Abstract
Background
Somatostatin is a tetradecapeptide exerting inhibitory action on endocrine and exocrine cell secretion and proliferation. Somatostatin receptors (SST) are widely expressed in various neoplasms including endocrine tumours. Using immunohistochemistry, the expression of SST1, SST2A, SST2B, SST3, SST4, and SST5 was studied in tissue microarrays (TMAs), using a series of 90 human pituitary adenomas producing growth hormone and/or prolactin, including 30 of each somatotroph, lactotroph, and mixed somatotroph/lactotroph adenoma type.
Methods
For immunohistochemistry, the standard avidin biotin complex method enhanced by tyramide was used, using polyclonal antisera for all SST types. A four point scoring system was used to assess the membranous immunopositivity.
Results
All SST types were positive in all tumour types, showing varying immunoreactivity scores. SST5 and SST2A were the predominant receptors, showing strong expression in high frequency in all three adenoma types. Strong expression of SST1 was higher in lactotroph adenomas than in other tumour types.
Conclusions
The immunohistochemical results of SST expression are in agreement with most findings of previous molecular studies. The fact that SST2A expression is predominant suggests that pharmaceutical octapeptide somatostatin analogues may act through this receptor, while the role of SST2B may be merely synergistic.
Keywords: adenomas, immunohistochemistry, pituitary, receptors, somatostatin
Somatostatin imhibits cell secretion and proliferation via five specific G‐protein coupled membrane receptors.1,2,3 Somatostatin receptors (SST) 1, 3, 4, and 5 are encoded by intronless genes, whereas SST2A and SST2B are generated through alternate mRNA splicing of the SST2 gene.4 All SST types have been detected by reverse transcriptase PCR mRNA analysis or binding assays in human endocrine tumours; pituitary adenomas, gastroenteropancreatic neuroendocrine tumours, medullary thyroid carcinomas, lung small cell carcinomas, and pheochromocytomas.5,6,7,8,9,10 Since the introduction of SST immunohistochemistry in 1998,11 a few studies on endocrine tumours have appeared in the literature.6,12,13,14,15,16,17,18,19
Tissue microarrays (TMAs) are collections of many tissue samples in a single paraffin block. The technique enables rapid, inexpensive, and large scale screening of archival material with histochemistry, immunohistochemistry in situ hybridisation, and fluorescent in situ hybridisation; .20,21,22,23
The aim of this study was to investigate using immunohistochemistry and TMAs the expression of SST types in a large number of pituitary adenomas producing growth hormone (GH) and/or prolactin (PRL). This study is the first immunohistochemical investigation of all SST types in functioning pituitary adenomas and in correlation with the histological type.
MATERIALS AND METHODS
In total, 30 unselected cases of somatotroph, 30 lactotroph, and 30 mixed somatotroph–lactotroph adenomas, from the last 5 years, were retrieved from the files of the Pituitary Tumor Reference Center, Department of Pathology, G. Gennimatas Athens General Hospital. All cases were classified by histology, histochemistry, and immunohistochemistry for all adenohypophysial hormones (GH, PRL, adrenocorticotropic hormone, β‐thyroid stimulating hormone, β‐follicle stimulating hormone, β‐luteinising hormone, and the α‐subunit of glycoprotein hormones).
Three TMAs were constructed, one for each adenoma type. For the TMAs, two samples of each tumour were selected from well preserved areas of the original paraffin block, after review of the haematoxylin and eosin stained diagnostic slides. For immunohistochemistry, polyclonal antisera against all SST types (1, 2A, 2B, 3, 4, and 5) were used (dilution 1:3000; Gramsch Lab, Schwabhausen, Germany). The standard ABC method using the Elite Vectastain detection system (Vector Laboratories Inc., Burlingame, CA, USA) was applied, with tyramide amplification, as described previously.24 Sections, microwaved in citrate buffer pH 6.0 for 20 min, were then incubated with the primary antibodies overnight at 4°C. A second series of sections, where the antiserum was substituted by citrate buffer, served as negative control. All SST antisera used in our study have been repeatedly tested by other investigators in various tumours.14,16,17,19,24 GH producing pituitary adenomas have been proved to be the most well established tumours for SST2 and SST5 expression by clinical and in vitro techniques, and also by octreoscan for SST2.6,10,25,26,27,28,29,30,31,32 Additional tissues from invasive duct carcinomas of the breast were used as controls for SST1 and SST3 and medullary thyroid carcinomas for SST415,24 (figs 1–3). For the assessment of SST membranous immunopositivity, a four point scoring system was used, similar to that used for Her‐2/neu in breast carcinomas (table 1).

Figure 1 Positive (A) and negative (B) control for SST1 in breast carcinoma (original magnification ×20).
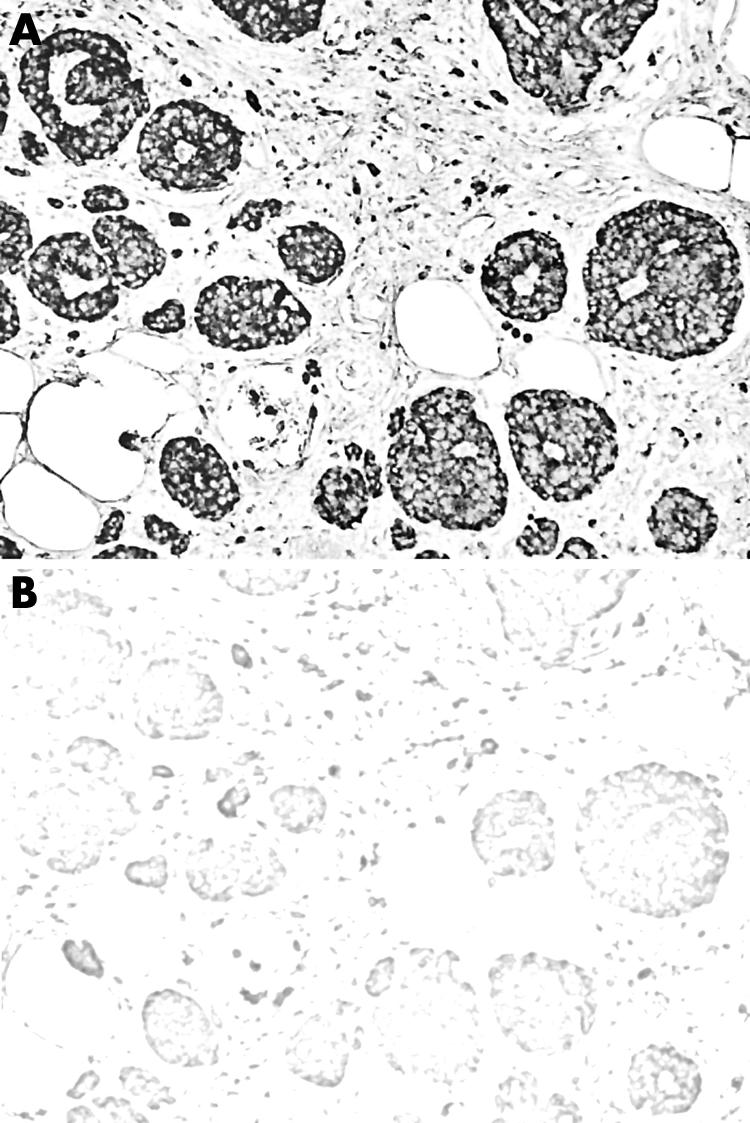
Figure 2 Positive (A) and negative (B) control for SST3 in breast carcinoma (original magnification ×20).
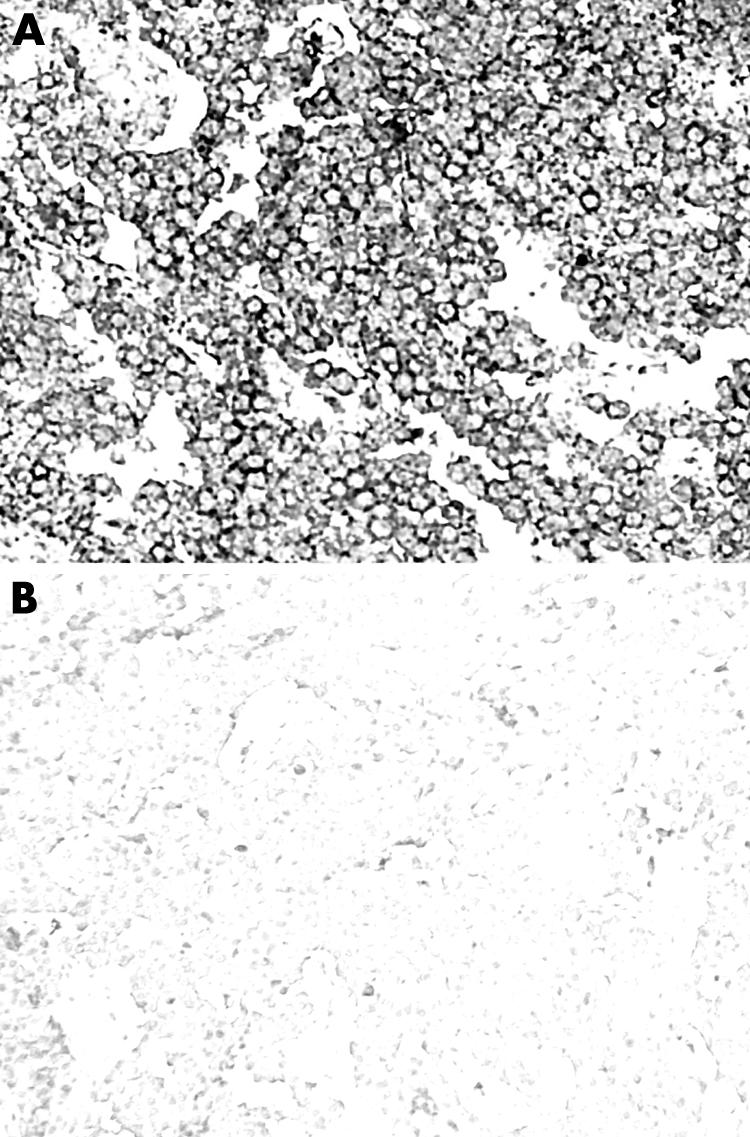
Figure 3 Positive (A) and negative (B) control for SST4 in medullary thyroid carcinoma (original magnification ×20).
Table 1 Scoring system for the evaluation of SST immunopositivity, similar to Her‐2/neu in breast carcinoma.
| Score | Staining results | % of cells stained | Membrane staining pattern | |||
|---|---|---|---|---|---|---|
| 0 | +/− | <10% | – | |||
| 1+ | + | >10% | Faint or incomplete | |||
| 2+ | + | >10% | Weak to moderate, complete | |||
| 3+ | + | >10% | Strong, complete |
RESULTS
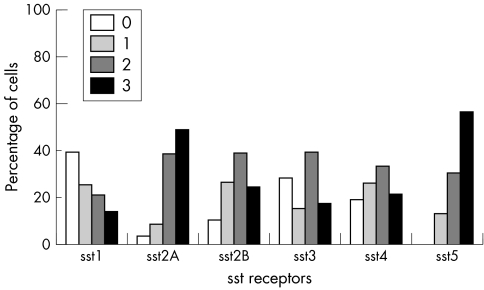
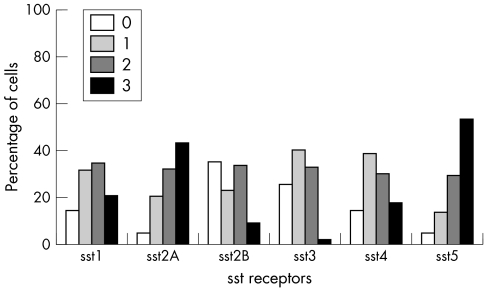
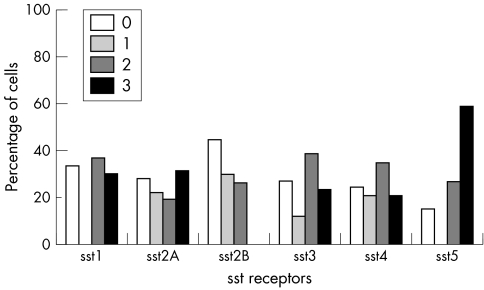
All SST types were positive in all tumour types showing varying immunoreactivity scores. In somatotroph adenomas, SST5 and SST2A predominated, positive in 100% and 96.5% of the tumours respectively, followed by SST2B, SST4, SST3, and SST1 (figs 4 and 5). SST5 and SST2A also showed the highest frequency in score 3+ (56.5% and 49% respectively). In mixed somatotroph lactotroph adenomas, the predominant receptors were also SST5 and SST2A (95.6%), followed by SST4, SST1, SST3, and SST2B (figs 6, 7). The highest numbers of 3+ scores were found in SST5 and SST2A (53.3% and 43.2% respectively). Lactotroph adenomas showed the highest expression of SST5 overall (85.3%) and expression with a score of 3+ (58.8%). Scores of 3+ occurred at lower frequency for SST2A and SST1, followed by SST3 and SST4, with an absence of SST2B (fig 8).
Figure 4 This histogram presents the frequency of each SST receptor in somatotroph adenomas. The vertical axis shows the percentage of positive tumours for each receptor according to the four point scoring system. The grey scale is immunopositivity.
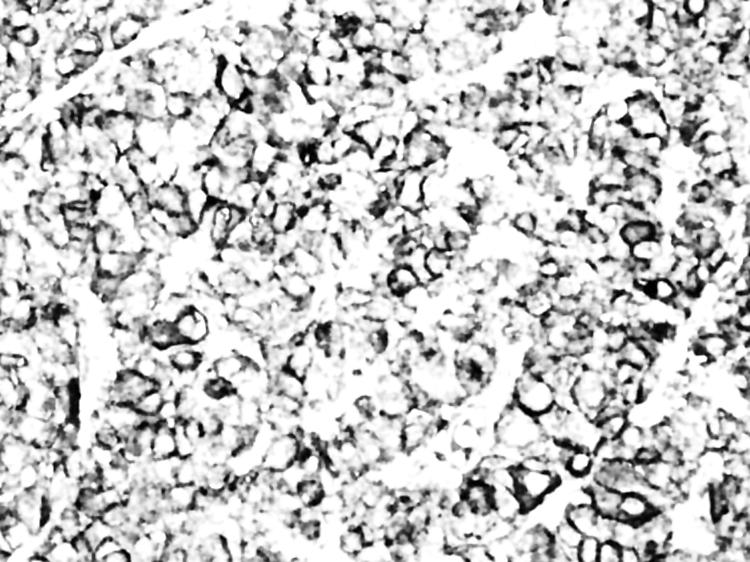
Figure 5 Score of 3+ for SST3 in a somatotroph adenoma. Complete and strong membranous immunopositivity was seen in the majority of adenoma cells (original magnification ×40).
Figure 6 SST type frequency in mixed somatotroph–lactotroph adenomas.

Figure 7 Moderate immunoreactivity for SST4 in a mixed somatotroph–lactotroph adenoma (original magnification ×20).
Figure 8 SST type frequency in lactotroph adenomas.
Heterogeneity of SST expression was detected among the various histological types and among tumours of the same type. SST5 was the predominant receptor in all three adenoma types (fig 9). Strong SST2A expression was mostly found in somatotroph and mixed somatotroph–lactotroph adenomas. SST2A expression exceeded that of SST2B in all adenoma types (figs 10, 11). Strong expression of SST1 was the highest in lactotroph adenomas (fig 12).
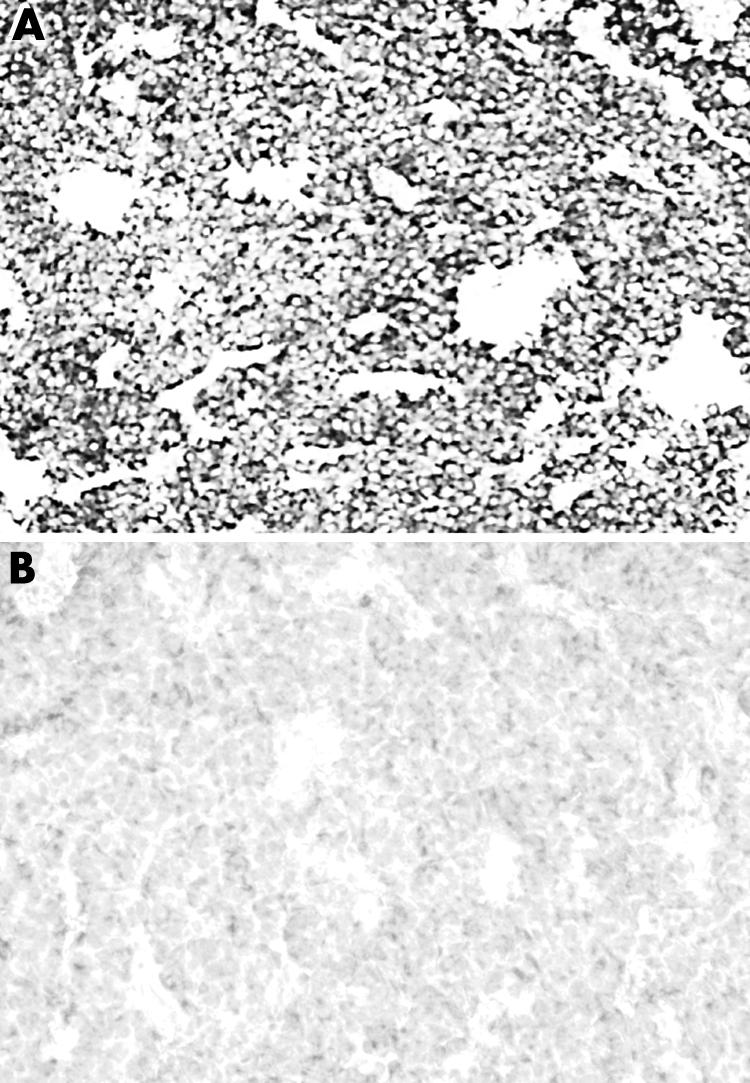
Figure 9 Strong immunoreactivity for SST5 in (A) a somatotroph adenoma; (B) the respective negative control (original magnification ×10).

Figure 10 Strong immunoreactivity for SST2A in (A) a mixed somatotroph–lactotroph adenoma; (B) the respective negative control (original magnification ×20).

Figure 11 Strong immunoreactivity for SST2B in (A) a pituitary somatotroph adenoma; (B) the respective negative control (original magnification ×20).

Figure 12 Moderate to strong membranous distribution of SST1 immunoreactivity in a lactotroph adenoma (original magnification ×20).
Cytoplasmic immunoreactivity was noted in the majority of tumours, occasionally making it difficult to detect incomplete or moderate membranous stain scores (1+ and 2+).
For further investigation of antigen heterogeneity, SST immunohistochemistry was performed on unselected cases using the original paraffin blocks. All SST types showed varying immunopositivity throughout the tissue section. However, comparison with the tissue microarray cores revealed no major differences in the immunohistochemical scoring. During sectioning of the microarray blocks, loss of one sample of the duplicate was detected in 15% of cases, while both samples were lost in about 4%.
DISCUSSION
Octapeptide somatostatin analogues are particularly effective in regulating hormonal hypersecretion in somatotroph and thyrotroph adenomas.25 Given that responsiveness to therapy is directly related to the receptor status of the adenoma, in vitro techniques for reliable SST detection on tumour cells are of clinical importance.26 Detection of SST can be achieved by: (a) receptor mRNA analysis using reverse transcriptase PCR or in situ hybridisation, and (b) receptor protein analysis using radioligand binding studies. These commonly used techniques are demanding in time and skill, involve radioactivity, and may produce results with poor cellular resolution. It should also be noted that tumour mRNA levels may not necessarily reflect the protein receptor levels.4,26 Therefore, morphological localisation of the receptor protein on cell membranes is necessary for precise evaluation of the tumour receptor status.26 Immunohistochemistry on formalin fixed paraffin embedded tissues enables the localisation of all receptor protein types with high cellular resolution and correlation with tissue morphology. However, careful optimisation is required before it can be applied to routine pathology.
In our study, TMAs enabled screening of all SST in a large series of adenomas. Duplicate samples from each donor tumour considerably decreased antigen heterogeneity and risk of complete tissue loss during sectioning.
In somatotroph adenomas, SST5 and SST2A predominated. The expression of SST5 and SST2A in somatotroph adenomas has also been frequently reported in previous studies with mRNA receptor analysis and receptor autoradiography.6,10,27,28,29,30,31,32 To our knowledge, this study is the first time that SST2B has been detected in somatotroph adenomas, because most molecular and binding studies have not discriminated between the two subtypes of receptor 2. In a single report of SST mRNA analysis in three somatotroph adenomas, the results were negative for SST2B, while SST2A was present.29 In our study, SST2B was expressed in the great majority of somatotroph adenomas, however, the level of 3+ expression was considerably less that that for SST2A. SST1 and SST3 were detected in a considerable number of cases in our series, although scores of 3+ were infrequent. Our results are keeping with previous SST mRNA studies, in which SST1 was also detected in GH secreting adenomas, whereas SST3 was more variably expressed.6,30,33,34,35 We also noted SST4 in many somatotroph adenomas, but scores of 3+ were infrequent. Previous mRNA studies in somatotroph adenomas reported SST4 in only a few sporadic cases.29,30 As emphasised previously, tumour mRNA levels may not necessarily reflect the protein receptor levels.4,26 Indeed, recent comparative studies of SST mRNA analysis and immunohistochemistry reported conflicting results in some tumours.14,17
Results from mixed somatotroph–lactotroph adenomas were similar to somatotroph adenomas. Mixed somatotroph–lactotroph adenomas in the literature are usually classified in the GH producing tumours. In a unique molecular study, which discriminated pure GH secreting adenomas from mixed GH/PRL secreting tumours,31 SST2 and SST5 were the predominant receptors in both adenoma groups. In addition, quantitative analysis showed no significant differences in the mRNA levels of these receptors between the two adenoma types. Analogous results with no major differences in SST2A and SST5 overall expression and scores of 3+ were observed in our study. Furthermore, Jaquet31 reported average SST5 mRNA levels 10 fold higher than SST2 levels, showing a predilection over SST5 expression in both types of tumours. This was comparable with our findings, where SST5 showed the highest frequency in both somatotroph and mixed somatotroph–lactotroph adenomas.
In lactotroph adenomas, the predominant receptor was also SST5, followed by SST2A. The stronger expression of SST1 compared with the acromegaly related tumours seems to be a characteristic of prolactinomas. According to mRNA studies the most frequently expressed receptors in human prolactinomas were SST1,27,29,34,36,37 SST5, 28,34,37 and SST2.29,30,33,34 Jaquet37 showed by quantitative mRNA analysis that SST2 levels were magnitudes lower compared with SST5 and SST1, which predominated. Our observations showing frequent and strong expression of SST5, SST2A, and SST1 are compatible with these results.
We detected cytoplasmic immunostaining of varying degree in several adenomas, an observation previously reported for other neuroendocrine tumours.12,17,18,19 Intracellular SST localisation is not an artefact, and it can be attributed to agonist induced receptor internalisation,26 a main mechanism for tumour tachyphylaxis, during the therapeutic management with somatostatin analogues.38 Given that internalised receptors are considered inactive for agonist binding, only membranous immunopositivity reflects the functional receptor status of the tumour.
The efficacy of octapeptide somatostatin analogues in acromegaly treatment is attributed to their high binding affinity, mainly to SST2, expressed in GH secreting adenomas.25,39 Our findings for the first time provide clear and direct morphological evidence for this assumption, demonstrating the presence of the SST2 protein on the cytoplasmic membrane of tumour cells. SST2A expression exceeded that of SST2B in all tumour types examined, suggesting that somatostatin analogues may exert their pharmaceutical action mainly through SST2A, while the role of SST2B may be merely synergistic.
In tissue culture studies, the SST5 preferential compound BIM‐23268, or the SST2 and SST5 bispecific compound BIM‐23244, achieved a more potent GH inhibition compared with octreotide in some adenoma experiments.40 Our results, showing high SST5 density in the majority of GH producing tumours, provide morphological support to these experimental observations. Assessment of SST tumour profile by immunohistochemistry may clarify the in vivo resistance of some adenomas from acromegalic patients to octreotide therapy. Furthermore, it may contribute to the application of novel therapeutic modalities with bispecific40 or universal somatostatin analogues, such as SOM 230,41 in the management of both GH and GH/PRL secreting adenomas.
Clinical experience has shown that octapeptide somatostatin analogues are ineffective in regulating hyperprolactinemia in patients with lactotroph adenomas.42 In our study, strong expression of SST2A was detected in one third of the tumours, suggesting that the receptor although present, may not play an active role in the regulation of PRL secretion. There is evidence from cultures of human pituitary adenomas that SST5 exclusively mediates PRL release, unlike GH, which is regulated by both SST2 and SST5.43 Besides the receptor density, other mechanisms such as formation of homodimers and heterodimers regulate the receptor signalling system and determine the response of hormone secretion to somatostatin analogues. In vitro studies have shown that SST5 and SST1 form heterodimers, resulting in increased ligand affinity and modified SST functionality.44 The investigators suggested that upregulation of certain receptors such as SST1 in heterodimers may lead to desensitisation of the other SST types, in order to maintain an overall normal somatostatin responsiveness in pituitary cells.44 Formation of such heterodimers with upregulated function could be an explanation for the possible desensitisation of SST2A in prolactinomas.
Immunohistochemistry, useful for SST characterisation of gastroenteropancreatic and lung endocrine tumours, generally has a good correlation with reverse transcriptase PCR results.14,17 In the present study, we showed that immunohistochemistry can serve as a tool for detecting SST expression in pituitary adenomas. Strong SST2 immunoreactivity was observed in a single insulinoma case that was very sensitive to octreotide treatment, indicating that the efficacy of somatostatin analogues depends on SST density on tumour cells.18 Based on this observation, we suggest that scores of 3+, corresponding to high receptor density, may indicate tumours that are more susceptible to therapy. However, extensive clinicopathological studies are necessary to correlate the SST immunohistochemical profile of pituitary adenomas with their responsiveness to somatostatin analogues.
TAKE HOME MESSAGES
Somatostatin is a tetradecapeptide exerting inhibitory action on endocrine and exocrine cell secretion and proliferation.
Somatostatin receptors (SST) are widely expressed in various neoplasms including endocrine tumours.
The expression of six SST types was examined in a tissue microarray series of 90 human pituitary adenomas producing growth hormone and/or prolactin, using polyclonal antibodies.
All SST types were positive in all tumour types, but SST5 and SST2A were the predominant receptors. Strong expression of SST1 was higher in lactotroph adenomas than in other tumour types.
The immunohistochemical results agree with most previous studies.
The study suggests that pharmaceutical octapeptide somatostatin analogues may act through the SST2A receptor, while the role of SST2B may be merely synergistic.
In conclusion, we provide morphological evidence for the predominance and high membrane density of SST2A and SST5 in GH producing adenomas, a result in keeping with the action of octapeptide somatostatin analogues through these receptor types in acromegaly. The higher SST2A expression compared with SST2B further suggests that SST2A may play a more critical role in the efficacy of these analogues. SST immunohistochemistry, applied in pituitary adenoma pathology, may become a useful tool for the selection of patients for complementary treatment with the present or new somatostatin analogues. The immunohistochemical demonstration of all SST types in pituitary adenomas may contribute to the design and validation of new SST type selective or consensus somatostatin analogues.
ACKNOWLEDGEMENTS
Pituitary hormone antibodies were donated by the National Hormone and Pituitary Program (NHPP) Torrance, CA, USA to G Kontogeorgos.
Abbreviations
GH - growth hormone
PRL - prolactin, SST, somatostatin receptors
TMA - tissue microarray
References
- 1.Reichlin S. Somatostatin. N Engl J Med 19833091495–1501. [DOI] [PubMed] [Google Scholar]
- 2.Reisine T, Bell G I. Molecular biology of somatostatin receptors. Endocr Rev 199516427–442. [DOI] [PubMed] [Google Scholar]
- 3.Patel Y C. Molecular pharmacology of somatostatin receptor subtypes. J Endocrinol Invest 199720348–367. [DOI] [PubMed] [Google Scholar]
- 4.Patel Y C. Somatostatin and its receptor family. Front Neuroendocrinol 199920157–198. [DOI] [PubMed] [Google Scholar]
- 5.Reubi J C, Schaer J C, Waser B.et al Expression and localization of somatostatin receptor SSTR1, SSTR2, and SSTR3 messenger RNAs in primary human tumors using in situ hybridization. Cancer Res 1994543455–3459. [PubMed] [Google Scholar]
- 6.Schaer J C, Waser B, Mengod G.et al Somatostatin receptor subtypes SST1, SST2, SST3 and SST5 expression in human pituitary, gastroentero‐pancreatic and mammary tumors: comparison of mRNA analysis with receptor autoradiography. Int J Cancer 199770530–537. [DOI] [PubMed] [Google Scholar]
- 7.Reubi J C, Häcki W H, Lamberts S W. Hormone‐producing gastrointestinal tumors contain a high density of somatostatin receptors. J Clin Endocrinol Metab 1987651127–1134. [DOI] [PubMed] [Google Scholar]
- 8.Mato E, Matias‐Guiu X, Chico A.et al Somatostatin and somatostatin receptor subtype gene expression in medullary thyroid carcinoma. J Clin Endocrinol Metab 1998832417–2420. [DOI] [PubMed] [Google Scholar]
- 9.Reubi J C, Kvols L K, Waser B.et al Detection of somatostatin receptors in surgical and percutaneous needle biopsy samples of carcinoids and islet cell carcinomas. Cancer Res 1990505969–5977. [PubMed] [Google Scholar]
- 10.Reubi J C, Waser B, Schaer J C.et al Somatostatin receptor SST1‐SST5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype‐selective ligands. Eur J Nucl Med. 2001;28: 836–46 (erratum in, Eur J Nucl Med 2001281433. [DOI] [PubMed] [Google Scholar]
- 11.Reubi J C, Kappeler A, Waser B.et al Immunohistochemical localization of somatostatin receptors SST2A in human tumors. Am J Pathol 1998153233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofland L J, Liu Q, Van Koetsveld P M.et al Immunohistochemical detection of somatostatin receptor subtypes SST1 and SST2A in human somatostatin receptor positive tumors. J Clin Endocrinol Metab 199984775–780. [DOI] [PubMed] [Google Scholar]
- 13.Kimura N, Pilichowska M, Date F.et al Immunohistochemical expression of somatostatin type 2A receptor in neuroendocrine tumors. Clin Cancer Res 199953483–3487. [PubMed] [Google Scholar]
- 14.Papotti M, Croce S, Macri L.et al Correlative immunohistochemical and reverse transcriptase polymerase chain reaction analysis of somatostatin receptor type 2 in neuroendocrine tumors of the lung. Diagn Mol Pathol 2000947–57. [DOI] [PubMed] [Google Scholar]
- 15.Papotti M, Kumar U, Volante M.et al Immunohistochemical detection of somatostatin receptor types 1–5 in medullary carcinoma of the thyroid. Clin Endocrinol 200154641–649. [DOI] [PubMed] [Google Scholar]
- 16.Papotti M, Croce S, Bello M.et al Expression of somatostatin receptor 2,3 and 5 in biopsy and surgical specimens of human lung tumors. Correlation with preoperative octreotide scintigraphy. Virchows Arch 2001439787–797. [DOI] [PubMed] [Google Scholar]
- 17.Papotti M, Bongiovanni M, Volante M.et al Expression of somatostatin receptors 1–5 in 81 cases of gastrointestinal and pancreatic tumors. A correlative immunohistochemical and reverse transcriptase polymerase chain reaction. Virchows Arch 2002440461–475. [DOI] [PubMed] [Google Scholar]
- 18.Oda Y, Tanaka Y, Naruse T.et al Expression of somatostatin receptor and effects of somatostatin analog on pancreatic endocrine tumors. Surg Today 200236690–694. [DOI] [PubMed] [Google Scholar]
- 19.Kulaksiz H, Eissele R, Rossler D.et al Identification of somatostatin receptor subtypes 1, 2A, 3 and 5 in neuroendocrine tumours with subtype specific antibodies. Gut 20025052–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kononen J, Bubendorf L, Kallioniemi A.et al Tissue microarrays for high‐throughput molecular profiling of tumor specimens. Nat Med 19984844–847. [DOI] [PubMed] [Google Scholar]
- 21.Schraml P, Kononen J, Bubendorf L.et al Tissue microarrays for gene amplification surveys in many different tumor types. Clin Cancer Res 199951966–1975. [PubMed] [Google Scholar]
- 22.Camp R L, Charette L A, Rimm D L. Validation of tissue microarray technology in breast carcinoma. Lab Invest 2000801943–1949. [DOI] [PubMed] [Google Scholar]
- 23.Simon R, Nocito A, Hubscher T.et al Patterns of her‐2/neu amplification and overexpression in primary and metastatic breast cancer. J Natl Cancer Inst 2001931141–1146. [DOI] [PubMed] [Google Scholar]
- 24.Schultz S, Schultz S, Schmitt J.et al Immunocytochemical detection of somatostatin receptors SST1, SST2A, SST2B and SST3 in paraffin‐embedded breast cancer tissue using subtype‐specific antibodies. Clin Cancer Res 199842047–2052. [PubMed] [Google Scholar]
- 25.Hofland L J, Lamberts S V. Molecular pathology of the pituitary. Front Horm Res 200432235–252. [DOI] [PubMed] [Google Scholar]
- 26.Reubi J C. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev 200324389–427. [DOI] [PubMed] [Google Scholar]
- 27.Greenman Y, Melmed S. Heterogeneous expression of two somatostatin receptors in pituitary. J Clin Endocrinol Metab 199478398–403. [DOI] [PubMed] [Google Scholar]
- 28.Greenman Y, Melmed S. Expression of three somatostatin receptor subtypes in pituitary adenomas: evidence for preferential SSTR5 expression in the mammosomatotroph lineage. J Clin Endocrinol Metab 199479724–729. [DOI] [PubMed] [Google Scholar]
- 29.Panetta R, Patel Y C. Expression of mRNA for all five human somatostatin receptors (hSSTR1‐5) in pituitary tumors. Life Sci 199556333–342. [DOI] [PubMed] [Google Scholar]
- 30.Murabe H, Shimatsu A, Ihara C.et al Expression of somatostatin receptor (SSTR) subtypes in pituitary adenomas: quantitative analysis of SSTR2 mRNA by reverse transcription‐polymerase chain reaction. J Neuroendocrinol 19968605–610. [PubMed] [Google Scholar]
- 31.Jaquet P, Saveanu A, Gunz G.et al Human somatostatin receptor subtypes in acromegaly: Distinct patterns of messenger ribonucleic acid expression and hormone suppression identify different tumoral phenotypes. J Clin Endocrinol Metab 200085781–792. [DOI] [PubMed] [Google Scholar]
- 32.Corbetta S, Ballare E, Mantovani G.et al Somatostatin receptor 2 and 5 in human GH‐secreting pituitary adenomas: analysis of gene sequence and mRNA expression. Eur J Clin Invest 200131208–214. [DOI] [PubMed] [Google Scholar]
- 33.Miller G M, Alexander J M, Bikkal H A.et al Somatostatin receptor subtype gene expression in pituitary adenomas. J Clin Endocrinol Metab 1995801386–1392. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen S, Mellemkjaer S, Rasmussen L M.et al Gene transcription of receptors for growth hormone‐releasing peptide and somatostatin in human pituitary adenomas. J Clin Endocrinol Metab 1998832997–3000. [DOI] [PubMed] [Google Scholar]
- 35.Matrone C, Pivonello R, Colao A.et al Expression and function of somatostatin receptor subtype 1 in human growth hormone secreting pituitary tumors deriving from patients partially responsive or resistant to long‐term treatment with somatostatin analogs. Neuroendocrinol 200479142–148. [DOI] [PubMed] [Google Scholar]
- 36.Hofland L J, de Herder W W, Visser‐Wisselaar H A.et al Dissociation between the effects of somatostatin (SS) and octapeptide SS‐analogs on hormone release in a small subgroup of pituitary and islet cell tumors. J Clin Endocrinol Metab 1997823011–3018. [DOI] [PubMed] [Google Scholar]
- 37.Jaquet P, Ouafik L, Saveanu A.et al Quantitative and functional expression of somatostatin receptor subtypes in human prolactinomas. J Clin Endocrinol Metab 1999843268–3276. [DOI] [PubMed] [Google Scholar]
- 38.Hofland L J, Lamberts S W. The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocr Rev 20032428–47. [DOI] [PubMed] [Google Scholar]
- 39.Shimon I, Taylor J E, Dong J Z.et al Somatostatin receptor subtype specificity in human fetal pituitary cultures. Differential role of SSTR2 and SSTR5 for growth hormone thyroid‐stimulating hormone and prolactin regulation. J Clin Inv 199799789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saveanu A, Gunz G, Dufour H.et al BIM‐23244, a somatostatin receptor subtype 2‐ and 5‐selective analog with enhanced efficacy in suppressing growth hormone (GH) from octreotide‐resistant human GH‐secreting adenomas. J Clin Endocrinol Metab 200186140–145. [DOI] [PubMed] [Google Scholar]
- 41.Hofland L J, van der Hoek J, van Koetsveld P M.et al The novel somatostatin analog SOM230 is a potent inhibitor of hormone release by growth hormone‐ and prolactin‐secreting pituitary adenomas in vitro. J Clin Endocrinol Metab 2004891577–1585. [DOI] [PubMed] [Google Scholar]
- 42.Lamberts S W, Zweens M, Klijn J G.et al The sensitivity of growth hormone and secretion to the somatostatin analogue SMS 201–995 in patients with prolactinomas and acromegaly. Clin Endocrinol (Oxf) 198625201–212. [DOI] [PubMed] [Google Scholar]
- 43.Shimon I, Yan X, Taylor J E.et al Somatostatin receptor (SSTR) subtype‐selective analogues differentially suppress in vitro growth hormone and prolactin in human pituitary adenomas. Novel potential therapy for functional pituitary tumors. J Clin Invest 19971002386–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rocheville M, Lange D C, Kumar U.et al Subtypes of the somatostatin receptor assemble as functional homo‐ and heterodimers. J Biol Chem 20002757862–7869. [DOI] [PubMed] [Google Scholar]