Abstract
Background and objectives
In patients with breast cancer (BC), the sentinel node (SN) is the first node in the axillary basin that receives the primary lymphatic flow and can be used to accurately assess the axillary nodal status without removal of the axillary contents. Currently, histology and/or immunohistochemistry are the routine methods of SN analysis. The primary objective of this study was to develop a reproducible reverse transcription (RT) PCR assay, with emphasis on achieving high specificity for accurate detection of BC micrometastases in the SN. To correct for the heterogeneity of BC cells, a multimarker approach was followed, with the further aim of improving the detection rate of the assay.
Methods
In total, 73 markers were evaluated, of which 7 were breast epithelial markers and 66 were either cancer testis or tumour associated antigens. Twelve BC cell lines and 30 SNs (from 30 patients) were analysed using RT‐PCR to determine the in vitro and in vivo detection rates for each of the markers. In addition, 20 axillary nodes obtained from a patient with brain death were used as controls to optimise the PCR cycle numbers for all the markers.
Results
Of the 30 SNs, 37% (11/30) were positive on haematoxylin and eosin analysis. Extensive immunohistochemical (IHC) analyses of the haematoxylin and eosin negative nodes confirmed the presence of very small numbers of BC cells in an additional 40% (12/30) of SNs. Molecular analysis with the hMAM‐A alone identified metastases in 70% (21/30) of SNs. Using MAGE‐A3 in combination with hMAM‐A identified metastases in 90% (27/30) of patients. Seven SNs (23%) were negative for micrometastases (with haematoxylin and eosin and IHC) but RT‐PCR positive for either hMAM‐A or MAGE‐A3.
Conclusions
As IHC analysis resulted in a 77% detection rate compared with 37% for haematoxylin and eosin analysis, we consider that IHC is essential in order not to miss SN micrometastases. Molecular analysis with hMAM‐A and MAGE‐A3 allows detection of BC micrometastases with a 90% detection rate. However, the clinical value of histologically negative but RT‐PCR positive SNs can only be determined with long term follow up.
Keywords: breast cancer, RT‐PCR, sentinel lymph node, specificity, micrometastases
Over the past decade, the mortality rate of breast cancer (BC) has not changed significantly in spite of efforts on many fronts to improve the prognosis.1,2 BC is still considered as one of the most potentially lethal diseases in women, despite the improvement in staging and diagnosis, and the recent advances in surgical treatment. The number of tumour involved axillary lymph nodes and the size of the largest nodal metastasis are currently the two most important prognostic factors for patients with BC.3,4,5 Of the patients presenting with a small operable breast mass without axillary nodal involvement, 50% may be cured by surgery alone; in 30% of these women, metastatic disease will recur within 5 years and the patient will eventually die of the disease.6 This clinical manifestation of relapse implies that these patients must have already developed subclinical/occult/micrometastases at the time of primary tumour excision. Thus, the search for these micrometastatic cells is an issue of significant clinical interest. The development of new methods to identify patients, who are node negative by conventional histological methods but are at increased risk of disease progression, has now become the focus of many studies.
It is well documented that the status of the regional axillary lymphatic basin is a reflection of the biological aggressiveness of the primary tumour.7 Once such nodal involvement becomes clinically evident, the 5 year survival rate decreases from 82.2% for node negative patients to 73% for those with 1–3 positive nodes and as low as 45.7% for patients with 4–12 positive nodes.8 This significant decrease in the survival rate necessitates a more accurate subclinical staging. This would help to stratify node negative patients into risk groups as the basis for decision making regarding the provision of adjuvant treatment and the administration of immunotherapy. In addition, those patients with no evidence of progressive disease will be spared the side effects of unnecessary surgical intervention and the cost and toxicity of radiation, chemotherapy, and immunotherapy. Therefore, it is crucial to identify those patients who harbour occult metastases at the time of primary tumour diagnosis, this strategy being the basis of sound cancer management. Thus, the challenge is to develop prognostic markers and techniques that will identify these high risk patients more accurately.
Currently, the detection of BC metastases is largely based on regular clinical breast examinations and radiological follow ups in the form of mammography. Even though these methods are of limited accuracy, they are of value in reducing the mortality. Several markers have been evaluated for the ability to detect occult BC cells in the peripheral blood by means of RT‐PCR. Some of these studies reported that CK 19 and CEA are both sensitive and specific for the detection of BC cells in leucopheresis samples.9,10,11 Considering the heterogeneity of BC cells, a combination of β‐human chorionic gonadotrophins, the oncogene receptor c‐Met, β1→4 N‐acetyl galactosamine transferase, and the tumour associated antigen MAGE‐A3 were evaluated in a multi‐marker RT‐PCR assay and found to enhance the detection of systemic metastases by 32%.12
It is now evident that analysis of the bone marrow and the draining lymphatic basin offers a more appealing approach to establish whether there are metastases in patients with early stage BC than does analysing the blood compartment and may provide better prognostic information.13 The sentinel node (SN) is the most likely site for lymphogenic metastases and can be identified with 98% accuracy using radioguided surgery.14 The status of the SN reflects the status of the remaining non‐sentinel axillary lymph nodes in as many as 40% of the patients.15,16 Although histopathology is the current standard of SN evaluation, it has been shown to underestimate the presence of metastases.17 Thus more sensitive, less labour intensive, and more cost effective methods are needed.
Although there has been progress on the development of molecular techniques for the detection of micrometastases in the SN, the lack of marker specificity and standardisation, and the variable tumour detection rates are currently preventing molecular evaluation of SN tissue from becoming clinically applicable. Therefore, the aim of this study was to develop a standardised and reproducible RT‐PCR assay, with the emphasis on achieving high specificity for accurate detection of BC micrometastases in the SNs. In recognition of the known heterogeneity of BC cells,18 a multimarker approach was followed, with a view to further improving the detection rate of the assay. Using the USA National Library of Medicine PubMed search engine, a thorough computerised literature search was conducted on all the molecular markers previously used for detection of BC cells in the blood and lymph nodes. In total, 73 markers were selected: seven were breast epithelial markers (hMAM‐A and ‐B, LPB, maspin, prolactin inducible protein (PIP), CK 19 and CK 20) and 66 were either cancer testis antigens (CTAs) or tumour associated antigens. By the process of elimination, all markers that gave rise to illegitimate or non‐specific transcripts were excluded. Thus, 17 markers were found to be specific. The presence of these markers was evaluated on 12 BC cell lines and 30 SNs from patients who had undergone primary tumour excision. We show that of the 17 markers, two (hMAM‐A and MAGE‐A3) proved ideal for the development of the multimarker assay.
MATERIAL AND METHODS
Cell lines
In total, 12 BC cell lines were used for this study. MCF7, MDA‐MB‐231, T47D, and ZR‐75‐1 were obtained from the American Type Culture Collection (Rockville, MD, USA). The UCT‐Br‐1 cell line was established at the University of Cape Town from a bone metastasis in a patient with adenocarcinoma of the breast.19 Seven other BC cell lines, SUM229PE, SUM225CWN, SUM159PT, SUM52PE, SUM185PE, SUM102PT, and SUM44PE, were developed at the University of Michigan. These cell lines were derived from primary tumours, chest wall recurrences, and pleural effusion metastases.20
MCF7, MDA‐MB231, T47D, ZR‐75‐1, and UCT‐Br‐1 were grown in RPMI‐1640 medium (Flow Laboratories, Irvine, UK) supplemented with 10% (v/v) heat inactivated (30 minutes at 56°C) fetal calf serum (FCS; Gibco, Grand Island, NY, USA), 50 IU/ml penicillin, and 20 μg/ml streptomycin at 37°C under 5% CO2, 95% air, and 90% humidity. The SUM cell lines were grown in Ham's F‐12 medium supplemented with 5% heat inactivated FCS, growth factors (5 μg/ml insulin, 1 mg/ml hydrocortisone, and 10 ng/ml epidermal growth factor), 5 μg/ml gentamycin and 0.5 μg/ml fungizone. For SUM44PE and SUM102PT cells, a serum free medium was used, to which 5 mmol/l ethanolamine, 10 mmol/l HEPES, 5 μg/ml transferrin, 10 nmol/l tri‐iodothyronine, 0.5 g/L bovine serum albumin, and 50 μmol/l sodium selenite were added. The immortalised mammary cell line MCF12A was grown in a 1:1 mixture of Dulbecco's modified Eagle's medium and Ham's F12 medium, 20 ng/ml epidermal growth factor, 100 ng/ml cholera toxin, 10 µg/ml insulin, 500 ng/ml hydrocortisone, and 5% horse serum.
Patients and tissue specimens
This study was approved by the ethics and research committee of the University of Cape Town (ref rec: 238/2001). Informed and written consent was obtained from all patients or from the next of kin of organ donors. Thirty SNs (each weighing 0.4–0.6 g) were obtained from 30 patients with stage I and II BC who had undergone sentinel lymphadenectomy in the Department of Surgery at the University of Cape Town and Groote Schuur Hospital academic health complex. SNs were detected by means of two way mapping with 1% lymphazurin vital blue dye and 99m‐technetium labelled colloid particles of human serum albumin, which were traced with a hand held gamma detection probe. These nodes were used to assess the in vivo marker detection rate at the early stage of disease progression. Each SN was bisected, and one half used to confirm the pathology by haematoxylin and eosin staining. The other half was used for molecular analysis. Twenty normal lymph nodes (each weighing 0.4–0.6 g) were obtained from the axillary and visceral regions of three organ donors. These were used to optimise the PCR cycle number for each marker.
Handling, homogenisation, and RNA extraction of nodal tissue
Immediately after SN biopsy, half of each lymph node was placed in a sterile 2 ml polypropylene cryovial, snap frozen in liquid nitrogen, and transported to the laboratory. The tissue was homogenised by adding a sterile 8 mm stainless steel ball together with 1.5 ml Tripure RNA extraction reagent (Roche Diagnostics, Mannheim, Germany) for every 0.15 g of tissue. After securing the cryovial in an Xpress homogeniser (Tekniva, South Africa), high speed oscillation was carried out for a total of 30 seconds, comprising six cycles of 5 seconds ‘on' followed by 10 seconds ‘off'. The remaining procedure for total RNA extraction was essentially as described by the manufacturer (a standard guanidinium thiocyanate/phenol/chloroform method). The purity and yield of the total RNA was measured spectrophotometrically at 260 and 280 nm. The RNA yield was relatively consistent, being approximately 200 µg total RNA for every 0.15 g of nodal tissue.
RT‐PCR
Reverse transcription and PCR reactions were carried out as previously described by Davids et al.21 The following markers were evaluated in the study: α‐A‐adaptin,22 B305D,23 B‐726P (NY‐Br‐1),24 BAGE,25 CAGE,26 CEA,27 CK 19,28 CK 20,29 CML28,30 CML66,31 CSAG‐ac,32 CT15,33 CT16,33 CT17,33 CTAGE‐1,34 CTP11,35 D40,36 ER‐alpha,37 ER‐beta,37 GABA‐pi,23 GAGE‐1,‐2,‐7,38,39 GAGE‐3,‐4,‐5,‐6,38,39 HCA90,40 HCA520,40 hCG,41 hMAM‐A,42 hMAM‐B,43 HMGIC,44 HSPC218,45 KIAA1416,33 KLK13,46 KM‐HN‐3,47 KM‐HN‐1,47 KNSL6,33 LPB,48 MAGE‐A1,49 MAGE‐A2,50 MAGE‐A3,51 MAGE‐A6,49 MAGE‐A1,‐2,‐3,‐4,‐5,‐6,52 MAGE‐A12,49 MAGE‐B4,34 MAGE‐C1,53 maspin,54 MDA‐7,55 MMA‐1ab,56 NY‐BR‐62,57 NY‐BR‐85,57 NY‐ESO‐1,58 PAGE‐1,59 PIP,60 PLU‐1,61 PRAME,62 PSE,63 PTI‐1,64 RAGE‐1,‐2,‐3,34 RAGE‐4,34 RBP1L1,65 SCP‐1,59,66 SSX‐1, ‐2, ‐3, ‐4, and ‐5,67 survivin‐2B,68 survivin‐δ Ex3,68 Sp17‐2,69 TRAG‐3,70 uPA‐R,71 XAGE‐1a,72 XAGE‐1b,72 XAGE‐1c,72 XAGE‐1d,72 and XAGE‐2.72
A table of the primer sequences for each marker can be obtained from us on request. Primers for each marker were synthesised and purified at the Department of Cellular and Molecular Biology, University of Cape Town. The primer pair for each marker was positioned in different exons and, if possible, was designed to lie on either side of a fairly large intron (>1 kb) to reduce the chance of genomic DNA amplification that might be present in the RNA sample. cDNA specificity controls (reverse transcription reactions without reverse transcriptase) were performed for each primer set to control for the possibility of genomic DNA amplification and to exclude the possibility that products were being amplified from pseudogenes.
Negative and positive PCR controls
Water (instead of the RNA template) was used as a negative control for each batch of samples to control for the possibility of false positive results that might arise from amplicon contamination. A positive control for each marker was included with each batch of samples to verify the efficiency of the RT‐PCR assay. Table 1 lists cell lines that were used as appropriate positive controls. The integrity of each RNA sample was verified by RT‐PCR for the housekeeping gene porphobilinogen deaminase (PBGD), also called hydroxymethylbilane synthase (HMBS).73
Table 1 Primers used in this study.
| mRNA target (Genbank accession no.) | Sequence from 5′ to 3′ end for the forward and reverse primers respectively | Nucleotide position (exon numbers) | Product size (bp) | Primer AT (°C) | PCR cycle no.† | Primer reference | Positive control cell lines | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CSAG‐ac (NM_153478)(a) (AF268419)(c) | TTGTAGAGAATGGCTGCGGGTCTCAGAGTGGCTGGATAGTGTTGG | 78–99 605–627(a) 34–55 767–789(c) | 550(a) 756(c) | 60 | 40 | 32 | SUM159 | |||||||
| GAGE‐1, ‐2, ‐7 (U19143) | GACCAAGACGCTACGTAGCCATCAGGACCATCTTCA | 118–135 344–361 | 244 | 56 | 30 | 38,39 | T47D | |||||||
| HMAM‐A (U33147) | AGCACTGCTACGCAGGCTCTATAAGAAAGAGAAGGTGTGG | 99–119 (Ex 1/2) 411–430 (Ex 3) | 332 | 60 | 40 | 42 | SUM159PT, SUM52PE, SUM225CWN, SUM44PE, SUM229PE, SUM102PT, ZR‐75‐1, UCT‐Br‐1, SUM185 | |||||||
| HMAM‐B (AF071219) | ACTCCTGGAGGACATGGTTGATCTGAGCCAAACGCCTTGGGT | 133–153 357–377 | 245 | 58 | 30 | 43 | UCT‐Br‐1, ZR‐75‐1 | |||||||
| MAGE‐A1 (BC017555) | AGCACCAAGGAGAAGATCTGCCGCTGGAACCCTCACTGGGTTGCC | 104–125 (Ex 2/3) 408–430 (Ex 3) | 327 | 60 | 30 | >Primer* <Primer 49 | T47D | |||||||
| MAGE‐A2 (L18920) | CATTGAAGGAGAAGATCTGCCTGAGTAGAAGAGGAAGAAGCGGT | 362–383 (Ex 3/4) 556–577 (Ex 4) | 216 | 60 | 30 | 50 | SUM159 | |||||||
| MAGE‐A3 (U03735) | GAAGCCGGCCCAGGCTCGGGAGTCCTCATAGGATTGGCTCC | 48–65 (Ex 1) 448–471(Ex 3) | 424 | 60 | 30 | 51 | SUM225CWN, MDA‐MB‐231 | |||||||
| MAGE‐C1 (AF056334) | GACGAGGATCGTCTCAGGTCAGCACATCCTCACCCTCAGGAGGG | 26–48 637–657 | 632 | 60 | 30 | 53 | UCT‐Mel‐1,‐3,‐6 | |||||||
| Maspin (U04313) | TCAAGCGGCTCTACGTAGACCCTCCACATCCTTGGGTAGT | 341–360 768–787 | 447 | 60 | 30 | 54 | MCF‐7, UCT‐Br1, MDA‐MB‐231, ZR‐75‐1, T47‐D | |||||||
| MMA‐1ab (AJ301615) | AACATGGGTGGCAAAAAGAGGTCACGGCATGAACTGAATG | 73–92 310–329 | 257 | 64 | 30 | 56 | SUM229, SUM159 | |||||||
| PBGD (NM_000190) | CTGGTAACGGCAATGCGGCTGCAGATGGCTCCGATGGTGA | 32–51 (Ex 1) 350–369 (Ex 5) | 338 | 60 | 30 | 73 | Housekeeping gene | |||||||
| PIP (NM_002652) | GCTCAGGACAACACTCGGAAATAACATCAACGACGGCTGC | 118–137 367–386 | 269 | 60 | 30 | 60 | SUM 159PT, SUM52PE, SUM225CWN, SUM44PE, SUM229PE, SUM 102PT, ZR‐75‐1, UCT‐Br‐1, SUM185PE, MCF7, T47D | |||||||
| PSE (AB031549) | AGTGCTCAAGGACATCGAGACgAGCCACTTCTGCACATTGCTG | 792–813 861–881 | 90 | 60 | 30 | 63 | UCT‐Br‐1 | |||||||
| PTI‐1 (L41498) | ATGGGGGTAGAGCACTGAATGAACACCAGCAGCAACAATCAG | 537–557 768–788 | 252 | 60 | 40 | 64 | MDA‐MB‐231, MCF7, T47D | |||||||
| RAGE‐4 (U46194) | CACACCCGCTCAGAAGATCGGACTCTAGCTGCCTTGTGG | 223–241 807–826 | 604 | 60 | 30 | 34 | MCF7, T47D, ZR‐75‐1 | |||||||
| SSX1‐5 (BC005325) | GCCCAAGAAGCCAGCAGAGGCTCTTCATAAACCACCAGCTGCTCTT CATAAATCACCAGCTG | 394–414 575–595 | 201 | 62 | 30 | 67 | T47D | |||||||
| XAGE‐1a (AF251237) | CTTTGGTGCCCACCTCAGTAAACCAGCTTGCGTTGTTTC | 92–110 540–559 | 468 | 60 | 30 | 72 | MDA‐MB‐231 | |||||||
| XAGE‐2 (AJ318880) | CAGCCGTCTGGACTCTTTCTTTGTGTTGTTTCAGCTTATCTTCC | 105–124 (Ex 1) 526–549 (Ex 5) | 445 | 60 | 30 | 72 | MCF7 |
*Designed by the authors using DNAMAN 5.1.0.0 (Lynnon Biosoft). †The PCR cycle number for each marker was optimised on 20 normal lymph nodes (10 of axillary and 10 of groin origin). AT, annealing temperature.
Pathological evaluation of the sentinel lymph nodes
Histopathological evaluation was carried out on one half of the SN using standard haematoxylin and eosin staining. Nodes found to be negative by histopathological analysis were then screened by immunohistochemistry (IHC). A maximum of 18 (2–4 μm thick) serial sections were stained, with every alternate section stained with CK 7 or MNF‐116 antibody, respectively. CK 7 is a monoclonal antibody that reacts with an intermediate filament protein of 54 kDa that recognises simple epithelium found in most glandular and transitional epithelia, but not in the stratified squamous epithelia. MNF116 is a monoclonal antibody that reacts with cytokeratins 5, 6, 8, 17, and probably also 19, and shows a broad pattern of reactivity with human epithelial tissue, from simple glandular to stratified squamous epithelium.
Statistical analysis
Stata8 software (StataCorp LP, College Station, TX, USA) was used to calculate standard errors and the corresponding binomial exact confidence intervals for the different analytical procedures.
RESULTS
Marker specificity
The first aim of this study was to design primers for each of the identified markers and to test each of them for suitability and specificity. Each primer set was tested on a panel of BC and melanoma cell lines (melanoma cell lines were included to increase the likelihood of finding a positive control cell line for each marker). Of the 73 markers initially selected for evaluation, eleven (α‐A‐adaptin, B‐726P, CSAG‐ac, CT16, CT17, HCA90, HCA520, KIAA1416, MAGE‐B4, NY‐ESO‐1, and RAGE‐1,2,3) were found not to be expressed in any cell line and were therefore excluded from further analysis. The remaining 62 markers were then tested for cDNA specificity by omitting the reverse transcriptase from the RT reaction. Two markers, namely BAGE and CTP11, gave rise to nonspecific products and therefore were excluded from further analysis.
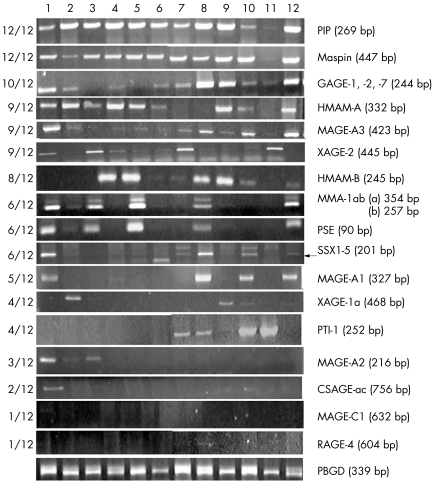
One of the major dilemmas with molecular detection of cancer cells in nodal tissue is that illegitimate transcripts and/or non‐specific transcripts from other low abundance nodal cell types can give rise to false positive results. To address this issue, we tested the remaining 60 markers on 20 normal lymph nodes. The optimum cycle number for each marker was determined by performing PCR reactions over a range of cycle numbers on the cDNA derived from normal lymph nodes. Initially, each marker was tested on five normal lymph nodes using 40, 35, and 30 PCR cycles. The PCR cycle number at which no false positives were generated was chosen for each marker. A further 15 normal lymph nodes were then evaluated to confirm the specificity of each marker at the chosen PCR cycle number. Markers found to be non‐specific at <30 PCR cycles were deemed to be unsuitable, as marker sensitivity would be severely compromised at this PCR cycle number. Our results showed that the following 17 markers were specific and potentially useful for detection of BC metastases (table 1): hMAM‐A and ‐B, CSAGac, GAGE‐1, ‐2 and –7 (consensus primer set), MAGE‐A1, ‐A2, ‐A3 and ‐C1, maspin, MMA‐1ab, PIP, PSE, PTI‐1, RAGE‐4, SSX‐1, ‐2, ‐3, ‐4 and ‐5 (consensus primer set), XAGE‐1a, and XAGE–2. All of the markers were specific at 30 PCR cycles, except for hMAM‐A and CSAGac, which were specific at 40 PCR cycles, and MAGE‐A1, which was specific at 35 PCR cycles.
Expression profile of selected markers on 12 BC cell lines and 30 SNs
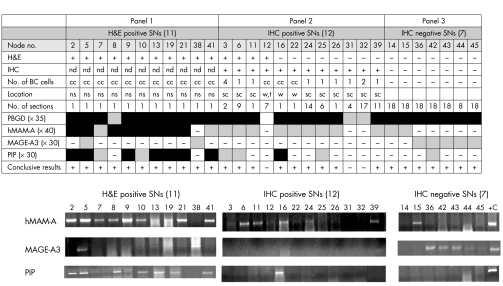
To further investigate the possible value of the 17 selected markers as diagnostic and/or prognostic tools in BC, we next tested these markers on a panel of 12 BC cell lines (fig. 1). Different levels of expression were observed for the different markers, of which 10 were expressed in at least half of the cell lines. To establish whether the expression profile of these markers could be used as a guideline to select markers for the detection of micrometastases in nodal tissue from patients with early stage BC, we tested our candidate markers on 30 SNs (fig 2, results for hMAM‐A, MAGE‐A3, and PIP shown only). An important observation was that although several markers, such as PIP, maspin, and GAGE, were widely expressed in the cell lines (fig 1), they had a low detection rate in the SNs (fig 2, results for PIP only shown).
Figure 1 Expression profile of 17 potentially useful molecular markers on 12 breast cancer cell lines. Lanes 1–12: SUM159, SUM52, SUM225, SUM44PE, SUM229, SUM102, MCF7, T47D, ZR‐75‐1, UCT‐Br1, MDA‐MB231, SUM185. All markers were tested at 30 PCR cycles. Cells were cultured, and the RNA extracted and subjected to RT‐PCR analysis for the markers indicted in the right hand column. The left column summarises the number of cell lines positive for each marker.
Figure 2 Expression profile of HMAM‐A, MAGE‐A3, and PIP on 30 SNs. Haematoxylin and eosin staining and immunohistochemical (IHC) analyses were carried out on 30 SNs as described in the text. Results were categorised as either haematoxylin and eosin positive (panel 1), IHC positive (panel 2), or IHC negative (panel 3). The number of breast cancer cells detected on an IHC section, the location of the BC cells, and the number of sections performed in order to obtain a positive IHC result is indicated in appropriate columns. PCR cycle numbers for each marker are indicated in brackets. Black and grey boxes represent strong and weak expression of markers respectively. Each marker was tested once in three independent experiments and the PCR result was scored as “positive” if at least two out of three results were positive. cc, cell clusters; sc, subcapsular sinus; w, within lymphoid tissue; t, trabeculae; nd, not determined; ns, not specified.
Molecular analyses of SNs versus histological analyses
The next aim of this study was to compare the detection rate of the molecular assay with standard histological analysis (fig 2). Of the 30 SNs analysed, 37% (11/30) were positive with histological analysis (fig 2, panel 1). Further immunohistochemical (IHC) analysis of the histologically negative nodes revealed the presence of very small numbers of BC cells in a further 40% (12/30) of SNs (fig 2, panel 2). Thus, the number of patients positive for BC micrometastases increased from 37% to 77%. It is important to note that an average of seven sections per SN was needed in order to obtain a positive IHC, when the cells were present as singles, rather than clusters.
The final aim of this study was to find an appropriate combination of molecular markers that would provide optimum detection of BC metastases in SN tissues. Of the markers studied, only hMAM‐A, MAGE‐A3, and PIP were found to be suitable for the detection of micrometastases in the SNs (fig 2). All the others were found to be unsuitable because of the low detection rate (or they did not improve the detection rate). Molecular analysis with hMAM‐A alone identified metastases in 70% (21/30) of the patients. MAGE‐A3 in combination with hMAM‐A identified metastases in 90%[27/30] of the patients. Although PIP had a relatively high detection rate (47%), it did not contribute to a higher detection rate when used in combination with either hMAM‐A or MAGE‐A3. Molecular analysis with hMAM‐A and MAGE‐A3 did not identify metastases in nodes 12, 31, and 32. The most likely explanation for these three negative PCR results is the poor quality RNA (as revealed by the PBGD internal control). A further seven SNs (23%) were IHC negative (even after 18 serial sections) but RT‐PCR positive for either hMAM‐A or MAGE‐A3 (fig 2, panel 3). The clinical value of histologically negative but RT‐PCR positive SNs is discussed later.
In order to determine which, if any of the analytical procedures were superior, we calculated standard errors and 95% binomial exact confidence intervals for the different procedures. As the confidence intervals for PCR (0.734 to 0.979) and both histological procedures (haematoxylin and eosin plus IHC, 0.577 to 0.901) did not overlap with the haematoxylin and eosin technique (0.199 to 0.561) alone, we are statistically confident (at the 5% level) that haematoxylin and eosin is an inferior technique compared with PCR alone or both histological techniques together. However, there is insufficient statistical evidence (at the 5% level) that PCR is superior to both histological techniques, probably because of the small sample size (n = 30), which affects the standard error. This analysis was performed assuming that all 30 patients were “true” positives. However, as nodes 14 and 15 did not express MAGE‐A3 (a true marker of malignancy), we recalculated the confidence intervals by excluding these two nodes (n = 28). Using this approach, the 95% confidence intervals did not change greatly, and thus the conclusions stand.
Expression profile of selected markers on normal breast epithelial and MCF12A cells
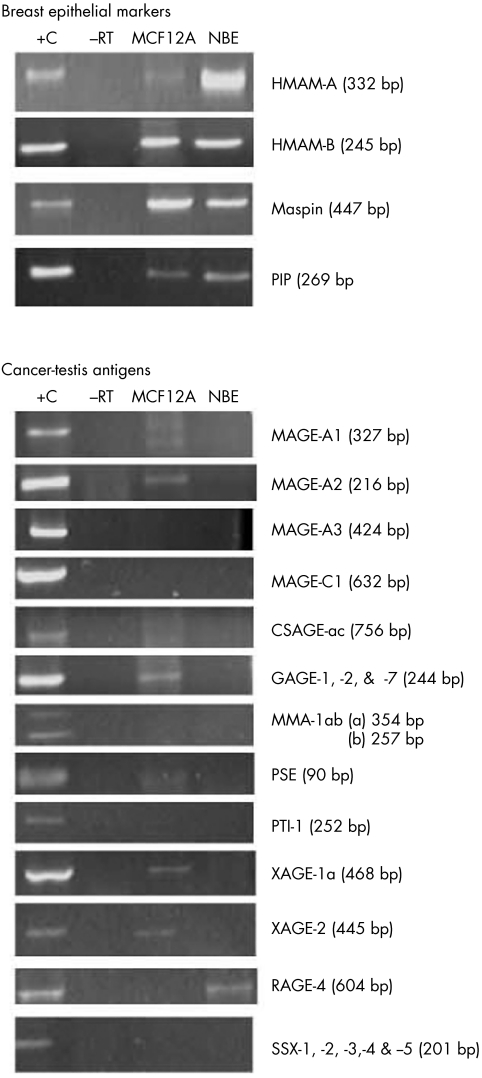
Although rare, normal breast epithelial cells (that is, epithelial inclusions) have been reported to be present in SNs that drain the respective epithelial tissue, whether this tissue is involved with benign or malignant disease.74 As the presence of such inclusions could result in false positives with IHC and molecular analysis (when epithelial markers are used), we wanted to establish whether any of the selected markers are expressed by normal breast epithelium. To address this issue, we tested our markers on the MCF12A immortalised normal mammary cell line and a normal breast tissue sample (fig 3). All the breast epithelial markers (hMAM‐A, hMAM‐B, maspin, and PIP) were expressed by the normal breast epithelium (fig 3). With the exception of RAGE‐4, all candidate CTAs were PCR negative when tested on the normal breast epithelium. This encouraging result indicates that markers not expressed by normal breast epithelium but expressed by BC would make it possible to differentiate between the benign and malignant epithelial components of nodal tissue.
Figure 3 Expression profile of 17 potentially useful molecular markers on normal breast epithelium (NBE) and the immortalised mammary breast epithelium cell line, MCF12A. Positive (+C) and cDNA negative controls (−RT) are indicated.
DISCUSSION
The early detection of tumour spread remains one of most challenging issues in oncology. Improved detection of micrometastases would contribute to a better understanding of patterns of tumour cell spread, and provide better insight into the true clinical significance of these cells. It would also greatly assist in the staging of patients and aid in proper choice of therapy. Despite extensive investigation, the patterns of micrometastatic disease spread remain unclear, partially because the techniques to explore these questions are inadequate. Recently developed sophisticated tracking methods have revealed that early metastatic spread is to the SN. This has led to a change in the trend for examining the lymphatic basin, with the focus now being on the SN and not the entire axillary lymphatic basin.
The current method for examination of SNs is a visual assessment of a single 5 μm section stained with haematoxylin and eosin. This is clearly inadequate, as many reports indicate that this approach significantly underestimates the true metastatic status of BC.17,75 The use of the IHC technique has been reported to upgrade approximately 20% of patients to node positive status.4 Similarly, we have shown that IHC examination of the SN with MNF116 and CK 7 increased the detection rate by 40%. Nevertheless, it is clear that these observer dependent methods are limited by low sensitivity, high cost, and labour intensity. Furthermore, only a small portion of the SN is examined.
Molecular analysis of the entire SN offers, in this respect, a more appealing approach to SN evaluation, as it is potentially more sensitive and cost effective, and less labour intensive. Proof of this requires vigorous testing, and rigorous proof of specificity is required. The first problem is that the specificity of marker mRNA detection would remain questionable if there were always the possibility that false positive results would be obtained because of the presence of occasional normal cells present in the SN that express the particular marker.76 Correct marker selection is therefore key to this problem. We confirmed that by using the correct markers we were able to amplify BC cell specific mRNA. We showed that a number of markers are BC cell specific by demonstrating that these markers are not expressed in normal tissues.
The second problem of molecular analyses of the SN arises from the possibility of illegitimate or non‐specific transcription of the marker genes in other nodal cell types. We reasoned that this problem could be overcome by optimising the PCR cycle number by using appropriate control tissue. In order to address this, we tested our panel of markers at various PCR cycle numbers on 20 normal lymph nodes. Of the large panel selected for this study, only 17 markers were found to be specific and therefore potentially useful for detection of BC metastases. All of these were specific at 30 PCR cycles, except for hMAM‐A and CSAGac, which were specific at 40 cycles and MAGE‐A1, which was specific at 35 cycles. Our results clearly demonstrate that appropriate and careful selection of the marker genes, and controlling for the possibility of illegitimate or non‐specific transcripts can be achieved by proper optimisation of PCR cycle number and the right choice of control tissue.
As previously reported,21 our study also highlights the need to evaluate sufficient numbers of control nodes in order to determine accurately the cutoff PCR cycle number for each marker. We have shown that the rate of detection of non‐specific/illegitimate transcripts in the nodal tissue was variable with increasing cycle numbers for the different markers and did not follow a regular pattern. For example, both MAGE‐A3 and MAGE‐A12 were specific at 30 cycles. However, at 35 cycles, MAGE‐A3 gave rise to non‐specific/illegitimate transcripts in only a quarter of the samples, whereas MAGE‐A12 gave rise to such transcripts in all samples.
We found that the expression profile of the markers as evaluated on the BC cell line panel (fig 1) was not entirely consistent with that as assessed on the SNs (fig 2, not all results shown). For example, although maspin was expressed in 100% of the cell lines, only 10% of the SNs had detectable maspin mRNA levels. This discrepancy might be attributed to the change in the biological behaviour and phenotype of the BC cell lines that could arise as a result of repeated cell culturing.77 In addition, differences in marker sensitivity may also contribute to this discrepancy. Thus, a marker assessment on a cell line panel is not necessary representative of the in vivo situation, and could be misleading when selecting appropriate markers for detection of micrometastases.
One of the aims of the present study was to select sensitive markers in order to ensure optimum detection of BC micrometastases. Eighteen markers proved to be valuable candidates as they provided good specificity and variable detection rates as assessed on the BC cell line panel (fig 1). Markers that could not be optimised at 30 PCR cycles were excluded as we found that a PCR cycle number <30 would always severely jeopardise sensitivity. For example, PRAME was found to be specific at 27 cycles, but gave a low detection rate at this cycle number. In addition CK 20 was previously reported to be useful for the detection of BC nodal metastases,29 but was excluded from our marker panel because of low sensitivity, even though it was found to be specific at 35 cycles (results not shown). Of the 18 markers selected, we have shown that hMAM‐A and MAGE‐A3 provided specific and sensitive detection in 90% of the patients when used in combination (fig 2). MAGE‐A3 was found to be more specific than hMAM‐A as it is not expressed in the normal mammary epithelium (fig 3). This is of particular importance because neither hMAM‐A nor IHC stains for cytokeratins can distinguish between benign epithelial inclusions and malignant epithelial cells. It has previously been shown that hMAM‐A used in combination with hMAM‐B improved the detection rate.78 However, we found that hMAM‐B did not improve the detection rate when used in combination with hMAM‐A (results not shown), as previously reported.78
One of the most difficult obstacles to overcome in developing assays for detection of metastatic tumour cells is the heterogeneity of marker expression, which could result in false negative results. It is therefore essential to develop a multimarker assay. Our study shows that using MAGE‐A3 in combination with hMAM‐A increased the detection rate for SN micrometastases from 70% to 90%. However, the simultaneous use of these two markers did not entirely eliminate false negative results, as three IHC positive nodes12,31,32 gave negative results with hMAM‐A and MAGE‐A3 (fig 2). However, the poor quality of the RNA, as represented by the weak PBGD signal and very low marker mRNA levels, may explain these negative results. This emphasises the value of using PBGD to control for quality. It is also possible that in these three false negative results, the portion of the node containing the metastatic focus had been sent for histopathological analysis, while the portion used for molecular analysis was indeed free of BC cells. If this were the case, analysing the whole node with RT‐PCR would ultimately eliminate the problem.
Our results showed that seven SNs (23%) were histologically negative (with haematoxylin and eosin and IHC) but RT‐PCR positive for either hMAM‐A or MAGE‐A3. Because MAGE‐A3, which can distinguish between benign epithelial inclusions and malignant epithelial cells, was expressed in five of these seven SNs,36,42,43,44,45 it is unlikely that they represent false positive results. Nevertheless, the possibility of such false positive results should be taken seriously and monitored closely, as it could result in unnecessary anxiety in the patient and potentially costly clinical investigations. A meaningful prognostic correlation of the clinical outcome of the histologically negative but RT‐PCR positive SNs, can only be determined with long ‐term follow up of these patients.
Of the nine SNs that were hMAM‐A negative,12,31,32,36,38,42,43,44,45 it is of particular interest that six of these were MAGE‐A3 positive.36,38,42,43,44,45 There seems to be a correlation between hMAM‐A downregulation and MAGE‐A3 upregulation. Considering the fact that the majority of the MAGE‐A3 positive SNs36,42,43,44,45 were also IHC negative, one might speculate on the possible involvement of MAGE‐A3 in early BC progression. Gene silencing (RNA interference) technology might help to clarify the role of MAGE‐A3 in BC progression.
TAKE HOME MESSAGES
In patients with breast cancer (BC), the sentinel node (SN) is the first node in the axillary basin that receives the primary lymphatic flow and can be used to accurately assess the axillary nodal status without removal of the axillary contents
We aimed to develop a reproducible reverse transcription (RT) PCR assay, with emphasis on achieving high specificity for accurate detection of BC micrometastases in the SN.
Use of MAGE‐A3 in combination with hMAM‐A identified metastases in 90% (27/30) of patients.
However, the clinical value of histologically negative but RT‐PCR positive SNs can only be determined with long term follow up
ACKNOWLEDGEMENTS
We owe a considerable amount of gratitude to Ms C Haniball and M Petersen from the Department of Anatomical Pathology (Faculty of Health Sciences/University of Cape Town) for their technical assistance regarding histological assessment of the SNs. We also wish to thank Drs E Muller and C Pienaar from the Department of Surgery (Groote Schuur Hospital) for providing normal lymph node tissues. This study was supported by a grant from the Medical Research Council of South Africa and a University Council Scholarship from the University of Cape Town.
Abbreviations
BC - breast cancer
CTA - cancer testis antigen
hMAM -
IHC - immunohistochemical
RT - reverse transcription
SN - sentinel lymph node
References
- 1.Parkin D M, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer 199980827–841. [DOI] [PubMed] [Google Scholar]
- 2.Boyle P, Leon M E, Maisonneuve P.et al Cancer control in women. Update 2003. Int J Gynaecol Obstet 200383(suppl 1)179–202. [DOI] [PubMed] [Google Scholar]
- 3.Braun S, Pantel K. Clinical significance of occult metastatic cells in bone marrow of breast cancer patients. Oncologist 20016125–132. [DOI] [PubMed] [Google Scholar]
- 4.Turner R R, Giuliano A E, Hoon D S.et al Pathologic examination of sentinel lymph node for breast carcinoma. World J Surg 200125798–805. [DOI] [PubMed] [Google Scholar]
- 5.Diel I J. Bone marrow staging for breast cancer: is it better than axillary node dissection? Semin Oncol 200128236–244. [DOI] [PubMed] [Google Scholar]
- 6.Fisher B, Bauer M, Wickerham D L.et al Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer 1983521551–1557. [DOI] [PubMed] [Google Scholar]
- 7.Kamath V J, Giuliano R, Dauway E L.et al Characteristics of the sentinel lymph node in breast cancer predict further involvement of higher‐echelon nodes in the axilla: a study to evaluate the need for complete axillary lymph node dissection. Arch Surg 2001136688–692. [DOI] [PubMed] [Google Scholar]
- 8.Cianfrocca M, Goldstein L J. Prognostic and predictive factors in early‐stage breast cancer. Oncologist 20049606–16 [DOI] [PubMed] [Google Scholar]
- 9.Lopez‐Guerrero J A, Gilabert P B, Gonzalez E B.et al Use of reverse‐transcriptase polymerase chain reaction (RT‐PCR) for carcinoembryonic antigen, cytokeratin 19, and maspin in the detection of tumor cells in leukapheresis products from patients with breast cancer: comparison with immunocytochemistry. J Hematother 1999853–61. [DOI] [PubMed] [Google Scholar]
- 10.Stathopoulou A, Vlachonikolis I, Mavroudis D.et al Molecular detection of cytokeratin‐19‐positive cells in the peripheral blood of patients with operable breast cancer: evaluation of their prognostic significance. J Clin Oncol 2002203404–3412. [DOI] [PubMed] [Google Scholar]
- 11.Xenidis N, Vlachonikolis I, Mavroudis D.et al Peripheral blood circulating cytokeratin‐19 mRNA‐positive cells after the completion of adjuvant chemotherapy in patients with operable breast cancer. Ann Oncol 200314849–855. [DOI] [PubMed] [Google Scholar]
- 12.Taback B, Chan A D, Kuo C T.et al Detection of occult metastatic breast cancer cells in blood by a multimolecular marker assay: correlation with clinical stage of disease. Cancer Res 2001618845–8850. [PubMed] [Google Scholar]
- 13.Umekita Y, Ohi Y, Sagara Y.et al Clinical significance of occult micrometastases in axillary lymph nodes in “node‐negative” breast cancer patients. Jpn J Cancer Res 200293695–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieweg O E, Tanis P J, Kroon B B. The definition of a sentinel node. Ann Surg Oncol 20018538–541. [DOI] [PubMed] [Google Scholar]
- 15.Veronesi U, Paganelli G, Viale G.et al A randomized comparison of sentinel‐node biopsy with routine axillary dissection in breast cancer. N Engl J Med 2003349546–553. [DOI] [PubMed] [Google Scholar]
- 16.Guenther J M, Hansen N M, DiFronzo L A.et al Axillary dissection is not required for all patients with breast cancer and positive sentinel nodes. Arch Surg 200313852–56. [DOI] [PubMed] [Google Scholar]
- 17.Dowlatshahi K, Fan M, Anderson J M.et al Occult metastases in sentinel nodes of 200 patients with operable breast cancer. Ann Surg Oncol 20018675–681. [DOI] [PubMed] [Google Scholar]
- 18.Hosch S B, Braun S, Pantel K. Characterization of disseminated tumor cells. Semin Surg Oncol 200120265–271. [DOI] [PubMed] [Google Scholar]
- 19.Wilson E L, Dutlow C, Dowdle E B. Effect of hormones on the secretion of plasminogen activator by a new line of human breast carcinoma cells UCT‐Br 1. Cold Spring Harbour conferences on cell proliferation: growth of cells in hormonally defined media 19829849–854. [Google Scholar]
- 20.Forozan F, Veldman R, Ammerman C A.et al Molecular cytogenetic analysis of 11 new breast cancer cell lines. Br J Cancer 1999811328–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davids V, Kidson S H, Hanekom G S. Accurate molecular detection of melanoma nodal metastases: an assessment of multimarker assay specificity, sensitivity, and detection rate. Mol Pathol 2003543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scorilas A, Levesque M A, Ashworth L K.et al Cloning, physical mapping and structural characterization of the human alpha(A)‐adaptin gene. Gene 2002289191–199. [DOI] [PubMed] [Google Scholar]
- 23.Zehentner B K, Dillon D C, Jiang Y.et al Application of a multigene reverse transcription‐PCR assay for detection of mammaglobin and complementary transcribed genes in breast cancer lymph nodes. Clin Chem 2002481225–1231. [PMC free article] [PubMed] [Google Scholar]
- 24.Jager D, Unkelbach M, Frei C.et al Identification of tumor‐restricted antigens NY‐BR‐1, SCP‐1, and a new cancer/testis‐like antigen NW‐BR‐3 by serological screening of a testicular library with breast cancer serum. Cancer Immun 200225. [PubMed] [Google Scholar]
- 25.van Baren N, Brasseur F, Godelaine D.et al Genes encoding tumor‐specific antigens are expressed in human myeloma cells. Blood 1999941156–1164. [PubMed] [Google Scholar]
- 26.Cho B, Lim Y, Lee D Y.et al Identification and characterization of a novel cancer/testis antigen gene CAGE. Biochem Biophys Res Commun 2002292715–726. [DOI] [PubMed] [Google Scholar]
- 27.Bostick P J, Hoon D S, Cote R J. Detection of carcinoembryonic antigen messenger RNA in lymph nodes from patients with colorectal cancer. N Engl J Med 19983391643–1644. [PubMed] [Google Scholar]
- 28.Datta Y H, Adams P T, Drobyski W R.et al Sensitive detection of occult breast cancer by the reverse‐transcriptase polymerase chain reaction. J Clin Oncol 199412475–482. [DOI] [PubMed] [Google Scholar]
- 29.Bostick P J, Chatterjee S, Chi D D.et al Limitations of specific reverse‐transcriptase polymerase chain reaction markers in the detection of metastases in the lymph nodes and blood of breast cancer patients. J Clin Oncol 1998162632–2640. [DOI] [PubMed] [Google Scholar]
- 30.Yang X F, Wu C J, Chen L.et al CML28 is a broadly immunogenic antigen, which is overexpressed in tumor cells. Cancer Res 2002625517–5522. [PMC free article] [PubMed] [Google Scholar]
- 31.Yang X F, Wu C J, McLaughlin S.et al CML66, a broadly immunogenic tumor antigen, elicits a humoral immune response associated with remission of chronic myelogenous leukemia. Proc Natl Acad Sci USA 2001987492–7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin C, Mak S, Meitner P A.et al Cancer/testis antigen CSAGE is concurrently expressed with MAGE in chondrosarcoma. Gene 2002285269–278. [DOI] [PubMed] [Google Scholar]
- 33.Scanlan M J, Welt S, Gordon C M.et al Cancer‐related serological recognition of human colon cancer: identification of potential diagnostic and immunotherapeutic targets. Cancer Res 2002624041–4047. [PubMed] [Google Scholar]
- 34.Eichmuller S, Usener D, Jochim A.et al mRNA expression of tumor‐associated antigens in melanoma tissues and cell lines. Exp Dermatol 200211292–301. [DOI] [PubMed] [Google Scholar]
- 35.Zendman A J, Cornelissen I M, Weidle U H.et al CTp11, a novel member of the family of human cancer/testis antigens. Cancer Res 1999596223–6229. [PubMed] [Google Scholar]
- 36.Takimoto M, Wei G, Dosaka‐Akita H.et al Frequent expression of new cancer/testis gene D40/AF15q14 in lung cancers of smokers. Br J Cancer 2002861757–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cullen R, Maguire T M, McDermott E W.et al Studies on oestrogen receptor‐alpha and ‐beta mRNA in breast cancer. Eur J Cancer 2001371118–1122. [DOI] [PubMed] [Google Scholar]
- 38.Gotte K, Usener D, Riedel F.et al Tumor‐associated antigens as possible targets for immune therapy in head and neck cancer: comparative mRNA expression analysis of RAGE and GAGE genes. Acta Otolaryngol 2002122546–552. [DOI] [PubMed] [Google Scholar]
- 39.Van den Eynde B, Peeters O, De Backer O.et al A new family of genes coding for an antigen recognized by autologous cytolytic T lymphocytes on a human melanoma. J Exp Med 1995182689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Han K J, Pang X W.et al Large scale identification of human hepatocellular carcinoma‐associated antigens by autoantibodies. J Immunol 20021691102–1109. [DOI] [PubMed] [Google Scholar]
- 41.Doi F, Chi D D, Charuworn B B.et al Detection of beta‐human chorionic gonadotropin mRNA as a marker for cutaneous malignant melanoma. Int J Cancer 199665454–459. [DOI] [PubMed] [Google Scholar]
- 42.Min C J, Tafra L, Verbanac K M. Identification of superior markers for polymerase chain reaction detection of breast cancer metastases in sentinel lymph nodes. Cancer Res 1998584581–4584. [PubMed] [Google Scholar]
- 43.Aihara T, Fujiwara Y, Miyake Y.et al Mammaglobin B gene as a novel marker for lymph node micrometastasis in patients with abdominal cancers. Cancer Lett 200015079–84. [DOI] [PubMed] [Google Scholar]
- 44.Rogalla P, Drechsler K, Frey G.et al HMGI‐C expression patterns in human tissues. Implications for the genesis of frequent mesenchymal tumors. Am J Pathol 1996149775–779. [PMC free article] [PubMed] [Google Scholar]
- 45.Somers V A, Brandwijk R J, Joosten B.et al A panel of candidate tumor antigens in colorectal cancer revealed by the serological selection of a phage displayed cDNA expression library. J Immunol 20021692772–2780. [DOI] [PubMed] [Google Scholar]
- 46.Chang A, Yousef G M, Scorilas A.et al Human kallikrein gene 13 (KLK13) expression by quantitative RT‐PCR: an independent indicator of favourable prognosis in breast cancer. Br J Cancer 2002861457–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monji M, Senju S, Nakatsura T.et al Head and neck cancer antigens recognized by the humoral immune system. Biochem Biophys Res Commun 2002294734–741. [DOI] [PubMed] [Google Scholar]
- 48.Zhao C, Nguyen T, Yusifov T.et al Lipophilins: human peptides homologous to rat prostatein. Biochem Biophys Res Commun 1999256147–155. [DOI] [PubMed] [Google Scholar]
- 49.Gibbs P, Hutchins A M, Dorian K T.et al MAGE‐12 and MAGE‐6 are frequently expressed in malignant melanoma. Melanoma Res 200010259–264. [PubMed] [Google Scholar]
- 50.Kufer P, Zippelius A, Lutterbuse R.et al Heterogeneous expression of MAGE‐A genes in occult disseminated tumor cells: a novel multimarker reverse transcription‐polymerase chain reaction for diagnosis of micrometastatic disease. Cancer Res 200262251–261. [PubMed] [Google Scholar]
- 51.Hoon D S, Wang Y, Dale P S.et al Detection of occult melanoma cells in blood with a multiple‐marker polymerase chain reaction assay. J Clin Oncol 1995132109–2116. [DOI] [PubMed] [Google Scholar]
- 52.Park M S, Park J W, Jeon C H.et al Expression of melanoma antigen‐encoding genes (MAGE) by common primers for MAGE‐A1 to ‐A6 in colorectal carcinomas among Koreans. J Korean Med Sci 200217497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jungbluth A A, Chen Y T, Busam K J.et al CT7 (MAGE‐C1) antigen expression in normal and neoplastic tissues. Int J Cancer 200299839–845. [DOI] [PubMed] [Google Scholar]
- 54.Ballestrero A, Coviello D A, Garuti A.et al Reverse‐transcriptase polymerase chain reaction of the maspin gene in the detection of bone marrow breast carcinoma cell contamination. Cancer 2001922030–2035. [DOI] [PubMed] [Google Scholar]
- 55.Huang E Y, Madireddi M T, Gopalkrishnan R V.et al Genomic structure, chromosomal localization and expression profile of a novel melanoma differentiation associated (mda‐7) gene with cancer specific growth suppressing and apoptosis inducing properties. Oncogene 2001207051–7063. [DOI] [PubMed] [Google Scholar]
- 56.de Wit N J, Weidle U H, Ruiter D J.et al Expression profiling of MMA‐1a and splice variant MMA‐1b: new cancer/testis antigens identified in human melanoma. Int J Cancer 200298547–553. [DOI] [PubMed] [Google Scholar]
- 57.Scanlan M J, Gout I, Gordon C M.et al Humoral immunity to human breast cancer: antigen definition and quantitative analysis of mRNA expression. Cancer Immun 200114. [PubMed] [Google Scholar]
- 58.Goydos J S, Patel M, Shih W. NY‐ESO‐1 and CTp11 expression may correlate with stage of progression in melanoma. J Surg Res 20019876–80. [DOI] [PubMed] [Google Scholar]
- 59.Yuasa T, Okamoto K, Kawakami T.et al Expression patterns of cancer testis antigens in testicular germ cell tumors and adjacent testicular tissue. J Urol 20011651790–1794. [PubMed] [Google Scholar]
- 60.Clark J W, Snell L, Shiu R P.et al The potential role for prolactin‐inducible protein (PIP) as a marker of human breast cancer micrometastasis. Br J Cancer 1999811002–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barrett A, Madsen B, Copier J.et al PLU‐1 nuclear protein, which is upregulated in breast cancer, shows restricted expression in normal human adult tissues: a new cancer/testis antigen? Int J Cancer 2002101581–588. [DOI] [PubMed] [Google Scholar]
- 62.Matsushita M, Ikeda H, Kizaki M.et al Quantitative monitoring of the PRAME gene for the detection of minimal residual disease in leukaemia. Br J Haematol. 2001 Mar 112916–926. [DOI] [PubMed] [Google Scholar]
- 63.Mitas M, Mikhitarian K, Hoover L.et al Prostate‐specific Ets (PSE) factor: a novel marker for detection of metastatic breast cancer in axillary lymph nodes. Br J Cancer 200286899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shen R, Su Z Z, Olsson C A.et al Identification of the human prostatic carcinoma oncogene PTI‐1 by rapid expression cloning and differential RNA display. Proc Natl Acad Sci USA 1995926778–6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cao J, Gao T, Stanbridge E J.et al RBP1L1, a retinoblastoma‐binding protein‐related gene encoding an antigenic epitope abundantly expressed in human carcinomas and normal testis. J Natl Cancer Inst 2001931159–1165. [DOI] [PubMed] [Google Scholar]
- 66.Tureci O, Sahin U, Zwick C.et al Identification of a meiosis‐specific protein as a member of the class of cancer/testis antigens. Proc Natl Acad Sci USA 1998955211–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Colleoni G W, Capodieci P, Tickoo S.et al Expression of SSX genes in the neoplastic cells of Hodgkin's lymphoma. Hum Pathol 200233496–502. [DOI] [PubMed] [Google Scholar]
- 68.Hirohashi Y, Torigoe T, Maeda A.et al An HLA‐A24‐restricted cytotoxic T lymphocyte epitope of a tumor‐associated protein, survivin. Clin Cancer Res 200281731–1739. [PubMed] [Google Scholar]
- 69.De Jong A, Buchli R, Robbins D. Characterization of sperm protein 17 in human somatic and neoplastic tissue. Cancer Lett 2002186201–209. [DOI] [PubMed] [Google Scholar]
- 70.Feller A J, Duan Z, Penson R.et al TRAG‐3, a novel cancer/testis antigen, is overexpressed in the majority of melanoma cell lines and malignant melanoma. Anticancer Res 2000204147–4151. [PubMed] [Google Scholar]
- 71.Chung H W, Wen Y, Ahn J J.et al Interleukin‐1beta regulates urokinase plasminogen activator (u‐PA), u‐PA receptor, soluble u‐PA receptor, and plasminogen activator inhibitor‐1 messenger ribonucleic acid expression in cultured human endometrial stromal cells. J Clin Endocrinol Metab 2001861332–1340. [DOI] [PubMed] [Google Scholar]
- 72.Zendman A J, Van Kraats A A, Weidle U H.et al The XAGE family of cancer/testis‐associated genes: alignment and expression profile in normal tissues, melanoma lesions and Ewing's sarcoma. Int J Cancer 200299361–369. [DOI] [PubMed] [Google Scholar]
- 73.de Vries T J, Fourkour A, Punt C J.et al Reproducibility of detection of tyrosinase and MART‐1 transcripts in the peripheral blood of melanoma patients: a quality control study using real‐time quantitative RT‐PCR. Br J Cancer 199980883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Layfield L J, Mooney E. Heterotopic epithelium in an intramammary lymph node. Breast J 2000663–67. [DOI] [PubMed] [Google Scholar]
- 75.Dowlatshahi K, Fan M, Bloom K J.et al Occult metastases in the sentinel lymph nodes of patients with early stage breast carcinoma: A preliminary study. Cancer 199986990–996. [PubMed] [Google Scholar]
- 76.Branagan G, Hughes D, Jeffrey M.et al Detection of micrometastases in lymph nodes from patients with breast cancer. Br J Surg 20028986–89. [DOI] [PubMed] [Google Scholar]
- 77.Vazquez S M, Mladovan A, Garbovesky C.et al Three novel hormone‐responsive cell lines derived from primary human breast carcinomas: functional characterization. J Cell Physiol 2004199460–469. [DOI] [PubMed] [Google Scholar]
- 78.Ouellette R J, Richard D, Maicas E. RT‐PCR for mammaglobin genes, MGB1 and MGB2, identifies breast cancer micrometastases in sentinel lymph nodes. Am J Clin Pathol 2004121637–643. [DOI] [PubMed] [Google Scholar]