cytoplasm and exhibit inconspicuous nucleoli. Blast cells are virtually absent. Note the atypical hypogranulated spindle shaped mast cells and the intermingled eosinophils in the upper right quadrant of the picture. (C) Anti‐tryptase antibody AA1 clearly decorates the compact cohesive nature of the mast cell infiltrate and demarcates it sharply from the surrounding lymphocytes. (D) A major proportion of lymphocytes expressed CD23, which can be regarded as clear indication of a neoplastic immunophenotype with a background of lymphocytic lymphoma. Original magnification: (A)×25; (B)×100; (C, D)×200.
Final pathological diagnosis
SM with involvement of bone marrow and duodenal mucosa, associated with B‐CLL (SM‐AHNMD/SM‐CLL).
Molecular studies
An activating point mutation of c‐kit (D816V) was detected in bone marrow MC but not in MC dissected from the gastric and duodenal mucosa. For the mucosal specimens, 10 and 15 amplification products, respectively, were examined by melting point analysis. Of the 15 samples from the duodenal mucosa, 10 consisted of microdissected, pooled lesional CD25+ MC of the compact infiltrate. None of the specimens carried the point mutation D816V. However, the mutation D816V was detected by melting point analysis in three of four amplification products from lesional MC and also in four of six amplification products from loosely scattered MC of the bone marrow. Microdissected CD23 positive lesional B lymphocytes of the bone marrow (10 samples) were found to contain a non‐mutated c‐kit gene (wild type D816V). However, a monoclonal rearrangement for IgH was found, thus confirming the presence of a neoplastic proliferation of B cells.
DISCUSSION
SM‐AHNMD is a recently defined subcategory of SM.11 Usually, both neoplasias are synchronously diagnosed on the basis of morphological evaluation of a bone marrow (bone marrow) trephine biopsy specimen.12 However, the definition of SM‐AHNMD also includes the possibility of a later development of an “AHNMD” in patients with long standing mastocytosis.
In our patient, the findings were very unusual with respect to the clinical notion of long standing urticaria pigmentosa‐like skin lesions, which were initially interpreted as cutaneous mastocytosis (without determination of the serum tryptase level and without analysis of bone marrow histology). Remarkably, the skin lesions had spontaneously regressed about 5 years prior to our diagnosis of SM‐AHNMD, which is extremely uncommon for the adult type of SM.
The overwhelming majority of AHNMDs are of myeloid origin, with chronic myelomomocytic leukaemia being the most common subtype.12 In contrast, the association of SM with malignant lymphoproliferative disorders is a rare finding.13,14,15,16,17 SM with an associated CLL has been previously described only once, in a 36 year old man who developed CLL 10 years after diagnosis of SM had been established on the basis of multifocal MC infiltrates in the bone marrow.18
The present case of SM‐CLL is unique in several clinical, morphological, and molecular aspects. To our knowledge, this is the first case of SM‐CLL diagnosed synchronously in one bone marrow trephine biopsy specimen. The histomorphology of the bone marrow at first glance resembled that of ISM, usually seen in patients with urticaria pigmentosa‐like skin lesions and multifocal compact MC infiltrates of the bone marrow with adjacent lymphocytic aggregates.19 In almost all cases, these lymphocytes have been found, using both immunophenotypical and molecular analyses, to be polyclonal in nature.5 In our case, however, immunohistochemical investigations revealed an aberrant immunophenotype of B cells with coexpression of CD5 and CD23, thus fulfilling the criteria for lymphocytic lymphoma of B cell origin, in particular of B‐CLL. Neoplastic lymphocytes within the compact infiltrates could not be distinguished from reactive lymphocytes by either distribution or cytomorphology, mainly due to the presence of mature appearing lymphocytes.
Another interesting morphological aspect in this woman was the involvement of the duodenal mucosa in SM, while in the gastric mucosa only marked MC hyperplasia was found. There were no compact MC infiltrates, and MC did not show aberrant expression of CD25. Surprisingly, a marked decrease of intramucosal MC in the stomach and duodenum in patients with SM was recently reported, a finding which clearly contrasts with our own experience (unpublished observations).20 The endoscopic appearance, with pronounced mucosal oedema, was the same in both tissue sites. Involvement of the gastroduodenal mucosa by the CLL could be ruled out by immunohistochemical demonstration of only a few loosely scattered B cells without atypical immunophenotype. Gastrointestinal symptoms, especially cramping and diarrhoea, are relatively frequent in patients with SM. Based on our histological findings, we believe that in the present case, the symptoms were indeed caused by mastocytosis.20,21
Molecular studies revealed the presence of an activating c‐kit mutation (D816V) in MC obtained from the compact and diffuse (loosely scattered) infiltrates within the bone marrow, but not in MC of the gastroduodenal mucosa, although a diagnostic compact MC infiltrate was detected in the lamina propria. However, these MC exhibited an aberrant immunophenotype with coexpression of CD25. The absence of the point mutation of c‐kit even after highly sensitive methods, including microdissection of MC, had been applied cannot be explained with certainty. One explanation could be that only non‐mutated MC subclones infiltrated the intestinal mucosa. The D816V point mutation of c‐kit was also found to be absent from neoplastic bone marrow B cells, contrasting with recently published findings obtained with non‐neoplastic B cells in SM.6,7 The absence of D816V in our patient may be explained by the fact that the B cells belonged to a separate clone.
Although histomorphologically mimicking ISM with reactive lymphocytosis, the present case clearly indicates that SM may very rarely be associated with a low grade malignant lymphoma/lymphocytic leukaemia. However, we do recommend extensive immunophenotypical and molecular sudies only in those patients with SM exhibiting also clinical signs of a malignant lymphoma (such as blood lymphocytosis in an elderly patient, as in our case) and/or when lymphocyte clusters are very numerous, large, irregularly outlined, or seen without associated MC infiltrates (such morphological findings, however, were not encountered in our case).
TAKE HOME MESSAGES
Although SM‐AHNMD usually includes an association of systemic mastocytosis and a myeloid malignancy, “AHNMD” is occasionally found to be a malignant lymphoma or lymphatic leukaemia.
Even in cases of ISM with typical histopathological findings of the bone marrow and demonstration of multifocal mixed mast cell/lymphocyte clusters, a low grade non‐Hodgkin's lymphoma or lymphocytic leukaemia can only be ruled out definitively after appropriate immunohistochemical and molecular analyses.
Abbreviations
AHNMD - associated clonal haematological non‐MC lineage disease
ISM - indolent systemic mastocytosis
MC - mast cell
SM - systemic mastocytosis
SM‐CLL - SM with associated B cell chronic lymphocytic leukaemia
References
- 1.Horny H‐P, Ruck M, Wehrmann M.et al Blood findings in generalized mastocytosis: evidence of frequent simultaneous occurrence of myeloproliferative disorders. Br J Haematol 199076186–193. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence J B, Friedman B S, Travis W D.et al Hematologic manifestation of systemic mast cell disease: a prospective study of laboratory and morphologic features and their relation to prognosis. Am J Med 199191612–624. [DOI] [PubMed] [Google Scholar]
- 3.Sperr W R, Horny H‐P, Lechner K.et al Clinical and biologic diversity of leukemias occurring in patients with mastocytosis. Leuk Lymphoma 200037473–486. [DOI] [PubMed] [Google Scholar]
- 4.Valent P, Horny H‐P, Li C Y.et al Mastocytosis (Mast cell disease). In: Jaffe ES, Harris NL, Stein H, Vardiman J, eds. World Health Organization (WHO) classification of tumours. Tumours of haematopoietic and lymphoid tissues 20011291–302. [Google Scholar]
- 5.Horny H ‐ P, Lange K, Sotlar K.et al Increase of bone marrow lymphocytes in systemic mastocytosis: reactive lymphocytosis or malignant lymphoma? Immunohistochemical and molecular findings on routinely processed bone marrow biopsy specimens. J Clin Pathol 200356575–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor M L, Sehgal D, Raffeld M.et al Demonstration that mast cells, T cells, and B cells bearing the activating kit mutaton D816V occur in clusters within the bone marrow of patients with mastocytosis. J Mol Diagn 20046335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akin C, Kirshenbaum A S, Semere T.et al Analysis of the surface expression of c‐kit and occurrence of the c‐kit Asp816Val activating mutation in T cells, B cells, and myelomonocytic cells in patients with mastocytosis. Exp Hematol 200028140–147. [DOI] [PubMed] [Google Scholar]
- 8.Hsu S, Raine L, Fanger H. Use of avidin‐biotin‐peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 198129577–580. [DOI] [PubMed] [Google Scholar]
- 9.Wang W, Kumar P, Schwarz M.et al PCR amplification of 40 year‐old paraffn‐embedded tumour tissues: comparison of four different DNA extraction and purfication methods. Int J Oncol 19945453–457. [DOI] [PubMed] [Google Scholar]
- 10.Sotlar K, Escribano L, Landt O.et al One‐step detection of c‐kit point mutations using PNA‐mediated PCR‐clamping and hybridization probes. Am J Pathol 2003162737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valent P, Horny H ‐ P, Escribano L.et al Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res 200125603–625. [DOI] [PubMed] [Google Scholar]
- 12.Horny H ‐ P, Sotlar K, Sperr W R.et al Systemic mastocytosis with associated clonal haematological non‐mast cell lineage diseases: a histopathological challenge. J Clin Pathol 200457604–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagen W, Schwarzmeier J, Walchshofer S.et al A case of bone marrow mastocytosis associated with multiple myeloma. Ann Haematol 199876167–174. [DOI] [PubMed] [Google Scholar]
- 14.Stellmacher F, Sotlar K, Balleisen L.et al Bone marrow mastocytosis associated with IgM Kappa plasma cell myeloma. Leuk Lymphoma 200445801–805. [DOI] [PubMed] [Google Scholar]
- 15.Cooper A J, Winkelmann R K, Wiltsie J C. Hematologic malignancies occurring in patients with urticaria pigmentosa. J Am Acad Dermatol 19827215–220. [DOI] [PubMed] [Google Scholar]
- 16.Travis W D, Li C Y, Bergstralh E J. Solid and hematologic malignancies in 60 patients with systemic mast cell disease. Arch Pathol Lab Med 1989113365–368. [PubMed] [Google Scholar]
- 17.Saletti P, Ghielmini M, Scali G.et al Hodgkin's and Castleman's disease in a patient with systemic mastocytosis. Ann hematol 19997897–100. [DOI] [PubMed] [Google Scholar]
- 18.Sanz M A, Valcarel D, Sureda A.et al Systemic mast cell disease associated with B‐chronic lymphocytic leukemia. Haematologica 2001861106–1107. [PubMed] [Google Scholar]
- 19.Horny H‐P, Valent P. Diagnosis of mastocytosis: general histopathological aspects, morphological criteria, and immunohistochemical findings. Leuk Res 200125543–551. [DOI] [PubMed] [Google Scholar]
- 20.Siegert S I, Diebold J, Ludolph‐Hauser D.et al Are gastrointestinal mucosal mast cells increased in patients with systemic mastocytosis? Am J Clin Pathol 2004122560–565. [DOI] [PubMed] [Google Scholar]
- 21.Jensen R T. Gastrointestinal anbnormalities and involvement in systemic mastocytosis. Hematol Oncol Clin North Am 200014579–623. [DOI] [PubMed] [Google Scholar]