Abstract
Background
Proteinuria is a common manifestation of renal disease which is a significant cause of morbidity in patients with sickle cell disease (SCD).
Objective
To evaluate and compare cystatin C, β2‐microglobulin, and creatinine as markers of renal disease in relation to the degree of proteinuria and other complications of SCD.
Methods
24 h urine collections were used for estimation of urine protein and creatinine clearance in 59 patients with SCD. Results were correlated with plasma cystatin C, β2‐microglobulin, creatinine, glomerular filtration rate (GFR; derived from plasma creatinine by Cockcroft‐Gault, MDRD formulae, and calculated cystatin C clearance), and clinical and haematological variables.
Results
Comparing the different methods of GFR, the proportion of patients with hyperfiltration (GFR >140 ml/min) were 30.5% (MDRD), 44.1% (Cockcroft‐Gault), and 10.2 % (calculated cystatin C clearance). Cystatin C was the most consistent marker of hyperfiltration. The endogenous markers of GFR showed an increasing trend with increasing proteinuria, but haematological variables were not correlated with cystatin C, β2‐microglobulin, or plasma creatinine. Urine protein excretion was correlated with age (r = 0.33) and significant proteinuria was present in 13.6% of patients. Patients with proteinuria had lower haemoglobin concentration (p = 0.027) than those without proteinuria but HbF was not related to the degree of proteinuria or to markers of GFR.
Conclusions
Markers of GFR show variable ability to identify hyperfiltration in patients with SCD, but cystatin C is the best endogenous marker. Proteinuria is associated with age, haemoglobin, and abnormalities of GFR. Routine screening is recommended to allow for early detection and intervention.
Keywords: cystatin C, β2‐microglobulin, glomerular filtration rate, sickle cell disease
Glomerulopathy and renal failure are frequent complications in patients with sickle cell disease (SCD), including those with homozygous sickle cell anaemia and compound sickle haemoglobinopathies such as haemoglobin SC disease and haemoglobin S with [β]‐thalassaemia (HbS/[β]‐thalassaemia).1,2,3
The main renal functional abnormalities in patients with SCD are alterations in the glomerular filtration rate (GFR) and proteinuria.1,2,3 However, accurate and convenient assessment of GFR in patients with SCD has always been elusive.
Although endogenous markers of GFR such as plasma creatinine, β2‐microglobulin, and endogenous creatinine clearance have been validated against reference methods for the determination of GFR,4 they are of limited usefulness for routine evaluation of GFR in patients with SCD. Plasma creatinine is affected by well known preanalytical and analytical factors, and its concentration increases only after the GFR has been reduced by about 50%. Furthermore, as tubular secretion is the predominant mode of creatinine excretion when the GFR is less than 40 ml/min/1.73 m2, plasma creatinine results would not give an assessment of GFR in older patients with SCD who may have disordered metabolism and tubular dysfunction.1,2,3,4,5 Although β2‐microglobulin has been shown to be better than plasma creatinine estimation in patients with SCD,4 the plasma concentration is affected by cellular proliferation.6,7
Several low molecular weight proteins (ribonuclease, β2‐microglobulin, retinol binding protein, free protein HC (α1‐microglobulin), and cystatin C) have been investigated for their value in detecting reduced GFR. 8,9,10,11 Cystatin C is a 13 kDa non‐glycosylated single chain protein produced by all nucleated cells. It has various characteristics that make it attractive for the estimation of GFR. It has a low molecular weight as well as a stable production rate.11,12 It is freely filtered in the glomeruli, completely absorbed and broken down by proximal tubular cells, and the plasma concentration increases reciprocally with reduction in GFR.13,14,15,16
Our main aim in this study was to evaluate and compare the usefulness of cystatin C, β2‐microglobulin, and creatinine as markers of GFR in patients with SCD and varying degrees of proteinuria and complications.
Methods
Patients
All patients gave informed voluntary consent to participate in the study, according to the protocol approved by the local ethics committee and in accordance with the ethical standards laid down in the Helsinki declaration. We studied 59 patients (25 male and 34 female) with SCD, aged 14 to 60 years. There were 28 (48%) with the SS genotype and 31 (52%) with S/thalassaemia genotype. Patients were recruited during their routine visit to haematology clinics. Those included in this study were in steady state and crisis‐free for at least two weeks before enrolment. Thus none of the patients was on drugs known to affect GFR (such as non‐steroidal anti‐inflammatory drugs), creatinine secretion (such as cimetidine), or general tubular function (for example, antibiotics). None of the patients was on hydroxyurea. Each participant attended in the morning after a 12 to 14 hour overnight fast, and was reviewed by a consultant haematologist who obtained data on age, smoking status, disease complications including recent acute events (for example, pain, priapism, acute chest syndrome, stroke), and treatments (emergency department visits, hospital admissions, and drugs). The height and weight in light clothing were recorded.
Laboratory analyses
Full blood counts (white cell count (WBC) and red cell count, haemoglobin concentration (Hb), haematocrit (hct), mean cell volume (MCV), mean cell Hb (MCH), red cell distribution width (RDW), and platelet count) were determined by Gen S Coulter counter (Beckman‐Coulter). The percentages of HbA, HbS, HbA2, and HbF were determined using the Paragon electrophoresis system (Beckman Coulter). Plasma cystatin C and β2‐microglobulin were determined by latex particle enhanced immunoturbidimetric assays (DAKO). The methods were adapted for use on the Hitachi 911 (Roche) automated analyser which was also used to determine plasma and urine creatinine, liver profile, and 24 hour urine protein.
Patients were given verbal and written instructions on 24 hour urine collection. Protein excretion >150 mg/24 h was considered to indicate significant proteinuria.
Creatinine clearance was calculated using the Cockcroft–Gault formula17 and the modified MDRD (modification of diet in renal disease) formula.18
The formula (87.1/plasma cystatin C) – 6.8719) was also used to calculate cystatin C clearance.
As done routinely, plasma creatinine >130 μmol/l in females or >140 μmol/l in males, or a measured creatinine clearance <60 ml/min, or both, were considered to indicate reduced GFR. Hyperfiltration was empirically defined as calculated GFR ⩾140 ml/min. Plasma creatinine concentration <40 μmol/l in females or <60 μmol/l in males and cystatin C and β2‐microglobulin ⩽0.8 mg/l were considered to be low and indicate hyperfiltration. Cystatin C ⩾1.3 mg/l or β2‐microglobulin ⩾2.2 mg/l were considered to be increased and to indicate reduced GFR.
Statistical methods
Statistical analyses were undertaken using the SPSS, version 12.0. The Kolmogorov–Smirnov test was used to test the data for normality. Comparison between groups was done by the Student's t test. Summary data are presented as mean and 95% confidence interval (CI). Pearson correlation and regression were used to study the association between the normally distributed variables (cystatin C, creatinine, MDRD GFR, and measured creatinine clearance). Haematological variables (WBC, MCV, RDW, and HbF) were not normally distributed and were log transformed to enable use of parametric tests. In view of expected differences between male and female patients with regard to some of the variables (for example, creatinine and Hb), partial correlation analyses correcting for sex were undertaken. Bland–Altman analyses20 were used to compare the different methods for evaluating or calculating GFR. Probability (p) values of <0.05 were considered statistically significant.
Results
The intra‐assay coefficient of variation for the cystatin C and β2‐microglobulin assays were 2.6% and 3.6%, respectively, at a plasma cystatin C concentration of 1.2 mg/l and a β2‐microglobulin concentration of 1.7 mg/l; the inter‐assay coefficient of variation were 2.9% and 4.2%, respectively.
Table 1 summarises the data for the patients. There were no sex differences in the plasma concentrations of cystatin C and β2‐microglobulin (p>0.05). There was also no significant difference between male and female patients for many variables.
Table 1 Characteristics of the patients according to sex.
| Variable | Male | Female | p Value |
|---|---|---|---|
| Age (years) | 25.7 (21 to 30) | 29.9 (26 to 33) | 0.142 |
| White blood cells (×109/l) | 10.48 (7.77 to 13.20) | 10.01 (8.35 to 11.67) | 0.803 |
| Red blood cells (×1012/l) | 4.13 (3.69 to 4.57) | 3.62 (3.39 to 3.85) | 0.017 |
| Hb (g/l) | 105 (97 to 113) | 98. (94 to 103) | 0.074 |
| Hct (l/l) | 0.33 (0.30 to 0.35) | 0.29 (0.28 to 0.31) | 0.004 |
| MCV (fl) | 80 (76 to 84) | 81 (78 to 84) | 0.642 |
| MCH (pg) | 27 (25 to 29) | 27 (26 to 28) | 0.574 |
| RDW (%) | 19 (17 to 20) | 17. (16 to 19) | 0.442 |
| Platelets (×109/l) | 307 (234 to 381) | 355 (276 to 435) | 0.427 |
| HbF (%) | 18 (13 to 23) | 15 (12 to 19) | 0.466 |
| HbS (%) | 77 (72 to 82) | 75 (70 to 81) | 0.516 |
| HbA2 (%) | 3 (3 to 4) | 3 (2. to 3) | 0.518 |
| Fasting blood sugar (mmol/l) | 5.6 (4.3 to 6.7) | 5.8 (4.7 to 6.9) | 0.767 |
| Plasma total protein (g/l) | 73 (70 to 76) | 71 (67 to 75) | 0.483 |
| Plasma albumin (g/l) | 42 (40 to 44) | 39 (36 to 42) | 0.109 |
| Plasma urea (mmol/l) | 3.1 (2.5 to 3.6) | 2.6 (2.2 to 3.0) | 0.132 |
| Plasma creatinine (μmol/l) | 67 (56 to 79) | 52 (46 to 58) | 0.016 |
| Cystatin C (mg/l) | 1.15 (0.98 to 1.32) | 1.13 (0.95 to 1.31) | 0.845 |
| β2‐microglobulin (mg/l) | 2.72 (2.41 to 3.03) | 2.64 (2.33 to 2.94) | 0.767 |
| 24 h urine protein (mg/24 h) | 181 (47 to 315) | 151 (58 to 243) | 0.617 |
| Measured Ccr (ml/min) | 120 (87 to 153) | 108 (88 to 129) | 0.508 |
| Cockcroft‐Gault GFR (ml/min) | 152 (132 to 171) | 154 (132 to 176) | 0.862 |
| MDRD GFR (mL/min/1.73 m2) | 122 (95 to 150) | 144 (120 to 169) | 0.22 |
| Calculated cystatin C clearance | 83 (72 to 95) | 106 (88 to 124) | 0.031 |
| (ml/min/1.73 m2) |
Values are mean (95% confidence interval).
Ccr, creatinine clearance; GFR, glomerular filtration rate; Hb, haemoglobin; Hct, haematocrit; MCH, mean cell haemoglobin; MCV, mean cell volume; MDRD, modified Modification of Diet in Renal Disease; RDW, red cell distribution width.
Markers of GFR
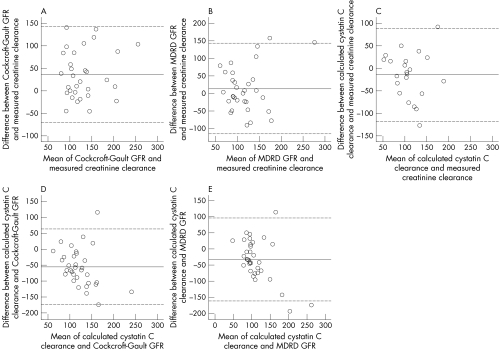
When compared with measured creatinine clearance, Bland–Altman analyses showed that the Cockcroft–Gault formula and the MDRD formula overestimated creatinine clearance (fig 1, panels A and B), while calculated cystatin C clearance (fig 1C) underestimated creatinine clearance. In view of the well documented excellent correlation of cystatin C with gold standard methods for the assessment of GFR,13,14,15,16 we decided to use calculated cystatin C clearance as the reference method and then repeated the Bland–Altman analyses. When this was done, Bland–Altman analyses showed that measured creatinine clearance, Cockcroft–Gault estimation, and MDRD estimation tended to overestimate the GFR (fig 1, panels C to E). The mean difference between calculated cystatin C clearance and the measured creatinine clearance, Cockcroft–Gault estimation, and MDRD estimation were −15 ml/min; −55 ml/min, and −33 ml/min respectively. The difference between calculated cystatin C clearance and creatinine derived clearances increased with increasing GFR (fig 1, panels C to E).
Figure 1 Bland–Altman plots of the relations between the different methods of calculating GFR. Solid horizontal lines indicate the mean of the differences and broken lines indicate ± 2SD. Some data points overlap.
Table 1 shows that calculated cystatin C clearance more closely approximates measured creatinine clearance in female patients than in male patients. It is difficult to explain this sex difference as there was no statistically significant difference in plasma cystatin C between male and female patients.
Table 2 (A) shows the Pearson correlations between different markers of GFR. In view of the sex difference in calculated cystatin C clearance, partial correlations were recalculated after correcting for sex (table 2 (B)). Correcting for sex affected some correlations observed between different markers of GFR.
Table 2 Pearson correlations between different markers of GFR.
| *Plasma creatinine | Cystatin C | *β2‐microglobulin | *Cockcroft‐Gault GFR | MDRD GFR | *Calculated CC clearance | Measured Ccr | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (μmol/l) | (mg/l) | (mg/l) | (ml/min) | (ml/min/1.73 m2) | (ml/min/1.73 m2) | (ml/min) | ||||||
| (A) Uncorrected | ||||||||||||
| Plasma creatinine (µmol/l) | r | |||||||||||
| p | ||||||||||||
| Cystatin C (mg/l) | r | 0.050 | ||||||||||
| p | 0.732 | |||||||||||
| β2‐Microglobulin (mg/l) | r | 0.331 | 0.677 | |||||||||
| p | 0.028 | <0.0001 | ||||||||||
| Cockcroft‐Gault GFR | r | −0.699 | −0.107 | −0.269 | ||||||||
| (ml/min) | p | <0.0001 | 0.475 | 0.081 | ||||||||
| MDRD GFR | r | −0.802 | −0.182 | −0.348 | 0.783 | |||||||
| (ml/min/1.73 m2) | p | <0.0001 | 0.217 | 0.021 | <0.0001 | |||||||
| Calculated CC clearance | r | −0.239 | −0.663 | −0.469 | 0.117 | 0.276 | ||||||
| (ml/min/1.73 m2) | p | 0.167 | <0.0001 | 0.004 | 0.517 | 0.115 | ||||||
| Measured Ccr (ml/min) | r | −0.207 | −0.150 | −0.033 | 0.434 | 0.271 | 0.271 | |||||
| p | 0.256 | 0.413 | 0.864 | 0.015 | 0.134 | 0.222 | ||||||
| (B) After correction for sex | ||||||||||||
| Plasma creatinine (μmol/l) | r | |||||||||||
| p | ||||||||||||
| Cystatin C (mg/l) | r | 0.044 | ||||||||||
| p | 0.767 | |||||||||||
| β2‐Microglobulin (mg/l) | r | 0.339 | 0.677 | |||||||||
| p | 0.026 | <0.0001 | ||||||||||
| Cockcroft‐Gault GFR | r | −0.739 | −0.106 | |||||||||
| (ml/min) | p | <0.0001 | 0.482 | 0.086 | ||||||||
| MDRD GFR | r | −0.803 | −0.180 | −0.346 | 0.791 | |||||||
| (ml/min/1.73 m2) | p | <0.0001 | 0.226 | 0.023 | <0.0001 | |||||||
| Calculated CC clearance | r | −0.147 | −0.687 | −0.480 | 0.115 | 0.236 | ||||||
| (ml/min/1.73 m2) | p | 0.408 | <0.0001 | 0.004 | 0.532 | 0.186 | ||||||
| Measured Ccr (ml/min) | r | −0.272 | −0.154 | −0.039 | 0.441 | 0.301 | 0.328 | |||||
| p | 0.138 | 0.407 | 0.845 | 0.015 | 0.100 | 0.147 |
*Log transformed before analysis.
CC, cystatin C; Ccr, creatinine clearance; GFR, glomerular filtration rate; MDRD, modified Modification of Diet in Renal Disease.
Hyperfiltration
The proportion of patients with hyperfiltration (GFR >140 ml/min/1.73 m2) were 30.5% (MDRD) and 44.1% (Cockcroft–Gault). When using calculated cystatin C clearance, six (10.2%) of the patients had hyperfiltration. All these patients had decreased cystatin C concentration (<0.8 mg/l). Four female patients (11.8%) had creatinine <40 μmol/l, and seven male patients (28.0%) had creatinine <60 μmol/l. No patient had decreased β2‐microglobulin (<0.8 mg/l), suggesting that this is a poor marker of hyperfiltration.
Proteinuria
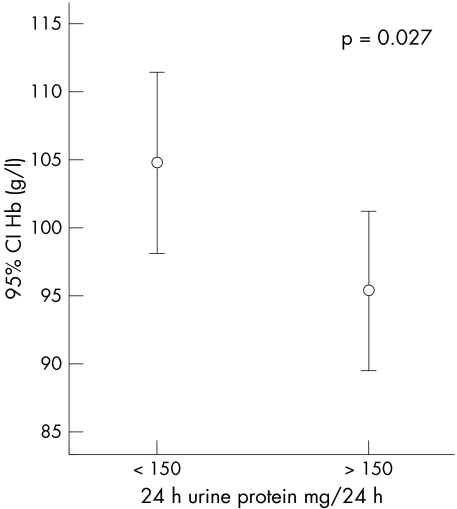
Significant proteinuria was present in eight of the patients (13.6%). Urine protein excretion (24 h urine protein) was significantly correlated with age (r = 0.33, p = 0.049). Patients with proteinuria had lower haemoglobin concentration (p = 0.027) than patients without proteinuria (fig 2). Although the endogenous markers of GFR showed an increasing trend with increases in the degree of proteinuria, the mean (95% CI) β2‐microglobulin (2.94 (2.32 to 3.56)) mg/l and cystatin C (1.43 (1.03 to 1.93)) mg/l were not significantly higher in patients with proteinuria than in patients without (mean (95% CI) β2‐microglobulin, 2.69 (2.34 to 3.05) mg/l; cystatin C, 1.10 (0.90 to 1.32)) mg/l.
Figure 2 Error bars showing the mean and 95% confidence interval (CI) of haemoglobin concentration in patients with and without proteinuria.
In contrast to previous reports,2 the degree of proteinuria was not significantly greater (p = 0.252) in patients with avascular necrosis of the hip (mean (95% CI), 206.75 (30.53 to 382.97)) mg/24h than in those without (104.50 (37.65 to 171.35)) mg/24h.
Correlations with clinical variables
None of the haematological variables showed significant correlation with cystatin C, β2‐microglobulin, or creatinine clearance. In particular, HbF concentration was not correlated with markers of GFR and the degree of proteinuria (r = −0.10; p = 0.60). In this regard the relatively high HbF in the patients suggests that they were not transfusion dependent.
Table 3 summarises the cross correlations between markers of GFR and haematological variables after correcting for sex.
Table 3 Partial correlation between markers of GFR and haematological parameters after correction for sex.
| Cockcroft‐Gault GFR (ml/min) | MDRD GFR (ml/min/1.73 m2) | Calculated CC clearance (ml/min/1.73 m2) | WBC (×109/l) | Hb (g/l) | MCV (fl) | RDW (%) | Platelets (×109/l) | HbF (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Cockcroft‐Gault | r | |||||||||
| GFR (ml/min) | p | |||||||||
| MDRD GFR | r | 0.764 | ||||||||
| (ml/min/1.73 m2) | p | <0.0001 | ||||||||
| Calculated CC clearance (ml/min/1.73 m2) | r | 0.037 | 0.026 | |||||||
| p | 0.838 | 0.883 | ||||||||
| WBC (×109/l) | r | −0.285 | −0.179 | 0.059 | ||||||
| p | 0.055 | 0.229 | 0.732 | |||||||
| Hb (g/l) | r | −0.067 | −0.219 | 0.086 | −0.577 | |||||
| p | 0.658 | 0.139 | 0.614 | <0.0001 | ||||||
| MCV (fl) | r | −0.006 | 0.071 | −0.015 | 0.224 | −0.038 | ||||
| p | 0.968 | 0.637 | 0.931 | 0.111 | 0.788 | |||||
| RDW (%) | r | 0.601 | 0.472 | −0.192 | 0.069 | −0.152 | −0.151 | |||
| p | <0.0001 | 0.002 | 0.301 | 0.644 | 0.306 | 0.311 | ||||
| Platelets (×109/l) | r | −0.023 | 0.180 | 0.252 | 0.658 | −0.424 | −0.012 | 0.092 | ||
| p | 0.881 | 0.225 | 0.138 | <0.0001 | 0.002 | 0.931 | 0.540 | |||
| HbF (%) | r | 0.205 | 0.023 | −0.266 | −0.364 | 0.341 | 0.082 | 0.031 | −0.347 | |
| p | 0.224 | 0.889 | 0.135 | 0.017 | 0.024 | 0.596 | 0.845 | 0.023 |
CC, cystatin C; GFR, glomerular filtration rate; Hb, haemoglobin; MCV, mean cell volume; MDRD, modified Modification of Diet in Renal Disease; RDW, red cell distribution width; WBC, white blood cell count.
Discussion
Assessment of renal function in patients with SCD is of great importance because the disease may present with a variety of types of renal dysfunction as a result of several structural and functional abnormalities that occur along the entire length of the nephron.1,2,3 Abnormal proximal tubular function, partly related to chronic use of analgesics, results in increased clearance of creatinine and possibly other markers of GFR.1,2,3 Young patients present with hyperfiltration, which declines towards adolescence and then declines with age.1,2,3 Monitoring renal function in these patients with the ubiquitously used plasma creatinine and creatinine clearance may lead to late detection of deteriorating renal function. This is because disordered metabolism and an increased rate of creatinine secretion by dysfunctional renal tubules may lead to a falsely normal plasma creatinine and creatinine clearance. In this study, we have shown that cystatin C is a reliable marker of the different aspects of SCD nephropathy—hyperfiltration and proteinuria.
As markers of GFR, our results are in agreement with several studies that have shown that cystatin C and β2‐microglobulin could be used as endogenous markers of GFR.4,5,11,12,13,14,15,16 Several studies have shown that plasma cystatin C correlates well with more precise methods, such as iohexol 125[I]iothalamate, or 51Cr‐EDTA clearance for the determination of GFR.13,14,15,16 However, these studies were done on mixed groups of patients with different renal diseases, and we are not aware of any study that included patients with SCD. The insignificant correlation between the endogenous markers of GFR and measured creatinine clearance is a reflection of the absence of patients with significantly reduced GFR in this study. Studies that include patients with markedly reduced GFR produce better correlations of GFR with endogenous markers.21 Although there was a significant correlation between plasma β2‐microglobulin and cystatin C in the patients in this study, cystatin C has the advantage of not being affected by cellular proliferation, especially in patients with haematological disorders and normal renal function.22
The glomerular hyperfiltration that occurs in patients with sickle cell disease plays an important role in the pathogenesis of sickle cell nephropathy; however, to date, there is no reliable endogenous marker of hyperfiltration. Although the GFR is expected to decline as early as the second decade of life, despite the persistence of high renal blood flow rates, some of our patients had hyperfiltration. Increased tubular secretion of endogenous markers of GFR may result in lower plasma levels1,2,3,23 and a resultant increase in measured and estimated clearances. Of all the endogenous markers of GFR studied, cystatin C was the best indicator of hyperfiltration. However, further studies using more accurate methods for the determination of GFR are required to confirm the usefulness of cystatin C as a marker of hyperfiltration in patients with SCD.
Take home messages
Markers of GFR show variable ability to identify hyperfiltration in patients with sickle cell disease, but cystatin C is the best endogenous marker.
Proteinuria is associated with age, haemoglobin, and abnormalities of GFR.
Routine screening is recommended to allow for early detection and intervention.
The MDRD and Cockcroft–Gault equations are the most widely recommended and used formulas for calculation of GFR. Compared with the Cockcroft‐Gault equation, the MDRD equations have been shown to be more precise and accurate for predicting GFR.24 However, in published studies, while the MDRD equations consistently underestimated GFR, the Cockcroft‐Gault equation is known to consistently overestimate measured GFR in people with normal renal function.23 These factors are probably responsible for the differences observed with the Bland–Altman analyses (fig 1) of the different methods for estimating the GFR. The variations in estimates of GFR could also reflect interindividual variability in plasma creatinine and GFR as well as measurement errors in the variables used in the equations for calculating GFR. Furthermore, the assay of plasma creatinine which is used in the equations is affected by several factors which may be critical in patients with SCD, and our study shows wide differences between measured and calculated creatinine clearance, especially in patients with hyperfiltration (fig 1). As cystatin C is not affected by these factors, calculated cystatin C clearance could prove to be more reliable than equations using plasma creatinine.
Proteinuria has been shown to be associated with SCD complications.1,2,3,23 In our patients proteinuria was correlated with age as well as with significantly lower haemoglobin concentrations (fig 2). However, we were unable to detect any relation between HbF levels and proteinuria or markers of GFR. Furthermore, the proportion of our patients with proteinuria (13.6%) is lower than that reported from other populations where rates of up to 30% have been reported.1,2,3 These findings could reflect the relatively high HbF level in our patients (table 1), as this is known to protect against severe systemic complications of the disease.25
Our study has some limitations. It was done in a relatively small sample with wide variability in clinical and laboratory variables. However, the patients are representative of the general population of patients with SCD and normal or mildly impaired kidney function. In addition, we did not use gold standard GFR methods to confirm our findings because most patients were unwilling to undergo further investigations. Our results should also be interpreted in relation to the limitations of the methods we used for estimating GFR. In particular, it is known that the method used for the estimation of creatinine has a significant impact on estimated GFR, as recently shown by Lamb et al.26 It is also known that estimates of GFR could be inaccurate in some non‐steady‐state clinical situations—for example, when there is hyperfiltration.27
Nevertheless, the National Kidney Foundation28 recommends that hospital laboratories should provide estimates of GFR from creatinine. Our data show that cystatin C could provide a reliable estimate of GFR in patients with SCD, especially if GFR is estimated from cystatin C.29
Conclusions
Evaluation of renal function is essential in patients with SCD because of the need to avoid excessive dosages of drugs, such as analgesics and nephrotoxic antibiotics that may cause toxic side effects or aggravate renal dysfunction. The unreliability of the ubiquitously used creatinine makes the assessment of GFR in patients with SCD difficult in clinical practice. As far as the authors are aware, this is the first study that evaluates the correlation of cystatin C with routine clinical measures and different methods of calculating creatinine clearance. Our study suggests that, although the different methods for estimating GFR are comparable, estimates derived from cystatin C could replace creatinine based methods for routine assessment of renal function in patients with SCD. cystatin C is not influenced by the non‐renal factors which affect β2‐microglobulin and creatinine.
We conclude that cystatin C is a good marker of renal disease in patients with SCD. Proteinuria is associated with abnormalities of GFR and we recommend routine screening to allow for early detection and intervention.
Acknowledgements
This project was supported by Kuwait University Research grant number MG 033. We thank Ms Hadeel El‐Muzaini, Dr Sunila George, and Ms Reema Matthew for clinical and technical assistance.
Abbreviations
GFR - glomerular filtration rate
MDRD - modification of diet in renal disease
SCD - sickle cell disease
References
- 1.Saborio P, Scheinman J I. Sickle cell nephropathy. J Am Soc Nephrol 199910187–192. [DOI] [PubMed] [Google Scholar]
- 2.Pham P T, Pham P C, Wilkinson A H.et al Renal abnormalities in sickle cell disease. Kidney Int 2000571–8. [DOI] [PubMed] [Google Scholar]
- 3.Ataga K I, Orringer E P. Renal abnormalities in sickle cell disease. Am J Hematol 200063205–211. [DOI] [PubMed] [Google Scholar]
- 4.Aparicio S, Mojiminiyi S, Kay J D.et al Measurement of GFR in patients with sickle cell anaemia: comparison of 51Cr‐EDTA clearance, creatinine clearance, serum β2 microglobulin and plasma creatinine. J Clin Pathol 199043370–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Jong P E, Jong‐van den Berg L T W, Sewrajsingh G S.et al Beta‐2‐microglobulin in sickle cell anemia. Evidence of increased tubular reabsorption. Nephron 198129138–141. [DOI] [PubMed] [Google Scholar]
- 6.Ffrench M, Ffrench P, Remy F.et al Plasma cell proliferation in monoclonal gammopathy: relations with other biologic variables – diagnostic and prognostic significance. Am J Med 19959860–66. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki T M, Pirsch J D, D'Alessandro A M.et al Increased beta 2‐microglobulin (B2M) is useful in the detection of post‐transplant lymphoproliferative disease (PTLD). Clin Transplant 19971129–33. [PubMed] [Google Scholar]
- 8.Jung K, Schulze B D, Sydow K.et al Diagnostic value of low‐molecular mass proteins in serum for detection of reduced glomerular filtration rate. J Clin Chem Clin Biochem 198725499–503. [DOI] [PubMed] [Google Scholar]
- 9.Ayatse J O, Kwan J T C. Relative sensitivity of serum and urine retinol binding protein and alpha 1 microglobulin in the assessment of renal function. Ann Clin Biochem 199128514–516. [DOI] [PubMed] [Google Scholar]
- 10.Grubb A. Diagnostic value of analysis of cystatin C and protein HC in biological fluids. Clin Nephrol 199238S20–S27. [PubMed] [Google Scholar]
- 11.Grubb A, Simonsen O, Sturfelt G.et al Serum concentration of cystatin C, factor D and beta 2‐microglobulin as a measure of glomerular filtration rate. Acta Med Scand 198540499–503. [DOI] [PubMed] [Google Scholar]
- 12.Abrahamson M, Olafsson I, Palsdottir A.et al Structure and expression of the human cystatin C gene. Biochem J 1990268287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kyhse‐Andersen J, Schmidt C, Nordin G.et al Serum cystatin C, determined by a rapid, automated particle‐enhanced turbidimetric method, is a better marker than serum creatinine for glomerular filtration rate. Clin Chem 1994401921–1926. [PubMed] [Google Scholar]
- 14.Newman D J, Thakkar H, Edwards R G.et al Serum cystatin C measured by automated immunoassay: a more sensitive marker of changes in GFR than serum creatinine. Kidney Int 199547312–318. [DOI] [PubMed] [Google Scholar]
- 15.Tenstad O, Roald A B, Grubb A.et al Renal handling of radiolabelled human cystatin C in the rat. Scand J Clin Lab Invest 199656409–414. [DOI] [PubMed] [Google Scholar]
- 16.Filler G, Witt I, Priem F.et al Are cystatin C and beta 2‐microglobulin better markers than serum creatinine for prediction of a normal glomerular filtration rate in paediatric subjects? Clin Chem 1997431077–1078. [PubMed] [Google Scholar]
- 17.Cockcroft D W, Gault M H. Prediction of creatinine clearance from serum creatinine. Nephron 19761631–41. [DOI] [PubMed] [Google Scholar]
- 18.Levey A S, Bosch J P, Lewis J B.et al A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999130461–470. [DOI] [PubMed] [Google Scholar]
- 19.Tan G D, Lewis A V, James T J.et al Clinical usefulness of cystatin C for the estimation of glomerular filtration rate in type 1 diabetes: reproducibility and accuracy compared with standard measures and iohexol clearance. Diabetes Care 2002252004–2009. [DOI] [PubMed] [Google Scholar]
- 20.Bland J M, Altman D G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 19861307–310. [PubMed] [Google Scholar]
- 21.Mojiminiyi O A, Abdella N. Evaluation of cystatin C and beta‐2 microglobulin as markers of renal function in patients with type 2 diabetes mellitus. J Diabetes Complications 200317160–168. [DOI] [PubMed] [Google Scholar]
- 22.Mojiminiyi O A, Marouf R, Abdella N.et al Serum concentration of cystatin C is not affected by cellular proliferation in patients with proliferative haematological disorders. Ann Clin Biochem 200239308–310. [DOI] [PubMed] [Google Scholar]
- 23.Sesso R, Almeida M A, Figueiredo M S.et al Renal dysfunction in patients with sickle cell anemia or sickle cell trait. Braz J Med Biol Res 1998311257–1262. [DOI] [PubMed] [Google Scholar]
- 24.Lin J, Knight E L, Hogan M L.et al A comparison of prediction equations for estimating glomerular filtration rate in adults without kidney disease. J Am Soc Nephrol 2003142573–2580. [DOI] [PubMed] [Google Scholar]
- 25.Ali S A. Milder variant of sickle‐cell disease in Arabs in Kuwait associated with unusually high level of foetal haemoglobin. Br J Haematol 197019613–619. [DOI] [PubMed] [Google Scholar]
- 26.Lamb E J, Wood J, Stowe H J.et al Susceptibility of glomerular filtration rate estimations to variations in creatinine methodology: a study in older patients. Ann Clin Biochem 20054211–18. [DOI] [PubMed] [Google Scholar]
- 27.Perrone R D, Madias N E, Levey A S. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem 1992381933–1953. [PubMed] [Google Scholar]
- 28.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification Part 5. Evaluation of laboratory measurements for clinical assessment of kidney disease. Guideline 4. Estimation of GFR ( www.ihs.gov/generalweb/webapps/sitelink/site.asp?link = http://www.kidney.org/professionals/doqi/kdoqi/toc.htm ), accessed July 2005
- 29.Grubb A, Nyman U, Bjork J.et al Simple cystatin C‐based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan‐Barratt prediction equations for children. Clin Chem 2005511420–1431. [DOI] [PubMed] [Google Scholar]