Abstract
Aim
To study the expression of claudins in mesothelioma and metastatic pleural adenocarcinoma.
Methods
Immunohistochemical staining of claudins 1, 2, 3, 4, 5, and 7 was studied in 35 malignant mesotheliomas and the expression compared with 24 cases of pleural metastatic adenocarcinoma. All cases were also immunostained with calretinin.
Results
Claudin 1, 2, 3, 4, 5, and 7 expression was seen in 40%, 80%, 18%, 23%, 14%, and 43% of mesotheliomas, respectively, while the corresponding figures for adenocarcinoma were 100%, 88%, 90%, 100%, 50%, and 92%. Claudins 1, 3, 4, 5, and 7 were significantly less positive in mesothelioma than in metastatic adenocarcinoma, while no difference was observed for claudin 2. Claudins 1, 3, 4, 5, and 7 were also inversely associated with calretinin positivity. Sarcomatoid and biphasic mesothelioma subtypes appeared more negative for these claudins than pure epithelioid subtypes. Claudin expression was not associated with survival of patients with malignant mesotheliomas.
Conclusions
The results show that malignant mesotheliomas have a lower expression of claudins 1, 3, 4, 5, and 7 than adenocarcinomas, and their expression could thus be used as an adjunct in differential diagnosis between the two. The difference was most evident for claudins 3 and 4, which were nearly as good as calretinin in mesothelioma detection. Sarcomatoid and biphasic mesotheliomas showed expression of these claudins only occasionally, which could be due to or contribute to their less epithelial appearance.
Keywords: claudin, mesothelioma, cancer, adhesion, tight junction
Claudins are tight junctional proteins that regulate the permeability of cell junctions in different epithelia and vascular endothelium. There are at least 23 different claudins known so far and their distribution in different epithelia may vary.1 In rat kidney, for instance, different claudins are expressed in different segments of the nephron, and their expression also varies during embryogenesis.1,2 Similarly, in intestinal organs and in gut, claudins show site specific expression.3 In rat liver, for instance, claudin 5 is present only in endothelial cells and claudin 4 is absent, while claudins 2 and 3 are expressed in hepatocytes.3 In rat pancreas, claudins 3 and 4 are uniformly expressed in epithelia, while claudin 5 is seen in acinar cells and claudin 2 in ductal cells.3
In rat lung, claudins 3, 4, and 5 are expressed in type II alveolar epithelial cells. In cell culture, they also showed weak claudin 1 and 2 expression.4 Claudins do, however, have opposing actions on the permeability of cellular junctions in human airways. According to Coyne et al, claudins 1 and 3 decrease solute permeability, while claudin 5 increases it.5 The function of claudin 1 is controlled by MAP kinase, which increases its barrier function.6 Claudin 5 also associates with endothelium, and it is important in vascular development and angiogenesis of the lung tissue.7
In tumours, claudin expression has not been studied extensively. Expression of various claudins is present in prostate, breast, ovarian, and pancreatic carcinoma. In breast cancer, loss of claudin 7 expression is associated with a higher histological grade of the tumours.8 Claudin 3 and 4 expression has also been detected in breast carcinoma, and they serve as receptors for Clostridium perfringens enterotoxin.9,10 In fact, administration of C. perfringens enterotoxin to breast or pancreatic carcinoma cells induces dose dependent cytolysis in tumour cells.9,11 Claudin 4 overexpression is also present in pancreatic adenocarcinoma and its precursor lesions.11,12 Claudin 3 and 4 overexpression has been found in prostate and ovarian carcinoma.13,14 In soft tissue tumours, claudin 1 expression can be used in the differential diagnosis of perineurinoma.15
In addition to epithelial and endothelial cells, mesothelium also contains tight junctions.16 The differential expression of claudins in mesothelial cells or tumours has not previously been studied. Given the site specific variation of claudins in different tissues, and the often problematic differential diagnosis between adenocarcinoma and mesothelioma, we tested whether claudins might be a useful adjunct in differentiating these tumours from each other. For this, we analysed 35 malignant mesotheliomas and 24 adenocarcinomas. Additionally, samples of non‐neoplastic pleural tissue were included.
MATERIALS AND METHODS
Study material
The study was approved by the ethics committee of Oulu University Hospital. The study material consisted of 35 surgically removed mesotheliomas, 24 metastatic pleural adenocarcinomas, and 6 non‐neoplastic surgical samples. The diagnosis of the mesothelioma cases was based on the WHO classification of lung and pleural tumours complemented with immunohistochemistry.17 There were 24 epithelioid, 4 sarcomatoid, and 7 biphasic subtypes. For metastatic adenocarcinomas, the diagnosis was based on morphology and immunohistochemistry. The malignant mesotheliomas were distinguished from metastatic adenocarcinomas on the basis of the presence of intracellular or extracellular hyaluronic acid in mesothelioma, while the adenocarcinomas contained intracellular, periodic acid‐Schiff positive and diastase resistant epithelial mucin. Immunohistochemical staining was also performed with carcinoembryonic antigen (CEA) and epithelial membrane antigen (EMA), cytokeratins (CK) 5/6 and 20, and calretinin. Metastatic adenocarcinomas of the pleura were diagnosed on the basis of CEA positivity and often contained intracellular EMA positivity, while mesotheliomas were usually CEA negative and showed membrane bound EMA positivity. Mesotheliomas characteristically showed positivity for CK 5/6, while they were negative for CK 20, which was present in the case of some metastatic adenocarcinomas originating, for example, from the gastrointestinal tract. A most important criterion was observing nuclear and cytoplasmic positivity for calretinin in mesotheliomas. The primary locations of the metastatic adenocarcinomas were lung, breast, kidney, gallbladder, pancreas, and ovarian tissue. The mean follow up was 22.0 months for mesothelioma and 22.5 months for metastatic adenocarcinoma.
Immunohistochemical staining
The primary antibodies used in the immunostaining were purchased from Zymed Laboratories Inc. (San Francisco, CA, USA) designed to be used in formalin fixed paraffin embedded tissues. They were polyclonal rabbit anti‐claudin 1 (clone JAY.8), monoclonal mouse anti‐claudin 2 antibody (clone 12H12), polyclonal rabbit anti‐claudin 3 (clone Z23.JM), monoclonal mouse anti‐claudin 4 (clone 3E2C1), monoclonal mouse anti‐claudin 5 (clone 4C3C2), and polyclonal rabbit anti‐claudin 7 (clone ZMD.241). The polyclonal rabbit anti‐calretinin antibody (catalogue no. 18‐0211) was also purchased from Zymed. Before application of the primary antibodies, the sections were heated in a microwave oven in 10 mmol/l citrate buffer, pH 6.0, for 10 minutes. After a 60 minute incubation with the primary antibody (dilution 1:50 for anti‐claudin 1, 2, 3, 4, 5, and 7 and 1:1000 for calretinin), a biotinylated secondary anti‐rabbit or anti‐mouse antibody and Histostain‐SP kit (Zymed) was used. For all the immunostains, the colour was developed by diaminobenzidine, and the sections were lightly counterstained with haematoxylin and mounted with Eukitt (Kindler, Freiburg, Germany).
Negative control stains were carried out by substituting non‐immune rabbit or mouse serum and phosphate buffered saline for the primary antibodies. As positive controls non‐neoplastic kidney, breast, skin and liver samples were used.
The immunostaining was assessed as follows; −, no immunostaining present; +, <25% of cells positive; ++, 25–50% of cells positive; +++, 50–100% of cells positive. In the evaluation, only membrane bound positivity was considered significant. The results were analysed by two pathologists who had no knowledge of the diagnosis at the time of the analysis (YS, PP).
Statistical analysis
SPSS for Windows software (Chicago, IL, USA) was used for statistical analysis. The significance of associations were determined using Fisher's exact probability test. Survival was analysed with the Kaplan‐Meier curve, and significance of associations with log rank, Breslow, and Tarone‐Ware tests.
RESULTS
Immunostaining revealed linear membrane bound positivity for all claudins except for claudin 2, which showed more granular membrane bound staining. With some claudins, such as claudins 1 and 3, some cytoplasmic positivity was also observed, but only membrane bound staining was considered significant. In histological samples of non‐neoplastic pleural mesothelial cells positivity for claudin 2 and weak membrane bound positivity for claudin 1 was seen. Claudins 3, 4, 5, and 7 appeared negative.
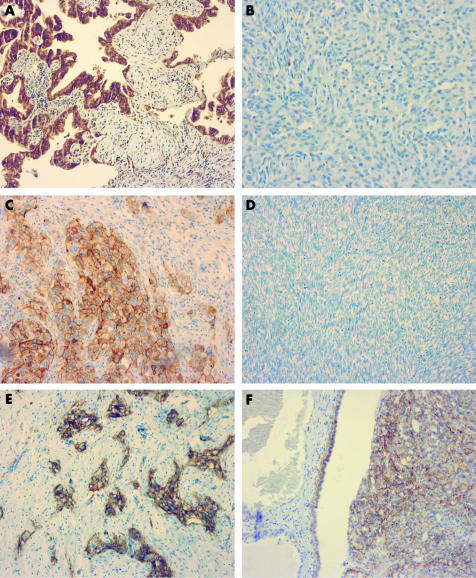
The results of the immunostaining are compiled in table 1. Of the mesotheliomas, 14/35 (40%) were positive for claudin 1, while 24/24 (100%) of the adenocarcinomas showed positivity (p<0.001) (fig 1A, B). With claudin 2, in contrast, no significant difference was observed in claudin expression, 28/35 (80%) of the cases being positive for claudin 2 in mesotheliomas and 21/24 (88%) in adenocarcinomas (p = 0.35). Claudin 3 was detected in 6/33 mesotheliomas (18%) and in 20/22 adenocarcinomas (90%), and there were significantly fewer positive cases in the mesothelioma group (p<0.001). Similar to this, claudin 4 expression was observed in 8/35 mesotheliomas (23%), but in all 23/23 adenocarcinomas (100%; p<0.001) (fig 1C, D). A similar trend was seen with claudin 7, 15/35 (43%) being positive in mesothelioma and 22/24 (92%) in adenocarcinoma (p<0.001). With claudin 5, 5/35 mesotheliomas (14%) and 11/22 adenocarcinomas (50%) were positive (p = 0.005) (fig 1E, F).
Table 1 Membrane‐bound expression of claudins 1, 2, 3, 4, 5, and 7 in malignant mesothelioma (MT) and metastatic adenocarcinoma (AC) of the pleura.
| Tumour | Positivity | Claudin | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 7 | |||||||||
| MT | +++ | 6 | 15 | 1 | 5 | 0 | 7 | |||||||
| ++ | 2 | 8 | 4 | 0 | 3 | 5 | ||||||||
| + | 6 | 5 | 1 | 3 | 2 | 3 | ||||||||
| − | 21 | 7 | 27 | 27 | 30 | 20 | ||||||||
| AC | +++ | 16 | 13 | 12 | 19 | 1 | 21 | |||||||
| ++ | 5 | 6 | 7 | 1 | 5 | 0 | ||||||||
| + | 3 | 2 | 1 | 3 | 6 | 1 | ||||||||
| − | 0 | 3 | 2 | 0 | 11 | 2 | ||||||||
Figure 1 (A) In a metastatic adenocarcinoma from the lung, strong membrane bound claudin 1 positivity can be seen around tumour cells. (B) In an epithelioid mesothelioma, membrane bound claudin 1 expression cannot be observed. (C) In another metastatic adenocarcinoma from the lung, strong membrane bound positivity for claudin 4 can be seen. (D) In a sarcomatoid mesothelioma, no expression for claudin 4 can be observed. (E) In a metastatic adenocarcinoma from the breast, membrane bound positivity for claudin 5 can be seen. (F) In this case of an epithelioid mesothelioma, claudin 5 positivity can also be observed. Original magnification×100.
Of the mesotheliomas, 28/34 (82%) were positive for calretinin and in adenocarcinomas 6/24 positive cases (25%) were found. Calretinin staining in mesotheliomas showed both cytoplasmic and nuclear positivity. The difference in the expression between mesothelioma and adenocarcinoma was significant (p<0.001), calretinin showing considerably more positivity in mesotheliomas. Calretinin expression was inversely associated with expression of claudins 1 (p<0.001), 3 (p<0.001), 4 (p<0.001), 5 (p = 0.003), and 7 (p = 0.002), but not with 2 (p = 0.63).
Of mesothelioma subtypes, epithelioid mesotheliomas showed most positive cases for claudins (table 2). All sarcomatoid subtypes were negative for claudins 1, 3, 5, and 7, while only one sarcomatoid subtype showed positivity for claudin 4. In contrast, all sarcomatoid subtypes were positive for claudin 2. All biphasic subtypes were negative for claudins 3, 4, and 5, while two of them showed positivity for claudin 7, two for claudin 1, and six for claudin 2. None of the claudins associated significantly with survival of the patients.
Table 2 Claudin expression in mesothelioma subtypes.
| Antibody | Immuno‐ staining | Epithelioid | Sarcomatoid | Biphasic | ||||
|---|---|---|---|---|---|---|---|---|
| Claudin 1 | Positive | 12 | 0 | 2 | ||||
| Negative | 12 | 4 | 5 | |||||
| Claudin 2 | Positive | 18 | 4 | 6 | ||||
| Negative | 6 | 0 | 1 | |||||
| Claudin 3 | Positive | 6 | 0 | 0 | ||||
| Negative | 17 | 3 | 7 | |||||
| Claudin 4 | Positive | 7 | 1 | 0 | ||||
| Negative | 17 | 3 | 7 | |||||
| Claudin 5 | Positive | 5 | 0 | 0 | ||||
| Negative | 19 | 4 | 7 | |||||
| Claudin 7 | Positive | 13 | 0 | 2 | ||||
| Negative | 11 | 4 | 5 |
When comparing immunoreactivity of claudins in the whole material, claudins 1 and 3 (p<0.001), 1 and 4 (p<0.001), 1 and 5 (p<0.001), 1 and 7 (p<0.001), 3 and 4 (p<0.001), 3 and 5 (p<0.001), 3 and 7 (p<0.001), 4 and 5 (p<0.001), 4 and 7 (p<0.001), and 5 and 7 (p = 0.021) associated significantly with each other. Of 17 mesotheliomas expressing positivity for claudins 1, 3, 4, 5, or 7, only three cases showed positivity for only one claudin; the rest showed expression of two and usually several claudins.
DISCUSSION
Claudins are tight junctional proteins present in endothelial and epithelial cells. They serve as barrier proteins and regulate the permeability of blood vessels and epithelium in various tissues. There are at least 23 different claudin types known so far, and their distribution in different tissue and cells may vary.1 Mesothelial cells also contain tight junctions.16 The aim of this study was to investigate the differential expression of claudins 1, 2, 3, 4, 5, and 7 in mesothelial cells and tumours and compare it with the expression found in metastatic adenocarcinomas of the pleura.
Previously, claudin expression has not been studied in mesotheliomas. Of other adhesion molecules, E‐cadherin is low and N‐cadherin higher in mesothelioma than in metastatic adenocarcinoma, whereas some other adhesion molecules, such as E‐selectin or vascular cell adhesion molecule are. Thus the former ones can be used in differential diagnosis between mesothelioma and adenocarcinoma.18,19 Of the mesothelioma subtypes, E‐cadherin is lower in the sarcomatoid than the epithelioid subtype, but neither E‐cadherin nor N‐cadherin distinguishes between malignant mesothelioma and mesothelial hyperplasia.20 β‐catenin and γ‐catenin associate with E‐cadherin and they are linked to the actin microfilaments by α‐catenin.20 In the report by Abutaily et al, α‐catenin and β‐catenin immunoreactivity were seen in 93% and 100% of malignant mesotheliomas, respectively, and β‐atenin expression was also cytoplasmic and sometimes nuclear, which differed from reactive mesothelial hyperplasia.20 The authors showed that in some cases mutations of the APC gene may contribute to the disturbed accumulation of β‐catenin.21
According to the results, claudin expression was different in cases diagnosed as malignant mesotheliomas compared with adenocarcinomas metastatic to pleural tissue. Claudins 1, 3, 4, 5, and 7 showed significantly less reactivity in mesotheliomas compared with metastatic adenocarcinomas, while there was no significant difference in the expression of claudin 2. The results indicate that claudins 1, 3, 4, 5, and 7 might be used as additional markers for differentiating metastatic adenocarcinoma from mesothelioma. It should, however, be borne in mind that the expression of different claudins in different types of carcinomas may vary. In breast carcinoma, for instance, claudins 3 and 4 are expressed in almost every case while expression of claudins 2 and 5 was found in only about half of cases.10 The difference between claudin expression in mesothelioma and carcinoma may also be partly quantitative, mesotheliomas expressing weaker reactivity owing to a lower concentration and perhaps to a lower number of tight junctional structures. Claudin 2 was similarly expressed in mesotheliomas and adenocarcinomas. This might, however, indicate that the number of tight junctions is not decisively different between the tumours.
In order to analyse further the putative differences between these lesions we also analysed separately tumours expressing calretinin to those without such an expression. Calretinin is widely used and has been considered a reliable marker for mesothelioma and usually differentiates mesothelioma from metastatic pleural tumours quite well. However, calretinin may sometimes be present in some non‐mesothelial tumours such as thymic carcinoma, and it is also present in a minority of lung adenocarcinomas.19,21 In our analysis, cases diagnosed as mesothelioma usually showed calretinin positivity, which agrees with previous reports.19 In line with the analysis of the cases based on histology and also other stains, cases showing positivity for calretinin showed an inverse association with claudins 1, 3, 4, 5, and 7. Thus membranous positivity for these claudins associates with calretinin negativity, suggesting that the tumour is a metastatic adenocarcinoma.
When comparing claudin expression in different mesothelioma subtypes, sarcomatoid and biphasic mesotheliomas had less claudin expression than the epithelioid subtype, except for claudin 2. Of other adhesion molecules, E‐cadherin also has lower expression in sarcomatoid than epithelioid mesotheliomas.20 This may indicate that lack of claudins and E‐cadherin together could in some way contribute to the non‐epithelial histological appearance of these tumours. There could also exist some functional relationship between E‐cadherin and claudins, as E‐cadherin has been shown to influence the formation of tight junctions and desmosomes.22,23
Claudin expression was not associated with survival of the patients with mesothelioma. This might indicate that claudin expression per se does not influence tumour behaviour to such an extent as to have prognostic value, even though epithelial mesotheliomas have a slightly better prognosis than sarcomatoid tumours. On the other hand, claudins 3 and 4 have been shown to serve as receptors for C. perfringens enterotoxin, and tumour cells with these proteins undergo apoptosis and necrosis when exposed to the toxin.9,11 In this way, these proteins can be linked to cell survival, and perhaps some day their expression can be used in the treatment of some malignant tumours.
In conclusion, we show that claudins 1, 3, 4, 5, and 7 are less frequently expressed in malignant mesotheliomas than in metastatic pleural adenocarcinomas. Thus, these claudins may be helpful in differentiating these tumours from one another. Of the mesothelioma subtypes, sarcomatoid and mixed types express less claudin immunoreactivity, which might partly contribute to the less epithelial character of these tumours.
TAKE HOME MESSAGES
Claudins are tight junctional proteins present in endothelial and epithelial cells, which serve as barrier proteins and regulate the permeability of blood vessels and epithelium in various tissues.
In this study, claudins 1, 3, 4, 5, and 7 showed significantly less reactivity in mesotheliomas compared with metastatic adenocarcinomas, while there was no significant difference in the expression of claudin 2.
This indicates that claudins 1, 3, 4, 5, and 7 might be used as additional markers for differentiating metastatic adenocarcinoma from mesothelioma.
ACKNOWLEDGEMENTS
The skilful technical assistance of E Tomperi, M Vahera and M Tuovinen is greatly appreciated.
Abbreviations
CEA - carcinoembryonic antigen
CK - cytokeratin
EMA - epithelial membrane antigen
References
- 1.Turksen K, Troy T‐C. Barriers built on claudins. J Cell Sci 20041172435–2447. [DOI] [PubMed] [Google Scholar]
- 2.Reyes J, Lamas M, Martin D.et al The renal segmental distribution of claudins changes with development. Kidney Int 200262476–487. [DOI] [PubMed] [Google Scholar]
- 3.Rahner C. Mitic LL. Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology 2001120411–422. [DOI] [PubMed] [Google Scholar]
- 4.Wang F, Daugherty B, Keise L L.et al Heterogeneity of claudin expression by alveolar epithelial cells. Am J Respir Cell Mol Biol 20032962–70. [DOI] [PubMed] [Google Scholar]
- 5.Coyne C B, Gambling T M, Boucher R C.et al Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol 2003285L1166–L1178. [DOI] [PubMed] [Google Scholar]
- 6.Fujibe M, Chiba H, Kojima T.et al Thr203 of claudin‐1, a putative phosphorylation site for MAP kinase, is required to promote the barrier function of tight junctions. Exp Cell Res 200429536–47. [DOI] [PubMed] [Google Scholar]
- 7.Favre C J, Mancuso M, Maas K.et al Expression of genes involved in vascular development and angiogenesis in endothelial cells of adult lung. Am J Physiol Heart Circ Physiol 20032851917–1938. [DOI] [PubMed] [Google Scholar]
- 8.Kominsky S L, Argani P.et al Loss of the tight junction protein claudin‐7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene 2003222021–2033. [DOI] [PubMed] [Google Scholar]
- 9.Kominsky S L, Vali M, Korz D.et al Clostridium perfringens enterotoxin elicits rapid and specific cytolysis of breast carcinoma cells mediated through tight junction proteins claudin3 and 4. Am J Pathol 20041641627–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soini Y. Claudins 2, 3, 4, and 5 in Paget's disease and breast carcinoma. Hum Pathol 2004351531–1616. [DOI] [PubMed] [Google Scholar]
- 11.Michl P, Buchholz M, Rolke M.et al Claudin 4: a new target for pancreatic cancer treatment using Clostridium perfringesn enterotoxin. Gastroenterology 2001121678–684. [DOI] [PubMed] [Google Scholar]
- 12.Terris B, Blaveri E, Crnogorac‐Jurcevic T.et al Characterization of gene expression profiles in intraductal papillary‐mucinous tumors of the pancreas. Am J Pathol 20021601745–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long H, Crean C D, Lee W ‐ H.et al Expression of Clostridium perfringens enterotoxin receptors claudin3 and claudin 4 in prostate cancer epithelium. Cancer Res 2001617878–7881. [PubMed] [Google Scholar]
- 14.Hough C D, Sherman‐Baust C A, Pizer E S.et al Large‐scale serial manalysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res 2000606281–6287. [PubMed] [Google Scholar]
- 15.Folpe A L, Billings S D, McKenney J K.et al Expression of claudin‐1, a recently described tight junction‐associated protein, distinguishes soft tissue perineurioma from potential mimics. Am J Surg Pathol 2002261620–1626. [DOI] [PubMed] [Google Scholar]
- 16.Thurlbeck W M, Churg A M.Pathology of the lung. 2nd ed. New York: Thieme Medical Publishers Inc, 1995
- 17.Travis W D, Brambilla E, Muller‐Hermelink H K.et alTumours of the lung, pleura, thymus and heart. Pathology and genetics. World Health Organization classification of tumours. International Agency for Research on Cancer (IARC). Lyon: IARC Press, 2004
- 18.Muller A M, Weichert A, Muller K M. E‐cadherin, E‐selectin and vascular cell adhesion molecule: immunohistochemical markers for differentiation between mesothelioma and metastatic pulmonary adenocarcinoma? Virchows Arch 200244141–46. [DOI] [PubMed] [Google Scholar]
- 19.Abutaily A S, Addis B J, Roche W R. Immunohistochemistry in the distinction between malignant mesothelioma and pulmonary adenocarcinoma: a critical evaluation of new antibodies. J Clin Pathol 200255662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abutaily A S, Collins J E, Roche W R. Cadherins, catenins and APC in pleural malignant mesothelioma J Pathol2003201355–362. [DOI] [PubMed] [Google Scholar]
- 21.Pan C C, Chen P C, Chou T Y.et al Expression of calretinin and other mesothelioma‐related markers in thymic carcinoma and thymoma. Hum Pathol 2003341155–1162. [DOI] [PubMed] [Google Scholar]
- 22.Wheelock M J, Jensen P J. Regulation of keratinocyte intercellular junction organization and epidermal morphogenesis by E‐cadherin. J Cell Biol 1992117415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol 19881071575–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]