Abstract
A large proportion of the samples tested in routine diagnostic microbiology laboratory are urine samples. The gold standard is bacterial culture, but a high proportion of samples cultured are negative. Unnecessary testing can be reduced and an improved service provided by an effective screening test. The Sysmex UF‐100 flow cytometer has been developed to count cells and casts accurately in urine samples. Its performance in a screening test was compared with bacterial culture by using 1005 consecutive urine samples, and cut‐off criteria were established. Cut‐off values of 3000 bacteria/μl and 111 WBC/μl provided the best discrimination. Of 1005 samples, 606 (60%) would be cultured. Sixteen samples that were not selected according to these criteria were culture positive. This was considered acceptable for our routine use. The use of a testing algorithm incorporating the Sysmex UF‐100 flow cytometer has improved the quality and efficiency of urine testing within the routine microbiology laboratory.
Urinary tract infection is common within community and hospital populations. About 50% of women state that they have experienced one infection in their lifetime and 27–48% have had recurrent infections.1 Catheter‐associated infections account for 40% of all infections acquired from hospitals.2 Therefore, urine samples constitute a large proportion of the samples tested by a routine microbiology laboratory. The gold standard is bacterial culture, which is based on bacterial counts.3 Although much time and effort are spent on the processing of urine samples by microscopy and culture most samples will yield no growth.3 Methods for selecting urine samples for culture to improve the efficiency of handling these samples in the laboratory were therefore developed. Examples of selective methods include the use of reagent strips and, more recently, the development of automated methods for detection and enumeration of white blood cells (WBC), red blood cells, epithelial cells, casts and micro‐organisms in urine.3 The aim of this study was to evaluate the Sysmex UF‐100 flow cytometer (Sysmex Corporation, Kobe, Japan)4 as a means of replacing routine microscopy and reducing the number of urine samples requiring culture.
Materials and methods
A total of 1005 consecutive urine samples in boric acid were tested in parallel by manual microscopy and routine culture and by the Sysmex UF‐100 flow cytometer from March to April 2004. The urine samples were from hospital and general practice patients and represented all age groups. Manual microscopy for WBC and red blood cells was carried out on a 2‐μl urine sample by using an in‐house calibrated hanging drop method based on the Neubauer chamber counting method.3 Cells were counted within one complete grid reference square of known area and depth and multiplied by a factor of 10 to equate to the number of cells/μl. Results were reported as <30 cells/μl, the absolute number or >1000 cells/μl. Semiquantitative culture was performed on one half of a culture plate of Chromogenic UTI clear agar (Oxoid, Basingstoke, UK) using a standard 2‐μl loop and incubated overnight at 37°C in air. Culture results were reported as no growth, no significant growth (<104 colony‐forming units (cfu)/ml), growth at 104–105 cfu/ml, or >105 cfu/ml. Single organism growth of ⩾104 cfu/ml was identified (Vitek 1, Biomérieux, UK) and antibiotic sensitivities were determined (Vitek 1, or by the Clinical Laboratory Standards Institute method).
The Sysmex UF‐100 flow cytometer is a second‐generation automated urine analyser. Details of the principles of the method have been published previously.5 The analyser can process 100 samples/h and is operated by trained medical laboratory assistants. The manufacturer's cut‐off values for bacteria and WBC were 8000 and 111/μl, respectively, below which urinary tract infection is deemed unlikely and culture not required. Sensitivity, specificity, positive predictive value and negative predictive value for a range of cut‐off values for bacteria and WBC were determined. The number of urine samples that would be recommended for culture at these cut‐off values was also determined. Ethics approval was not required for this study.
Results
Table 1 shows the results of the Sysmex UF‐100 flow cytometer test using a constant WBC count (111/μl) and a variable bacteria count as the cut‐off compared with bacterial culture. Forty‐one (96%) of 427 samples with positive cultures were not flagged for culture by the Sysmex UF‐100 flow cytometer when the manufacturer's cut‐off values were used. At a cut‐off value of 3000/μl for bacteria, the number of false‐negative Sysmex UF‐100 flow cytometer results was 16. The culture results for these false‐negative samples were Escherichia coli (one), coliform (four), Streptococcus spp/Enterococcus spp (four), Staphylococcus spp (one), yeasts (one) and mixed growth (five). The Sysmex UF‐100 flow cytometer flagged the sample containing yeasts and so it would have been cultured routinely. A negative predictive value of 96% allowed for the reporting of negative results without culture being performed. This reduced the turnaround times for these samples from 24 h to same‐day reporting.
Table 1 Comparison of Sysmex UF‐100 flow cytometer and bacterial culture results using variable bacterial counts as the cut‐off values.
| Cut‐off value (number of bacteria/μl) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 8000 | 7000 | 6000 | 5000 | 4 000 | 3 000 | 2 000 | 1 000 | |
| Number of culture positive/Sysmex positive | 265 | 268 | 269 | 275 | 281 | 290 | 297 | 304 |
| Number of culture negative/Sysmex positive | 162 | 176 | 198 | 223 | 263 | 316 | 420 | 591 |
| Number of culture negative/Sysmex negative | 537 | 523 | 501 | 476 | 436 | 383 | 279 | 108 |
| Number of culture positive/Sysmex negative | 41 | 38 | 37 | 31 | 25 | 16 | 9 | 2 |
| Sensitivity | 86 | 87 | 88 | 90 | 92 | 92 | 97 | 99 |
| Specificity | 77 | 75 | 72 | 68 | 62 | 55 | 40 | 15 |
| Positive predictive value | 62 | 60 | 56 | 55 | 52 | 47 | 41 | 34 |
| Negative Predictive value | 93 | 93 | 93 | 94 | 94 | 96 | 97 | 98 |
| Number of urine samples flagged for culture, total (%) | 427 (42) | 444 (44) | 467 (46) | 498 (49) | 544 (54) | 606 (60) | 717 (71) | 895 (89) |
Reducing the WBC cut‐off to 50 or 20 WBC/μl at 3000 bacteria/μl lowered the number of false‐negative Sysmex UF‐100 flow cytometer results by two or four, but increased the number of culture plates to be set up by 21 or 72, respectively. Five (0.5%) samples were not read by the Sysmex UF‐100 flow cytometer and required the use of the manual microscopy method to produce a WBC or red blood cell count. These samples were either very small or turbid. Nearly one third (31.4%) of the samples were culture negative/Sysmex positive. Some reasons for this discrepancy may be antibiotic treatment before sampling, inhibitory substances in the urine and fastidious organisms. Also, samples with pyuria will be Sysmex positive but the pyuria may not necessarily be caused by an infection.
Discussion
Variable cut‐off values for bacteria have been reported for use with the UF‐100 flow cytometer compared with the manufacturer's recommendations (173–8000 bacteria/μl).5,6,7 This study identified cut‐off values for bacteria (3000/μl) and WBC (111/μl) as giving a reasonable balance between an acceptable negative predictive value (96%) and reducing the number of urine samples requiring culture by 40%. This cut‐off is similar to a smaller study by Koken et al6 of urine samples from 260 patients, with a bacteria cut‐off of 3800/μl. Of the 16 false‐negative results in this study, yeast was isolated from one sample that was flagged by the flow cytometer and the remaining 15 had low WBC counts (<40/μl) by microscopy. Of these, five were mixed growths.
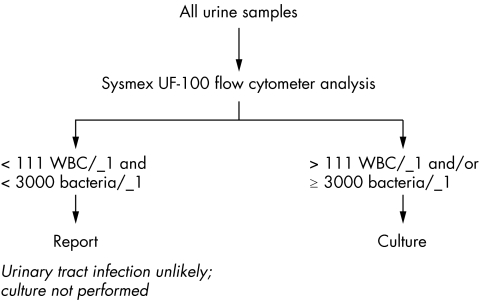
A testing algorithm was established once the appropriate cut‐off values for bacteria and WBC were determined (fig 1). All suprapubic aspirates and ureteric samples are cultured regardless of the bacteria and WBC counts because the presence of any isolate may be considered as clinically relevant.8 Similarly, samples from patients who are potentially neutropenic (haematology/oncology wards) are routinely cultured. Samples from children aged 5 years or under are routinely cultured because of the potential for lasting renal damage associated with urinary tract infection in this age group.9
Figure 1
Culture remains the gold standard for the detection of urinary tract infection in urine samples. The demands of human and financial resources within the microbiology laboratory, however, have meant that alternative methods have had to be introduced. The adoption of this method has improved the efficiency and quality of the service, improved turnaround times in that 40% of the urine reports are issued rapidly and released staff for other tasks.
Acknowledgements
We are grateful to the Biomedical Scientists and medical laboratory assistants of the Bacteriology Laboratory, Raigmore Hospital for their technical expertise during this project.
Abbreviations
WBC - white blood cells
References
- 1.Hooton T M. Pathogenesis of urinary tract infections: an update. J Antimicrobial Chemother 200046S1–S9. [PubMed] [Google Scholar]
- 2.Stamm W E, Norrby S R. Urinary tract infections: disease panorama and challenges. J Infect Dis 2001183S1–S4. [DOI] [PubMed] [Google Scholar]
- 3.Davies E M, Lewis D A. Bacteriology of urine. In: Hawkey P, Lewis DA, eds. Medical bacteriology. Oxford: Oxford University Press, 20041–25.
- 4.Okada H, Sakai Y, Miyazaki S.et al Detection of significant bacteriuria by automated urinalysis using flow cytometry. J Clin Microbiol 2000382870–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manoni F, Valverde S, Antico F.et al Field evaluation of a second‐generation cytometer UF‐100 in diagnosis of acute urinary tract infections in adult patients. Clin Microbiol Infect 20028662–668. [DOI] [PubMed] [Google Scholar]
- 6.Koken T, Aktepe O C, Serteser M.et al Determination of cut‐off values for leucocytes and bacteria for urine flow cytometer (UF‐100) in urinary tract infections. Int Urol Nephrol 200234175–178. [DOI] [PubMed] [Google Scholar]
- 7.Regeniter A, Haenni V, Risch L.et al Urine analysis performed by flow cytometry: reference range determination and comparison to morphological findings, dipstick chemistry and bacterial culture results – a multicenter study. Clin Nephrol 200155384–392. [PubMed] [Google Scholar]
- 8.Graham J C, Galloway A. The laboratory diagnosis of urinary tract infection. J Clin Pathol 200154911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smellie J M, Prescod N P, Shaw P J.et al Childhood reflux and urinary infection: a follow‐up of 10–41 years in 226 adults. Pediatr Nephrol 199812727–736. [DOI] [PubMed] [Google Scholar]