Abstract
Aims
To evaluate the diagnostic significance of anti‐filamentous actin antibodies (A‐FAA) assessed with a commercial ELISA in comparison with immunofluorescence reactivity and patterns of anti‐smooth muscle antibodies (SMA); and to correlate A‐FAA positivity with clinical, immunogenetic, laboratory, and histological features in patients with autoimmune hepatitis type 1 (AIH‐1).
Methods
We studied 78 consecutive untreated AIH‐1 patients and 160 controls: 22 with autoimmune hepatitis type 2 (AIH‐2), 51 with hepatitis C, 17 with coeliac disease (CD), 20 with primary biliary cirrhosis (PBC) and 50 blood donors. SMA was evaluated by indirect immunofluorescence (IIF) on frozen sections of rat tissues, and A‐FAA with a modified commercial ELISA.
Results
SMA was detected by IIF in 61 (78%) of 78 AIH‐1 patients, of whom 47 (60%) had the SMA‐T/G and 14 (18%) the SMA‐V pattern. Of the pathological controls, 32 (20%) had the SMA‐V pattern (25 with hepatitis C, 2 with AIH‐2, 2 with PBC, 3 with CD). A‐FAA were present in 55 AIH‐1 patients (70.5%; 46 with SMA‐T/G, 7 with SMA‐V, and 2 SMA‐negative), and in 10 controls (6%), of whom five had hepatitis C, two AIH‐2, two PBC and one CD. The association between A‐FAA and the SMA‐T/G pattern was statistically significant (p<0.0001). A‐FAA levels were higher in SMA‐T/G positive than SMA‐V positive AIH‐1 patients and controls (p<0.0001). A‐FAA positivity was significantly associated with higher γ‐globulin and IgG levels, but did not correlate with other considered parameters.
Conclusion
The modified A‐FAA ELISA strictly correlates with the SMA‐T/G pattern and is a reliable and operator independent assay for AIH‐1. Detection of A‐FAA, even if devoid of prognostic relevance, may be useful when interpretative doubts of standard IIF arise.
Keywords: Autoimmune hepatitis, anti‐filamentous actin antibodies, smooth muscle antibodies, indirect immunofluorescence, ELISA
Smooth muscle antibodies (SMA) and antinuclear antibodies are immunoserological markers of autoimmune hepatitis type 1 (AIH‐1).1 SMA are conventionally detected by indirect immunofluorescence (IIF) on snap frozen sections of rat liver, kidney, and stomach.2 The antigenic targets of the SMA immunomorphological patterns are various cellular cytoskeleton components, namely microfilaments (actin and vinculin), intermediate filaments (vimentin and desmin), and microtubules (tubulin).3 More specific SMA staining patterns for AIH‐1 are the peritubular (SMA‐T) and the glomerular (SMA‐G) patterns, which mainly react with filamentous (polymerised) actin (F‐actin).4
Although actin is the most obvious candidate autoantigen of AIH specific SMA patterns, over the past few years, several actin based assays have been found to give inconsistent results.5,6,7 A possible explanation is the fact that the actin epitopes recognised by SMA are mainly conformational, therefore they are irreversibly denatured during the extractive process of monomeric/glomerular actin. At present, IIF remains the most sensitive technique to detect SMA,8 but the interpretation of the results largely depends on the operator's experience, and standardisation of the IIF technique with reference sera is still in progress.9
In this study, a commercially available ELISA detecting anti‐F‐actin antibodies (A‐FAA) was compared with standard IIF. In addition, we tried to correlate A‐FAA positivity with clinical, immunogenetic, laboratory, and histological features in AIH‐1 patients.
MATERIALS AND METHODS
Study population
Informed consent was obtained from each patient before inclusion in the study. We studied 78 consecutive patients with AIH‐1 referred to our department at the time of diagnosis. All 78 patients (82% female, median age 39 years, range 7–82) had a score for “definite” AIH (median score 17, range 16–22), according to the criteria issued by the International Autoimmune Hepatitis Group.1 In addition, 110 pathological controls were studied at the time of diagnosis: 22 patients with AIH type 2 (AIH‐2), 51 patients with chronic hepatitis C, 20 with primary biliary cirrhosis (PBC), and 17 patients with coeliac disease (CD). Sera from 50 blood donors were the normal controls.
In all AIH‐1 patients clinical (age, female sex, response to treatment), laboratory (conventional laboratory tests of liver inflammation and function), immunogenetic (human leucocyte antigens; HLA) and histological parameters (severe hepatitis and/or cirrhosis) were correlated with anti‐actin positivity. Hepatitis B surface antigen (HBsAg) (Abbott Diagnostics, North Chicago, IL, USA) and antibodies to hepatitis C virus (Ortho version HCV 3.0 ELISA; Ortho‐Clinical Diagnostics, Inc, Raritan, NJ, USA) were absent. Hepatitis C virus (HCV) RNA testing (nested PCR with primers derived from the highly conserved 5′ non‐coding region) was negative for all patients. Class I (A, B, and C loci) and class II (DR and DQ loci) HLA alleles were detected by microlymphocytotoxicity and PCR as previously reported.10
In total, 61 patients (78%) were = treated with high dose prednisone alone (initial dose 1 mg/kg body weight per day, tapered to a maintenance dose of 4–12 mg/day), and 17 patients (22%) received prednisone in combination with 50 mg/day azathioprine (initial dose of prednisone 30 mg/day, tapered to a maintenance dose of 4–12 mg/day). All patients achieving biochemical remission were maintained on low dose steroids (4 mg/day). Complete remission and relapse were defind according to the IAIHG guidelines.1
Detection of SMA on rat tissue sections
SMA were detected by IIF as previously described.11 Briefly, sera diluted 1:40 in phosphate buffered saline (PBS) were tested on cryostat frozen sections of rat liver, kidney, and stomach. A fluorescein conjugated secondary antibody either polyspecific or IgG‐specific (anti‐human polyvalent immunoglobulins IgA, IgG, IgM, and anti‐IgG fluorescein isothiocyanate conjugate, respectively, diluted 1:100 in PBS; Sigma ImmunoChemicals, St. Louis, MO, USA). SMA positivity was assessed using a fluorescence microscope (Orthoplan; Leitz, Wetzlar, Germany).
The identification of the SMA patterns was made according to Bottazzo et al.4 (a) SMA‐V (vessels): staining of small/medium sized vessel walls; (b) SMA‐G (glomeruli): in addition to vessels, staining of glomerular mesangial cells; and (c) SMA‐T (tubuli): staining of vessels, and glomerular and peritubular structures. Detection of SMA antibodies was performed blindly by two independent investigators (FC and AG).
Detection of anti‐F‐actin antibodies by ELISA
A‐FAA IgG antibodies were detected by a commercially available ELISA kit (Quanta Lite Actin IgG assay; Inova Diagnostics, San Diego, CA, USA) following the manufacturer's instructions. According to the manufacturer, purified F‐actin is coated to the wells under conditions that preserve the antigen in its native state. On each plate, a high and low positive control and a negative control were added. Anti‐F‐actin reactivity was determined by: sample absorbance/low positive absorbance × 25 (number of units assigned to the low positive control).
According to the manufacturer's instructions, a test is to be considered positive if the OD corresponds to more than 30 arbitrary units (AU), weakly positive between 20 and 30 AU, negative under 20 AU. The cutoff value of 20 AU was obtained by the manufacturer testing 150 normal serum samples, of which three were strongly positive, two had a moderate to strong result (31 and 75 AU) and one was weakly positive (21 AU). The average value for 150 normal samples tested by the manufacturer was 7.3 AU.
To adapt the sensitivity and specificity of the commercial ELISA to our patient and control populations, we tested 100 blood donors (different from those included in the study population) and defined our own cutoff as the mean optical density at 450 nm ± 5SD.
Histological assessment
Liver tissue was obtained by percutaneous needle biopsy, and a single experienced liver pathologist evaluated the specimens. Inflammatory activity was scored as mild, moderate, or severe. The diagnosis of cirrhosis required fibrosis and the presence of a complete regenerative nodule.12 The patients were divided in four groups: (a) mild/moderate inflammatory activity, (b) severe inflammatory activity, (c) mild/moderate fibrosis, and (d) severe fibrosis/cirrhosis.
Serological follow up study
After treatment, we re‐tested 46 (58.9%) of the 78 AIH‐1 patients. For each, the determination of A‐FAA was assessed once during follow up (12–30 months following initiation of immunosuppression). A‐FAA behaviour (unmodified, appearance, disappearance), was correlated with disease activity (complete remission or relapse) at the time of re‐evaluation to determine their importance in predicting treatment outcome. All the 46 patients were also re‐assessed for viral markers and again tested seronegative.
Statistical analyses
The comparison of categorical variables was performed using χ2 and Fisher's exact tests as applicable. The Mann‐Whitney U test was used for the comparison of continuous data. Nominal variables were correlated by contingency tables. Significance was set at p<0.05. Statistical analysis was performed using GraphPad InStat (version 3.0a for Macintosh), and StatView (version 5.0.1 for Macintosh; SAS Institute Inc).
RESULTS
SMA was detected by IIF in 61 of 78 patients (78%) with AIH‐1 (47 (60%) had SMA‐T/G, 14 (18%) had SMA‐V), and in 32 (20%) of the 160 controls (25 with hepatitis C, 2 with AIH‐2, 2 with PBC, and 3 with CD; all 32 had the SMA‐V pattern). SMA reactivity was of the IgG class in all but two coeliac patients.
The mean optical density of the A‐FAA ELISA for the 100 blood donors was 0.250, and the resulting OD cutoff was calculated at 0.500, higher than the “low positive” control reported in the manufacturer's instructions (0.420). The absorbance of 0.500, corresponding to 30 AU (500/420×25 = 30 AU), was therefore chosen as our modified cutoff level.
The prevalence of SMA and of A‐FAA is summarised in table 1.
Table 1 Prevalence of A‐FAA and SMA in the study population.
| Patients | A‐FAA+ (⩾20 AU) | A‐FAA+ (⩾30 AU) | SMA+* | SMAT/G+* | ||||
|---|---|---|---|---|---|---|---|---|
| AIH‐1 (n = 78) | 63 (81%) | 55 (70.5%)† | 61 (78%)† | 47 (60%)† | ||||
| AIH‐2 (n = 22) | 6 (27%) | 2 (9%) | 2 (9%) | 0 | ||||
| HCV (n = 51) | 15 (30%) | 5 (10%) | 25 (49%) | 0 | ||||
| CD (n = 17) | 5 (29%) | 1 (6%) | 3 (17%) | 0 | ||||
| PBC (n = 20) | 6 (30%) | 2 (10%) | 2 (10%) | 0 | ||||
| BD (n = 50) | 3 (6%) | 0 | 0 | 0 |
*The same results were obtained using IgG specific secondary antibodies. †A‐FAA v SMA: 70.5% v 78%, p = 0.3; A‐FAA v SMA‐T/G: 70.5% v 60%, p = 0.2.
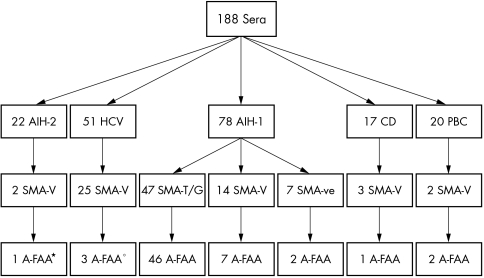
According to our modified cutoff, A‐FAA were present in 55 (70.5%) patients with AIH‐1 (mean (SD) 90 (35) AU) (46 with SMA‐T/G, 7 with SMA‐V, and 2 SMA negative), and in 10 (6%) of the controls (mean (SD) 42 (6)) (7 with SMA‐V and 3 SMA negative; namely 5 HCV positive patients, 2 with AIH‐2, 2 with PBC, and 1 with CD), as shown in fig 1. The inter‐assay coefficient of variation (CV) of the ELISA was 8.7% and the intra‐assay CV was 4.2%.
Figure 1 Serological study by IIF (anti‐total human Ig as secondary antibodies) and A‐FAA ELISA assay. *A second AIH‐2 A‐FAA positive patient was negative for SMA. °Two further HCV patients were A‐FAA positive but SMA negative. Fifty blood donors were also tested and were negative for SMA and A‐FAA.
A‐FAA sensitivity was lower than that of SMA, but higher than that of SMA‐T/G, however without reaching statistical significance (70.5% v 78%, p = 0.3 and 70,5% v 60, p = 0.2 respectively). Interestingly, 46 (98%) of the 47 SMA‐T/G positive patients with AIH‐1 also had A‐FAA, the correlation between SMA‐T/G and A‐FAA being statistically significant (p<0.0001, Fisher's exact test).
The level of absorbance (AU) for SMA‐T/G positive AIH‐1 patients was significantly higher than that for SMA‐V positive AIH‐1 patients and controls (median 89 AU (range 12 to 178) v 20.7 (range 2 to157), respectively; p<0.0001, Mann‐Whitney test). However, no correlation was observed between A‐FAA optical density readings, AU, and SMA titre.
According to the manufacturer's cutoff (20 AU), A‐FAA were detected in 63 of the 78 AIH‐1 patients (46 with SMA‐T/G, 10 with SMA‐V, and 7 SMA negative), but also in 35 controls (namely 15 HCV positive patients, 6 with AIH‐2, 6 with PBC, 5 with CD and 3 blood donors). Table 2 shows patient by patient results with the two different assays.
Table 2 Reactivities of the 78 patients with AIH‐1.
| No.* | SMA T/G† | SMA V† | Titre | A‐FAA | ELISA‡ | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 53 | − | − | 0 | − | 1.70 | |||||
| 78 | − | + | 1:40 | − | 2.00 | |||||
| 29 | − | − | 0 | − | 2.00 | |||||
| 71 | − | − | 1:640 | − | 4.00 | |||||
| 52 | − | − | 0 | − | 5.00 | |||||
| 77 | − | − | 0 | − | 5.00 | |||||
| 30 | − | − | 0 | − | 7.00 | |||||
| 70 | − | − | 0 | − | 7.60 | |||||
| 18 | − | + | 1:5120 | − | 7.75 | |||||
| 50 | − | + | 80 | − | 8.10 | |||||
| 28 | − | − | 0 | − | 9.40 | |||||
| 51 | − | − | 0 | − | 10.00 | |||||
| 31 | − | + | 1:40 | − | 11.20 | |||||
| 49 | + | − | 1:640 | − | 12.00 | |||||
| 17 | − | − | 0 | − | 14.00 | |||||
| 54 | − | − | 0 | − | 20.50 | |||||
| 46 | − | + | 1:80 | − | 20.70 | |||||
| 27 | − | + | 1:160 | − | 23.00 | |||||
| 55 | − | − | 0 | − | 23.00 | |||||
| 32 | − | − | 0 | − | 23.60 | |||||
| 47 | − | + | 1:40 | − | 25.00 | |||||
| 19 | − | − | 0 | − | 27.00 | |||||
| 48 | − | − | 0 | − | 28.10 | |||||
| 72 | + | − | 1:80 | + | 31.00 | |||||
| 40 | − | − | 0 | + | 34.50 | |||||
| 69 | + | − | 1:160 | + | 35.00 | |||||
| 16 | + | − | 1:10240 | + | 38.95 | |||||
| 56 | + | − | 1:320 | + | 45.00 | |||||
| 41 | + | − | 1:1280 | + | 47.00 | |||||
| 45 | − | + | 1:320 | + | 50.00 | |||||
| 61 | + | − | 1:5120 | + | 54.00 | |||||
| 1 | + | − | 1:320 | + | 54.60 | |||||
| 20 | + | − | 1:5120 | + | 55.60 | |||||
| 42 | + | − | 1:320 | + | 56.00 | |||||
| 44 | − | − | 0 | + | 57.95 | |||||
| 57 | + | − | 1:320 | + | 58.00 | |||||
| 15 | + | − | 1:40 | + | 64.70 | |||||
| 43 | + | − | 1:160 | + | 65.00 | |||||
| 59 | − | + | 1:160 | + | 65.00 | |||||
| 14 | + | − | 1:160 | + | 67.00 | |||||
| 58 | + | − | 1:160 | + | 67.80 | |||||
| 73 | + | − | 1:5120 | + | 69.40 | |||||
| 21 | + | − | 1:320 | + | 71.15 | |||||
| 74 | + | − | 1:320 | + | 72.80 | |||||
| 33 | + | − | 1:320 | + | 73.00 | |||||
| 2 | + | − | 1:160 | + | 74.65 | |||||
| 68 | − | + | 1:80 | + | 80.00 | |||||
| 13 | + | − | 1:640 | + | 83.30 | |||||
| 37 | − | + | 1:5120 | + | 85.20 | |||||
| 60 | + | − | 1:640 | + | 89.00 | |||||
| 22 | + | − | 1:640 | + | 89.00 | |||||
| 3 | + | − | 1:320 | + | 89.00 | |||||
| 38 | + | − | 1:1280 | + | 89.90 | |||||
| 34 | + | − | 1:2560 | + | 90.30 | |||||
| 4 | − | + | 1:160 | + | 92.95 | |||||
| 62 | + | − | 1:160 | + | 95.00 | |||||
| 9 | + | − | 1:160 | + | 98.00 | |||||
| 39 | + | − | 1:160 | + | 98.00 | |||||
| 23 | − | + | 1:320 | + | 101.00 | |||||
| 63 | + | − | 1:640 | + | 102.00 | |||||
| 75 | + | − | 1:640 | + | 102.00 | |||||
| 5 | + | − | 1:320 | + | 103.00 | |||||
| 67 | + | − | 1:1280 | + | 103.00 | |||||
| 8 | + | − | 1:640 | + | 107.00 | |||||
| 64 | + | − | 1:5120 | + | 123.00 | |||||
| 12 | + | − | 1:640 | + | 126.00 | |||||
| 24 | + | − | 1:640 | + | 126.00 | |||||
| 6 | + | − | 1:320 | + | 126.00 | |||||
| 11 | + | − | 1:640 | + | 130.00 | |||||
| 35 | + | − | 1:320 | + | 131.00 | |||||
| 65 | + | − | 1:640 | + | 135.00 | |||||
| 25 | + | − | 1:320 | + | 137.00 | |||||
| 7 | + | − | 1:640 | + | 141.00 | |||||
| 66 | + | − | 1:640 | + | 141.00 | |||||
| 36 | + | − | 1:1280 | + | 144.00 | |||||
| 76 | + | − | 1:640 | + | 148.00 | |||||
| 26 | − | + | 1:1280 | + | 157.00 | |||||
| 10 | + | − | 1:5120 | + | 178.00 | |||||
A‐FAA AU cutoff >30; SMA titre cutoff 1:40. *Patient number; †IIF with anti‐total human IgG as secondary antibodies; ‡measured in AU.
Positivity for A‐FAA with our modified cutoff was associated with higher γ‐globulin (median 26 g/l, range 10 to 59 versus 20 g/l, range 13 to 34, p<0.005, Mann‐Whitney test) and IgG levels (median 3060 mg/dl (range 1296 to 7344) versus 2050 mg/dl (range 1280 to 3402), p<0.002, Mann‐Whitney test) in AIH‐1 patients, but not in pathological controls (median γ‐globulin 12.6 g/l (range 9 to 19) versus 13.6 g/l (range 9.5 to 21) g/l, p = 0.6; median IgG levels 3060 mg/dl (range 1296 to 7344) versus 2050 mg/dl (range 1280 to 3402), p = 0.3, Mann‐Whitney test). In addition, in AIH‐1 patients A‐FAA positivity did not correlate with any other parameter, whether clinical (age, female sex, complete remission, relapse), laboratory (alanine transferase, aspartate aminotransferase, alkaline phosphatase, γ‐glutamiltranspeptidase, albumin, bilirubin, γ‐globulin), histological (inflammatory activity and fibrosis staging), or immunogenetic (DRB1*0301 and DRB1*0401 alleles).
Median duration of follow up of the 78 AIH‐1 patients calculated from the first visit at time of diagnosis was 8 years (range 1 to 16). The frequency of complete remission and that of relapse within the observation period was similar in A‐FAA positive and A‐FAA negative AIH‐1 patients (table 3). Four patients (two A‐FAA positive and two A‐FAA negative) had to stop treatment because of steroid related side effects (osteoporosis, brittle diabetes).
Table 3 Clinical, biochemical, histological, and immunogenetic parameters of A‐FAA+ve and A‐FAA‐ve AIH‐1 patients.
| A‐FAA+ve (n = 55) | A‐FAA‐ve (n = 23) | p* | ||||
|---|---|---|---|---|---|---|
| Age (years)† | 35 (7–76) | 43 (15–82) | NS | |||
| Female sex | 82% | 82.6% | NS | |||
| AST (× UNV)† | 10.36 (2.1–72) | 11.36 (2.3–69) | NS | |||
| ALT (× UNV)† | 14.59 (1.9–81) | 13.38 (2–79) | NS | |||
| Bilirubin (× UNV)† | 5.52 (0,4–26) | 4.11 (0.6–28) | NS | |||
| ALP (× UNV)† | 1.49 (0.17–8.6) | 1.54 (0.2–8.2) | NS | |||
| γ‐GT (× UNV)† | 3.15 (0.4–11) | 3.36 (0.3–10.3) | NS | |||
| Albumin (g/dl)† | 3.6 (2.5–4.3) | 3.6 (2.3–4.9) | NS | |||
| γ‐globulin (g/l)† | 26 (10–59) | 20 (13–34) | < 0.005 | |||
| IgG (mg/dl)† | 3060 (1296–7344) | 2050 (1280–3402) | < 0.002 | |||
| IgA (mg/dl)† | 284 (36–840) | 292 (43–750) | NS | |||
| IgM (mg/dl)† | 184 (53–552) | 193 (49–539) | NS | |||
| Mild/moderate inflammatory activity | 61%‡ | 60%§ | NS | |||
| Severe inflammatory activity | 39%‡ | 40%§ | NS | |||
| Mild/moderate fibrosis | 62%‡ | 64§ | NS | |||
| Severe fibrosis/cirrhosis | 38%‡ | 40%§ | NS | |||
| DRB1†0301 | 16 (29%) | 8 (35%) | NS | |||
| DRB1†0401 | 11 (20%) | 8 (35%) | NS | |||
| Complete remission | 45 (82%) | 19 (83%) | NS | |||
| Relapse | 28 (51%) | 12 (52%) | NS |
*Mann‐Whitney test; †median (range); ‡evaluation on 48 samples; §evaluation on 18 samples. UNV, upper normal level; AST, aspartate aminotransferase; ALT, alanino trasferase; ALP, alkaline phosphatase; γ‐GT, γ‐glutamyl transpeptidase; NS, not significant.
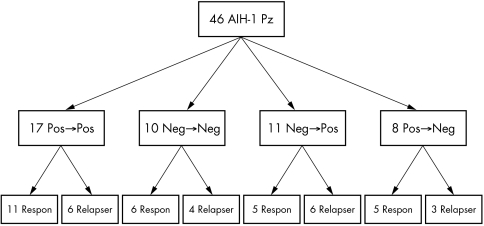
Of the 46 sera of AIH‐1 patients re‐evaluated during follow up, 17 maintained their original positivity, 10 maintained their original negativity, 11 expressed A‐FAA that was absent at presentation, and 8 lost a previously positive A‐FAA (fig 2). None had to stop treatment because of treatment side‐effects or other reason. Appearance or disappearance of A‐FAA during follow up did not reflect disease activity (complete remission or relapse) at the time of re‐evaluation.
Figure 2 Follow up study. In total, 46 AIH‐1 patients were re‐evaluated for A‐FAA during follow‐up: 17 mantained their original positivity (Pos→Pos), 10 maintained their original negativity (Neg→Neg), 11 expressed A‐FAA that has not been present at presentation (Neg→Pos), and 8 lost A‐FAA (Pos→Neg). Appearance or disappearance of A‐FAA during follow up did not reflect complete remission (Respon) or relapse (Relapser).
DISCUSSION
IIF represents the standard technique to detect non organ‐specific autoantibodies such as SMA, and is a cornerstone of the immunoserology of AIH, as recently outlined in a consensus statement.9 However, the interpretation of the immunofluorescence patterns is dependent on the observer's experience, particularly in evaluating the SMA reactivity, and many laboratories are not used to defining the SMA patterns. The SMA‐T/G pattern is highly predictive of AIH‐1, with a sensitivity of approximately 80% and a specificity higher than 90%,8,11 whereas the SMA‐V pattern is considered less specific.13 As previously demonstrated8 the SMA‐T/G pattern is the immunomorphological expression of an autoreaction targeting F‐actin. Therefore, an easy and reliable diagnostic tool to detect anti‐F‐actin reactivity would be very useful to support the diagnosis of AIH‐1.14
We found that a commercially available anti‐F‐actin ELISA, with the cutoff adjusted to our population, strictly correlated with the SMA‐T/G reactivity, being positive for all but one of the AIH‐1 sera with SMA‐T/G. The overall sensitivity of A‐FAA for AIH‐1 was lower than that of SMA not further characterised, but this difference was not statistically significant. The SMA‐T/G pattern was not detected in any of the controls and had the highest disease specificity. In our hands, A‐FAA detected with this modified ELISA also showed excellent disease specificity (94%). The elevated sensitivity of this ELISA is probably due to the preservation of the three dimensional structure of F‐actin and conservation of the most immunogenic autoepitopes. In addition, it is possible that previous discrepancies between different assays detecting anti‐actin antibodies may be due to a thermolabile F‐actin depolymerising factor described in the serum of AIH patients.15,16
In keeping with our previous retrospective study, some 20% of SMA positive AIH‐1 patients lacked anti‐F‐actin reactivity.8 Even so, we found a very high degree of correlation between A‐FAA and SMA‐T/G, an observation further supporting the actin specificity of the SMA‐T/G pattern, its strong association with AIH‐1, and its important diagnostic value. There are two possible explanations for the lack of correlation between A‐FAA and the SMA titres: (a) the SMA‐V pattern is usually a result of a mixture of antibodies against cytoskeleton proteins whereas AIH specific SMA‐T/G could also react to antigens other than actin; and (b) the immunodominant epitopes on the F‐actin coated onto the ELISA wells may be better exposed and more easily recognised than in the cryopreserved rat tissue, explaining why nine AIH patients positive for A‐FAA were negative for SMA‐T/G (of these, seven were SMA‐V positive). It is of interest that two AIH‐1 patients were SMA negative but A‐FAA positive; the potential diagnostic value of A‐FAA in IIF‐negative patients with AIH‐1 should be evaluated further in larger series.
The positivity for A‐FAA in our series of AIH‐1 patients was associated with higher γ‐globulin and IgG levels but did not correlate with any other clinical, laboratory, histological, or immunogenetic parameter, therefore it does not appear to identify a particular subset of the disease among AIH‐1 patients. This observation contrasts with that of Czaja et al, who found anti‐actin reactivity to be strongly associated with HLA‐B8 and DR3 alleles and with a worse prognosis.15 The higher frequency of HLA DR3 in North American patients10 and the different techniques used to detect anti‐actin reactivities (IIF on HEp‐2 cells, IIF on sections of liver from rats injected with phalloidin, and counterimmunoelectrophoresis for the detection of XR117), may explain this discrepancy. On the other hand, the kinetics of A‐FAA appearance and disappearance during treatment and follow up is in keeping with a recent study.18
TAKE HOME MESSAGES
Indirect immunofluorescence (IIF) is the standard technique to detect non organ‐specific autoantibodies such as smooth muscle antibodies (SMA), and is a cornerstone of the immunoserology of autoimmune hepatitis (AIH).
The SMA‐T/G pattern is highly predictive of AIH‐1, with a sensitivity of approximately 80% and a specificity higher than 90%, whereas the SMA‐V pattern is considered less specific.
We found that a commercially available anti‐F‐actin ELISA, with the cutoff adjusted to our population, strictly correlated with the SMA‐T/G reactivity, being positive for all but one of the AIH‐1 sera with SMA‐T/G.
In conclusion, in virtue of its higher sensitivity IIF remains the first level screening technique, as it also allows the detection of other autoantibodies relevant in the setting of autoimmune liver disorders such as anti‐, anti‐mitochondrial, anti‐liver kidney microsomes type 1, and anti‐liver cytosol type 1 antibodies. Even if the modified A‐FAA detection cannot replace IIF and does not appear to have prognostic significance, it is a reliable and operator independent assay that could be helpful when interpretative doubts of standard immunofluorescence technique arise.
Abbreviations
A‐FAA - anti‐filamentous actin antibodies
AIH‐1 - autoimmune hepatitis type 1
AIH‐2 - autoimmune hepatitis type 2
AU - arbitrary unit
CD - coeliac disease
F‐actin - filamentous actin
HBsAg - hepatitis B surface antigen
HCV - hepatitis C virus
HLA - human leucocyte antigen
IIF - indirect immunofluorescence
PBC - primary biliary cirrhosis
SMA - smooth muscle antibodies
References
- 1.Alvarez F, Berg P A, Bianchi F B.et al International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol 199931929–938. [DOI] [PubMed] [Google Scholar]
- 2.Czaja A J, Freese D K. Diagnosis and treatment of autoimmune hepatitis. Hepatology 200236479–497. [DOI] [PubMed] [Google Scholar]
- 3.Toh B H. Smooth muscle autoantibodies and autoantigens. Clin Exp Immunol 197938621–628. [PMC free article] [PubMed] [Google Scholar]
- 4.Bottazzo G F, Florin‐Christensen A, Fairfax A.et al Classification of smooth muscle autoantibodies detected by immunofluorescence. J Clin Pathol 197629403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bretherton L, Brown C, Pedersen J S.et al ELISA assay for IgG autoantibody to G‐actin: comparison of chronic active hepatitis and acute viral hepatitis. Clin Exp Immunol 198351611–616. [PMC free article] [PubMed] [Google Scholar]
- 6.Leibovitch L, George J, Levi Y.et al Anti‐actin antibodies in sera from patients with autoimmune liver diseases and patients with carcinomas by ELISA. Immunol Lett 199548129–132. [DOI] [PubMed] [Google Scholar]
- 7.Zamanou A, Tsirogianni A, Terzoglou C.et al Anti‐smooth muscle antibodies (ASMAs) and anti‐cytoskeleton antibodies (ACTAs) in liver diseases: a comparison of classical indirect immunofluorescence with ELISA. J Clin Lab Anal 200216194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muratori P, Muratori L, Agostinelli D.et al Smooth muscle antibodies and type 1 autoimmune hepatitis. Autoimmunity 200235497–500. [DOI] [PubMed] [Google Scholar]
- 9.Vergani D, Alvarez F, Bianchi F B.et al Liver autoimmune serology: a consensus statement from the committee for autoimmune serology of the International Autoimmune Hepatitis Group. J Hepatol 200441677–683. [DOI] [PubMed] [Google Scholar]
- 10.Muratori P, Czaja A J, Muratori L.et al Genetic distinctions between autoimmune hepatitis in Italy and North America. World J Gastroenterol 2005111862–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassani F, Cataleta M, Valentini P.et al Serum autoantibodies in chronic hepatitis C: comparison with autoimmune hepatitis and impact on the disease profile. Hepatology 199726561–566. [DOI] [PubMed] [Google Scholar]
- 12.Czaja A J, Carpenter H A. Sensitivity, specificity, and predictability of biopsy interpretations in chronic hepatitis. Gastroenterology 19931051824–1832. [DOI] [PubMed] [Google Scholar]
- 13.Toh B H. Anti‐cytoskeletal autoantibodies: diagnostic significance for liver diseases, infections and systemic autoimmune diseases. Autoimmunity 199111119–125. [DOI] [PubMed] [Google Scholar]
- 14.Silvestrini R A, Benson E M. Whither smooth muscle antibodies in the third millennium? J Clin Pathol 200154677–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cancado E L, Vilas‐Boas L S, Abrantes‐Lemos C P.et al Heat serum inactivation as a mandatory procedure for antiactin antibody detection in cell culture. Hepatology 1996231098–1104. [DOI] [PubMed] [Google Scholar]
- 16.Cancado E L, Abrantes‐Lemos C P, Vilas‐Boas L S.et al Thermolabile and calcium‐dependent serum factor interferes with polymerized actin, and impairs anti‐actin antibody detection. J Autoimmun 200117223–228. [DOI] [PubMed] [Google Scholar]
- 17.Czaja A J, Cassani F, Cataleta M.et al Frequency and significance of antibodies to actin in type 1 autoimmune hepatitis. Hepatology 1996241068–1073. [DOI] [PubMed] [Google Scholar]
- 18.Czaja A J. Behavior and significance of autoantibodies in type 1 autoimmune hepatitis. J Hepatol 199930394–401. [DOI] [PubMed] [Google Scholar]