Abstract
Objective
To evaluate the role of c‐myc oncogene amplifications in the progression of invasive breast carcinomas.
Methods
c‐myc gene copy number was evaluated in a series of 49 primary breast carcinomas and the corresponding local recurrences using fluorescence in situ hybridisation.
Results
11 of the primary carcinomas (22%) harboured c‐myc amplifications; these tumours typically were hormone receptor negative and occurred in younger patients (43 v 53 years). At the time of relapse, six additional tumours had acquired a c‐myc amplification. The mean recurrence‐free survival was 24 months; c‐myc amplified tumours relapsed significantly earlier than carcinomas without amplification (18 v 27 months). Univariate analysis showed a worse overall survival in these patients.
Conclusions
While c‐myc amplifications can be observed in early stage breast cancer, especially in younger patients, they often occur later in tumour development and appear to be associated with disease progression.
Keywords: breast cancer, c‐myc, fluorescence in situ hybridisation
Over the last 10 to 15 years, the surgical treatment of invasive breast cancer has undergone major changes. Owing to the increasing quality of radiological examination, breast cancer awareness and the establishment of screening programmes, the typical size of primary breast carcinomas has decreased substantially.1 On the other hand, encouraging results with breast conserving surgery, in combination with adjuvant therapy, have led to the application of this treatment for larger tumours as well.2 Currently, data from large follow up studies show local recurrence rates of approximately 5% for breast conserving surgery.3 While there still is a higher risk of relapse compared with mastectomy, it is commonly acknowledged that the benefits of a less aggressive treatment approach outweigh the possibility of recurrent disease.
Clinical studies focusing on local failure of breast cancer treatment have revealed a large number of cases with distant metastases at the time of intramammary, axillary, or chest wall (following mastectomy) recurrence, and reported a poor overall survival in these patients.4,5 Addressing these problems, several studies have identified possible clinicopathological or biological predictors of local recurrence.5,6,7,8,9,10 However, little is known about biological changes in the tumour during progression of breast carcinomas.
Methods
Patients
Between 1992 and 1998, 2996 patients with primary breast carcinoma were treated at the Department of Gynaecology and Obstetrics of Heidelberg University. Between 1996 and 2001, 67 of these patients were diagnosed with locally (intramammary, chest wall, or axillary) recurrent breast carcinoma; 14 of the tumours recurred more than once in the given time span. On the basis of the availability of representative paraffin embedded tissue from both primary and recurrent tumours at the Department of Pathology of the University of Heidelberg, 49 individual patients were selected. The tumours were classified according to the World Health Organisation,11 and pathological staging was based on the fifth edition of the TNM classification. For fluorescence in situ hybridisation (FISH), four tissue microarrays were created containing both primary tumours and local recurrences using 1.5 mm punch needles on a semiautomatic system (Beecher Instruments, Silver Spring, Maryland, USA) as described previously.12
Immunohistochemistry
Slides containing full tissue sections of representative tumour areas were dewaxed and rehydrated using xylene and a series of graded alcohols, followed by heat induced antigen retrieval using citrate buffer (DakoCytomation, Glostrup, Denmark) in a microwave oven. Staining was done on an automated staining system (Techmate 500+, DakoCytomation) with the avidin‐biotin‐complex peroxidase technique using AEC (3‐amino‐9‐ethyl‐carbazol) for visualisation and haematoxylin for counterstaining. Dilutions for the primary antibodies were 1:500 for Her2 (A0485, DakoCytomation), 1:200 for Ki‐67 (MIB‐1, DakoCytomation), 1:50 for oestrogen receptor (1D5, DakoCytomation) and progesterone receptor (PgR636, DakoCytomation), and 1:100 for p53 (DO7, DakoCytomation).
Fluorescence in situ hybridisation
Approximately 5 μm sections of the tissue microarrays were mounted on coated slides (DakoCytomation) and dried overnight at 37°C. Deparaffinised slides were pretreated using 1 M sodium thiocyanate (NaSCN, Merck, Darmstadt, Germany) at 80°C for 30 minutes, followed by digestion with proteinase K (Roche, Mannheim, Germany; 100 μg/ml, 37°C, 17 minutes). DNA obtained from a cosmid clone containing the c‐myc gene sequence (cos‐myc 72)13 was labelled using tetramethyl‐rhodamine‐5‐dUTP (Roche) with nick translation.14 Slides and probe DNA (approximately 0.1 μg) were co‐denatured in the presence of 10 μg Cot1‐DNA (Roche) on an in situ polymerase chain reaction (PCR) thermocycler (Perkin Elmer, Monza, Italy) and hybridised at 37°C for 16 to 24 hours in a humid chamber. Post‐hybridisation washes and counterstaining using DAPI (4,6‐diamidino‐2‐phenylindole) were carried out as described previously.12
Statistical evaluation
Data were analysed using the R software package (version 1.7.1, http://www.r‐project.org). For count data, Fisher's exact test (two sided) was used; continuous data were analysed using the two sided Wilcoxon rank sum test. Differences between primary tumours and recurrences were calculated using the paired Wilcoxon signed rank test. The Kaplan–Meier method was applied to calculate survival rates for both local recurrences and overall survival; for multivariate analysis, the Cox proportional hazards regression model was used. Univariate survival data were tested for significance using the Mantel–Haenszel log rank test. Probability (p) values less than 0.05 were considered significant.
Results
Clinicopathological data
The clinical characteristics of the primary tumours are summarised in table 1. Twenty six patients were initially treated using breast conserving surgery, 21 by mastectomy, and two by subcutaneous mastectomy. Information on adjuvant treatment was available in 37 cases (table 1). There were 42 invasive ductal, six invasive lobular, and one tubular carcinoma. Tumours were often poorly differentiated (31 cases, 63%). Only 13 carcinomas (27%) measured less than 2 cm (pT1). In 23 cases, multifocal or multicentric tumour growth was observed. Lymphatic vessel invasion was present in 28 cases, and 34 carcinomas had metastasised to the axillary lymph nodes (table 2). Local recurrences occurred after an average of 23.7 months (range 5 to 63 months); there were 24 intramammary, 16 intracutaneous (chest wall), five axillary, two infraclavicular or supraclavicular, and two contralateral recurrences. Twelve of the cohort suffered from a second local relapse between five and 44 months later; one tumour recurred a third time four months after the second relapse. Further follow up information was available in 28 cases (median 15 months, range 3 to 42).
Table 1 Clinical data on 49 breast carcinomas with local recurrences.
| Age (years) (median (range)) | 50 (26 to 85) |
| Surgical treatment | |
| Breast conserving surgery | 26 (53%) |
| Mastectomy | 23 (47%) |
| Axillary dissection | 47 (96%) |
| Adjuvant treatment | |
| Antihormonal therapy | 18/37 (49%)* |
| Radiation therapy | 20/37 (54%)* |
| Cytostatic chemotherapy | 29/37 (78%)* |
| Time to local recurrence (months) | 24 (5 to 63) |
| (mean (range)) | |
| Local recurrence | |
| Intramammary | 24 (49%) |
| Chest wall | 16 (33%) |
| Axillary | 5 (10%) |
| Other | 4 (8%) |
Values are n (%) unless stated otherwise.
*In 12 cases, no information on adjuvant treatment was available.
Table 2 Histopathological characteristics of 49 breast carcinomas with local recurrences.
| Tumour type | |
|---|---|
| Invasive ductal carcinoma | 42 (86%) |
| Invasive lobular carcinoma | 6 (12%) |
| Tubular carcinoma | 1 (2%) |
| Histological grading | |
| G1 | 1 (2%) |
| G2 | 17 (35%) |
| G3 | 31 (63%) |
| Tumour extent | |
| pT1 (pT1a,pT1b,pT1c) | 13 (27%) |
| pT2 | 24 (49%) |
| pT3 | 6 (12%) |
| pT4 (pT4a, pT4b, pT4c, pT4d) | 6 (12%) |
| Multifocal | 16 (33%) |
| Multicentric | 7 (14%) |
| Free margins | 42 (86%) |
| Lymph node status | |
| pN0 | 13 (27%) |
| pN1 | 32 (49%) |
| pN2 | 2 (4%) |
| pNX | 2 (4%) |
| Lymphatic vessel invasion | 28 (57%) |
Values are n (%).
Immunohistochemical characterisation
Immunohistochemical staining results are summarised in table 3. Hormone receptor staining was evaluated using a semiquantitative scoring system, resulting in scores ranging from 0 to 12.15 Immunoreactivity scores (IRS) greater than 2 were considered positive. Twenty five of the primary tumours (51%) showed expression of the oestrogen receptor (21 cases) or progesterone receptor (15 cases). At the time of recurrence, 14 of these cases were scored negative for both oestrogen receptor and progesterone receptor (p<0.0003, both receptors combined). Overexpression of the Her2 protein product was diagnosed in 17 cases (35%) which showed moderate to strong circumferential membrane staining (Dako score 3+) while the rest of the cases showed no staining or only weak and partial membrane staining (30 cases with Dako score of 0, two cases scored 1). There was no staining difference between primary tumours and recurrences: the same 17 cases showed an overexpression of Her2 at the time of relapse while all other cases remained negative. Nuclear accumulation of the p53 protein in more than 50% of the tumour cells was observed in 18 (37%) of primary tumours and in 20 recurrences (41%). Two tumours with initial p53 overexpression stained negative at the time of relapse; four tumour recurrences which had initially been negative had strong nuclear staining. On average, primary tumours showed a proliferative activity of 51% Ki67 positive nuclei; hormone receptor positive tumours were associated with a lower growth rate (35% v 64%, p = 0.0002) and overexpression of the p53 protein was associated with an increased proliferative activity (70% v 40%, p = 0.0004). In addition, tumour recurrences showed a slight reduction in proliferative activity (45% v 51%, p = 0.028).
Table 3 Immunohistochemical characteristics of 49 breast carcinomas with local recurrences.
| Primary | Recurrences | p Value* | |
|---|---|---|---|
| tumours | |||
| Oestrogen receptor expression | 21 cases | 8 cases | 0.0024 |
| Progesterone receptor expression | 15 cases | 9 cases | 0.244 |
| Her2 overexpression | 17 cases | 17 cases | 1 |
| p53 overexpression | 18 cases | 20 cases | 0.300 |
| Average Ki67 expression (% positive nuclei) | 51% | 45% | 0.028 |
*Calculated using the paired Wilcoxon signed rank test.
c‐myc Oncogene amplification
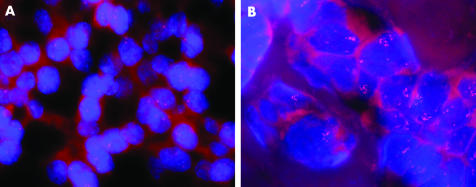
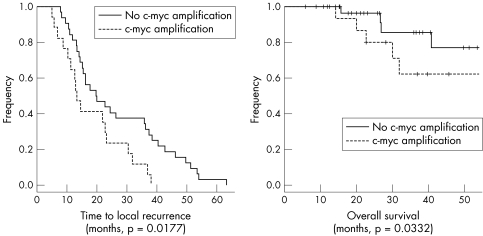
FISH results were evaluated by counting the average number of nuclear signals in at least 50 tumour cell nuclei (in 10 cases with only few tumour cell nests included in the tissue array sections fewer nuclei were evaluated). Typical hybridisation results are shown in fig 1. Eleven (22%) of the primary tumours (10 invasive ductal and one invasive lobular carcinoma, nine high grade and two intermediate grade tumours) showed between five and 12 signals per nucleus (7.2 on average) and were considered amplified. These tumours were associated with a lower age at the time of diagnosis (43 v 53 years, p = 0.0223) and typically lacked hormone receptor expression (two of 11 cases compared with 16 of 38 cases; p = 0.0373). At the time of recurrence, six additional cases showed c‐myc oncogene amplifications. With respect to the overall number of cases, this increase (35% v 22%) reached significance (p = 0.0197). In primary tumours with c‐myc amplifications, there was no loss of or further increase in gene copy number. In the six cases with acquired c‐myc amplification (three chest wall, two intramammary, one axillary recurrence), the average age at the time of diagnosis (51 years) did not vary from that of the remainder of the patients. Three of these six tumours had primarily been oestrogen or progesterone receptor positive; however, all three cases had lost hormone receptor expression at the time of relapse. Patients with c‐myc amplified primary tumours or recurrences had a shorter recurrence‐free survival compared with those with normal gene copy number (18 v 27 months, p = 0.0177, fig 2A) and a worsened overall survival following recurrence (p = 0.0332, fig 2B). Using multivariate analysis, c‐myc amplified primary tumours were associated with earlier local failure (p = 0.041, table 4). However, the only factor independently influencing overall survival following local recurrence was UICC stage (p = 0.039).
Figure 1 c‐myc Fluorescence in situ hybridisation. (A) Invasive breast carcinoma showing no c‐myc amplification; (B) c‐myc amplified breast tumour with 10 to 12 signals per nucleus.
Figure 2 Recurrence free (A) and overall (B) survival stratified by c‐myc amplification. Crosses indicate follow up end points with patients alive.
Table 4 Cox proportional hazards regression model analysing factors influencing the time to local recurrence.
| Hazard ratio | p Value | |||
|---|---|---|---|---|
| (95% CI) | ||||
| UICC stage | 0.97 (0.52 to 1.79) | 0.920 | ||
| c‐myc Amplification (primary tumour) | 2.39 (1.04 to 5.48) | 0.041 | ||
| c‐myc Amplification (acquired) | 2.66 (0.97 to 7.27) | 0.057 | ||
| High grade | 1.22 (0.48 to 3.08) | 0.680 | ||
| Positive margins | 3.11 (0.87 to 11.14) | 0.081 | ||
| Lymphatic vessel invasion | 1.09 (0.45 to 2.62) | 0.850 | ||
| Positive hormone receptor status | 1.03 (0.45 to 2.62) | 0.940 | ||
| Her2 Overexpression | 0.91 (0.42 to 1.97) | 0.810 | ||
| p53 Overexpression | 1.65 (0.76 to 3.59) | 0.210 | ||
| High proliferative activity (Ki67>25%) | 0.97 (0.38 to 2.51) | 0.950 |
UICC, International Union Against Cancer.
Discussion
Locoregional breast cancer recurrences typically occur years after initial treatment and can be a potential source of distant metastases and potentially disease related deaths in these patients.16 Using comparative genomic hybridisation and analysis of loss of heterozygosity, an increased complexity of karyotypes has been observed during the progression of breast cancer.17,18 During the time span between initial surgery and locoregional recurrence, residual tumour cells are subjected to different conditions, applying selective pressure including surgery related influences such as local hypoxaemia and inflammatory reactions, as well as radiation induced DNA damage, cytostatic chemotherapy, or antihormonal treatment.
The MYC proto‐oncogene functions as a promotor of cell replication by activating positive cell cycle regulators like the cyclin protein family.19 Activation of the c‐myc gene in tumours leads to a higher proliferative activity as well as to an increased rate of apoptosis.20 However, the oncogenetic potential of c‐myc alterations alone has been found to be low.21 Different experiments on transgenic mice have shown that additional genetic changes are needed to promote tumour formation. Among these additional alterations are activation of the Her222 and BCL223 oncogenes, as well as inactivation of the p53 tumour suppressor gene,21 which are commonly observed in breast cancer.
In our series of locally recurrent breast carcinomas, 11 of the primary tumours (22%) harboured c‐myc oncogene amplifications. This frequency is slightly higher than in previous studies, which found c‐myc amplifications in 5–15% of the cases,24,25,26 possibly reflecting the selection of a more aggressive subset of breast carcinomas. Most of these 11 myc‐amplified primary tumours were hormone receptor negative, high grade carcinomas. Interestingly, the average age at diagnosis was only 43 years in these cases, compared with 53 years in the primary tumours without myc‐amplifications. Recently, Grushko et al reported a high frequency of 53% c‐myc amplifications in BRCA1 associated breast carcinomas.27 Adem et al observed an association of myc alterations with both BRCA1 and BRCA2 related tumours.28 However, as we have no data on BRCA1 or BRCA2 mutations in the patients included in this study, we can only speculate on the reason for the observed difference in patient age.
While amplifications of c‐myc have been observed in a subset of pure intraductal breast carcinomas,12,29 Naidu et al30 and Robanus‐Maandag et al31 have reported several cases of myc amplified invasive ductal carcinoma with an intraductal tumour component lacking c‐myc amplifications. Based on these findings, an association of c‐myc with the progression from in situ to invasive carcinoma has been proposed.31 We have detected amplifications of the c‐myc oncogene in six local recurrences of primary breast carcinoma with normal myc copy number. Contrasting the findings in primarily amplified tumours, these cases were not associated with lack of hormone receptors or younger patient age at the time of diagnosis. Thus it appears likely that in these cases c‐myc amplifications are based on an increased chromosomal instability and are involved in tumour progression rather than in tumour initiation. In addition, we observed an association between breast carcinomas with increased myc copy number, either at the time of primary diagnosis or on recurrence, and a shortened relapse‐free time both using univariate and multivariate analysis. This suggests that, owing to hypoxic or adjuvant treatment related cellular damage, an increased selective pressure may lead to the accumulation of genetic alterations such as c‐myc amplifications that provide a growth advantage or permit survival under cell damaging conditions. Recently, an increased expression of c‐myc has been found to facilitate human mammary epithelial cells with radiation induced DNA damage to inappropriately enter mitosis by attenuating the G2/M arrest.32 Furthermore, in another experiment, c‐myc overexpression was also shown to weaken the G1/S arrest in the same mammary epithelial cells and thus may promote the replication of damaged DNA by an increased S‐phase entry.33 Using MCF‐7 breast cancer cells, Venditti et al observed that induced c‐myc gene expression conferred a resistance to antioestrogenic treatment.34 These results may explain the increased frequency of c‐myc amplifications both in the progression of in situ to invasive breast carcinomas and in our series of locally recurring tumours.
In conclusion, our results indicate that while c‐myc amplifications can be observed in early stage breast cancer, especially in hormone receptor negative, high grade tumours in younger patients, they often occur later in tumour development and appear to be associated with disease progression and early local failure.
Acknowledgements
We thank Marie‐Luise Diarra and Annemarie Wiss for technical support in the procession of the tissue array slides and Marin Bentz for providing the c‐myc cosmid DNA. This work was supported by a grant from the Heidelberg University (3542.2).
Abbreviations
FISH - fluorescence in situ hybridisation
IRS - immunoreactivity scores
UICC - International Union against Cancer
References
- 1.Legorreta A P, Chernicoff H O, Trinh J B.et al Diagnosis, clinical staging, and treatment of breast cancer: a retrospective multiyear study of a large controlled population. Am J Clin Oncol 200427185–190. [DOI] [PubMed] [Google Scholar]
- 2.Fredriksson I, Liljegren G, Arnesson L G.et al Time trends in the results of breast conservation in 4694 women. Eur J Cancer 2001371537–1544. [DOI] [PubMed] [Google Scholar]
- 3.Huston T L, Simmons R M. Locally recurrent breast cancer after conservation therapy. Am J Surg 2005189229–235. [DOI] [PubMed] [Google Scholar]
- 4.Voogd A C, van Tienhoven G, Peterse H L.et al Local recurrence after breast conservation therapy for early stage breast carcinoma: detection, treatment, and outcome in 266 patients. Dutch Study Group on Local Recurrence after Breast Conservation (BORST). Cancer 199985437–446. [DOI] [PubMed] [Google Scholar]
- 5.Haffty B G, Hauser A, Choi D H.et al Molecular markers for prognosis after isolated postmastectomy chest wall recurrence. Cancer 2004100252–263. [DOI] [PubMed] [Google Scholar]
- 6.Sinn H P, Anton H W, Magener A.et al Extensive and predominant in situ component in breast carcinoma: their influence on treatment results after breast‐conserving therapy. Eur J Cancer 199834646–653. [DOI] [PubMed] [Google Scholar]
- 7.Isaacs C, Stearns V, Hayes D F. New prognostic factors for breast cancer recurrence. Semin Oncol 20012853–67. [DOI] [PubMed] [Google Scholar]
- 8.Le M G, Arriagada R, Spielmann M.et al Prognostic factors for death after an isolated local recurrence in patients with early‐stage breast carcinoma. Cancer 2002942813–2820. [DOI] [PubMed] [Google Scholar]
- 9.Engel J, Eckel R, Aydemir U.et al Determinants and prognoses of locoregional and distant progression in breast cancer. Int J Radiat Oncol Biol Phys 2003551186–1195. [DOI] [PubMed] [Google Scholar]
- 10.Fredriksson I, Liljegren G, Palm‐Sjovall M.et al Risk factors for local recurrence after breast‐conserving surgery. Br J Surg 2003901093–1102. [DOI] [PubMed] [Google Scholar]
- 11.Ellis I O, Schnitt S J, Sastre‐Garau X.et al Invasive breast carcinoma. In: Tavassoli FA, Devilee P, editors. Pathology and genetics of tumours of the breast and female genital organs (WHO classification of tumours). Lyon: IARC Press, 200313–59.
- 12.Aulmann S, Bentz M, Sinn H P. C‐myc oncogene amplification in ductal carcinoma in situ of the breast. Breast Cancer Res Treat 20027425–31. [DOI] [PubMed] [Google Scholar]
- 13.Joos S, Falk M H, Lichter P.et al Variable breakpoints in Burkitt lymphoma cells with chromosomal t(8;14) translocation separate c‐myc and the IgH locus up to several hundred kb. Hum Mol Genet 19921625–632. [DOI] [PubMed] [Google Scholar]
- 14.Lichter P, Bentz M, Joos S. Detection of chromosomal aberratins by means of molecular cytogenetics: painting of chromosomes and chromosomal subregions and comparative genomic hybridization. Methods Enzymol 1995254334–359. [DOI] [PubMed] [Google Scholar]
- 15.Remmele W, Dietz M, Schmidt F.et al Relation of elastosis to biochemical and immunohistochemical steroid receptor findings, Ki‐67 and epidermal growth factor receptor (EGFR) immunostaining in invasive ductal breast cancer. Virchows Arch A Pathol Anat Histopathol 1993422319–326. [DOI] [PubMed] [Google Scholar]
- 16.Vicini F A, Kestin L, Huang R.et al Does local recurrence affect the rate of distant metastases and survival in patients with early‐stage breast carcinoma treated with breast‐conserving therapy? Cancer 200397910–919. [DOI] [PubMed] [Google Scholar]
- 17.Nishizaki T, DeVries S, Chew K.et al Genetic alterations in primary breast cancers and their metastases: direct comparison using modified comparative genomic hybridization. Genes Chromosomes Cancer 199719267–272. [DOI] [PubMed] [Google Scholar]
- 18.Regitnig P, Moser R, Thalhammer M.et al Microsatellite analysis of breast carcinoma and corresponding local recurrences. J Pathol 2002198190–197. [DOI] [PubMed] [Google Scholar]
- 19.Guo Q M, Malek R L, Kim S.et al Identification of c‐myc responsive genes using rat cDNA microarray. Cancer Res 2000605922–5928. [PubMed] [Google Scholar]
- 20.Jamerson M H, Johnson M D, Dickson R B. Dual regulation of proliferation and apoptosis: c‐myc in bitransgenic murine mammary tumor models. Oncogene 2000191065–1071. [DOI] [PubMed] [Google Scholar]
- 21.McCormack S J, Weaver Z, Deming S.et al Myc/p53 interactions in transgenic mouse mammary development, tumorigenesis and chromosomal instability. Oncogene 1998162755–2766. [DOI] [PubMed] [Google Scholar]
- 22.Cardiff R D, Sinn E, Muller W.et al Transgenic oncogene mice. Tumor phenotype predicts genotype. Am J Pathol 1991139495–501. [PMC free article] [PubMed] [Google Scholar]
- 23.Jager R, Herzer U, Schenkel J.et al Overexpression of Bcl‐2 inhibits alveolar cell apoptosis during involution and accelerates c‐myc‐induced tumorigenesis of the mammary gland in transgenic mice. Oncogene 1997151787–1795. [DOI] [PubMed] [Google Scholar]
- 24.Isola J, Chu L, DeVries S.et al Genetic alterations in ERBB2‐amplified breast carcinomas. Clin Cancer Res 199954140–4145. [PubMed] [Google Scholar]
- 25.Rummukainen J K, Salminen T, Lundin J.et al Amplification of c‐myc oncogene by chromogenic and fluorescence in situ hybridization in archival breast cancer tissue array samples. Lab Invest 2001811545–1551. [DOI] [PubMed] [Google Scholar]
- 26.Al‐Kuraya K, Schraml P, Torhorst J.et al Prognostic relevance of gene amplifications and coamplifications in breast cancer. Cancer Res 2004648534–8540. [DOI] [PubMed] [Google Scholar]
- 27.Grushko T A, Dignam J J, Das S.et al MYC is amplified in BRCA1‐associated breast cancers. Clin Cancer Res 200410499–507. [DOI] [PubMed] [Google Scholar]
- 28.Adem C, Soderberg C L, Hafner K.et al ERBB2, TBX2, RPS6KB1, and MYC alterations in breast tissues of BRCA1 and BRCA2 mutation carriers. Genes Chromosomes Cancer 2004411–11. [DOI] [PubMed] [Google Scholar]
- 29.Fiche M, Avet‐Loiseau H, Maugard C M.et al Gene amplifications detected by fluorescence in situ hybridization in pure intraductal breast carcinomas: relation to morphology, cell proliferation and expression of breast cancer‐related genes. Int J Cancer 200089403–410. [DOI] [PubMed] [Google Scholar]
- 30.Naidu R, Wahab N A, Yadav M.et al Protein expression and molecular analysis of c‐myc gene in primary breast carcinomas using immunohistochemistry and differential polymerase chain reaction. Int J Mol Med 20029189–196. [PubMed] [Google Scholar]
- 31.Robanus‐Maandag E C, Bosch C A, Kristel P M.et al Association of C‐MYC amplification with progression from the in situ to the invasive stage in C‐MYC‐amplified breast carcinomas. J Pathol 200320175–82. [DOI] [PubMed] [Google Scholar]
- 32.Sheen J H, Woo J K, Dickson R B. c‐Myc alters the DNA damage‐induced G2/M arrest in human mammary epithelial cells. Br J Cancer 2003891479–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheen J H, Dickson R B. Overexpression of c‐Myc alters G(1)/S arrest following ionizing radiation. Mol Cell Biol 2002221819–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Venditti M, Iwasiow B, Orr F W.et al C‐myc gene expression alone is sufficient to confer resistance to antiestrogen in human breast cancer cells. Int J Cancer 20029935–42. [DOI] [PubMed] [Google Scholar]