Abstract
Aims
The introduction of clearly defined histopathological criteria for a standardised evaluation of the periprosthetic membrane, which can appear in cases of total joint arthroplasty revision surgery.
Methods
Based on histomorphological criteria, four types of periprosthetic membrane were defined: wear particle induced type (detection of foreign body particles; macrophages and multinucleated giant cells occupy at least 20% of the area; type I); infectious type (granulation tissue with neutrophilic granulocytes, plasma cells and few, if any, wear particles; type II); combined type (aspects of type I and type II occur simultaneously; type III); and indeterminate type (neither criteria for type I nor type II are fulfilled; type IV). The periprosthetic membranes of 370 patients (217 women, 153 men; mean age 67.6 years, mean period until revision surgery 7.4 years) were analysed according to the defined criteria.
Results
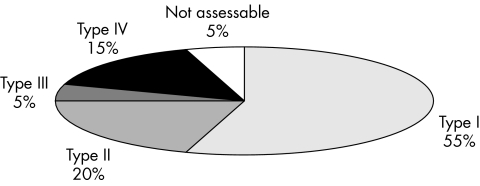
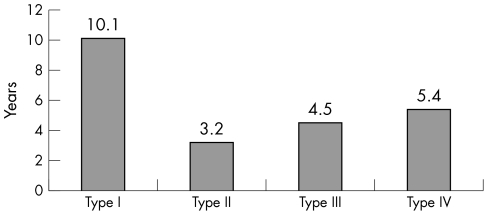
Frequency of histopathological membrane types was: type I 54.3%, type II 19.7%, type III 5.4%, type IV 15.4%, and not assessable 5.1%. The mean period between primary arthroplasty and revision surgery was 10.1 years for type I, 3.2 years for type II, 4.5 years for type III and 5.4 years for type IV. The correlation between histopathological and microbiological diagnosis was high (89.7%), and the inter‐observer reproducibility sufficient (85%).
Conclusion
The classification proposed enables standardised typing of periprosthetic membranes and may serve as a tool for further research on the pathogenesis of the loosening of total joint replacement. The study highlights the importance of non‐infectious, non‐particle induced loosening of prosthetic devices in orthopaedic surgery (membrane type IV), which was observed in 15.4% of patients.
Keywords: total joint replacement, aseptic loosening, septic loosening, classification, histopathology
In the past few decades, total joint replacement (TJA) has become a frequently performed operation, and the number of operations is increasing. Worldwide, about 1.3 million hip endoprostheses are implanted annually.1 In Germany alone, more than 150 000 total hip replacements are carried out each year.2 At the same time, the average lifetime of a hip endoprosthesis has notably increased. In a study of 2000 American patients, a 10 year survival rate of 94% for the prosthesis was reported.1 In German speaking countries, survival rates of 88% and 95% have been found.3,4
A major drawback of TJA is so called "aseptic loosening".5 The aetiology of this condition is blamed on two major factors (a) particles of polyethylene (PE), bone cement, ceramics, or metal resulting from wear (wear particles) stimulate macrophages to produce resorption stimulating factors and thus lead to periprosthetic osteolysis;6,7,8,9,10 or (b) lack of initial stability, which is considered essential for osseointegration.11,12
Apart from aseptic loosening, loosening may be induced by periprosthetic infection (septic loosening), which originates from minimal bacterial contamination during surgery or from bacteria that reach the prosthesis via the bloodstream or lymphatic system. The clinical feature of periprosthetic infection is categorised by the symptoms resulting. If typical symptoms of infectious disease result, it is considered high grade infection. In contrast, low grade infection is considered if only unspecific symptoms, often difficult to distinguish from aseptic loosening, occur.13 Well documented cases of infection due to bacteraemia are reported after tooth extraction.14,15,16
In both aseptic and septic loosening of TJA, a fringe of connective tissue of varying width develops between the bone and prosthesis – the periprosthetic membrane. Even well fixed implants may have these membranes; however they are considerably smaller (0.1–0.3 mm at the shaft of the femoral component, increasing with the survival period, and >1.0 mm at the acetabular component).17,18,19 In each case, concomitant bone defects surrounding the prosthesis appear, ranging from mild forms to wide ranging osteolysis. These defects result from cellular activity and enzymatic processes in the periprosthetic membrane or from micromovement in the interface between bone and prosthesis (fig 1).8,20
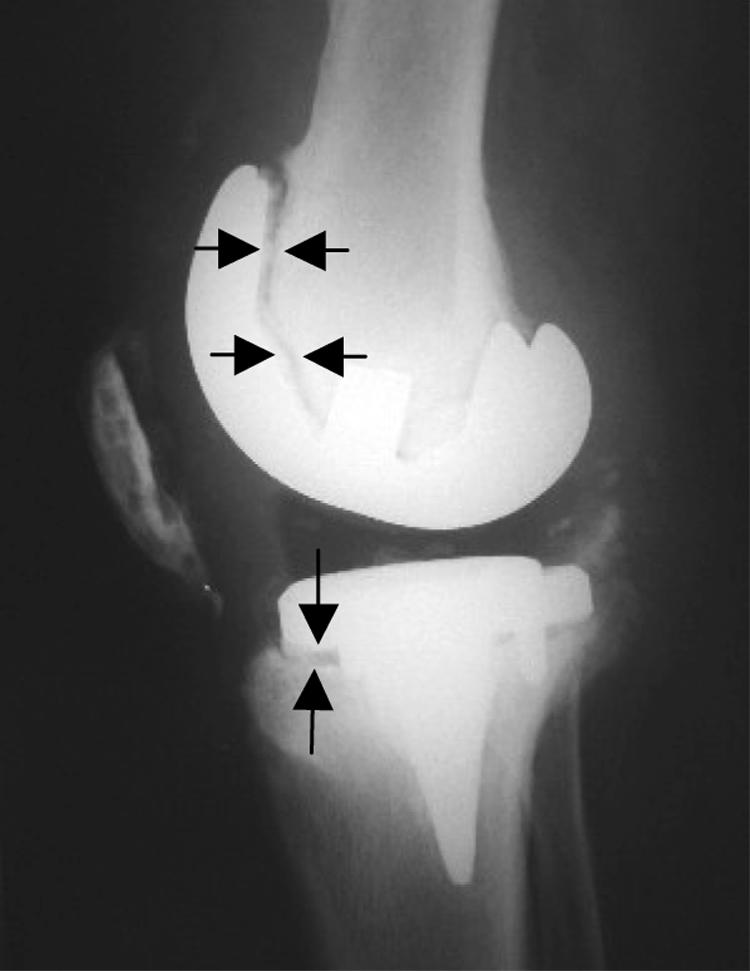
Figure 1 Radiological aspect of a loose knee endoprosthesis with osteolyses in the ventral femoral and tibial areas (arrows). Histological analysis of this case revealed a periprosthetic membrane of the wear particle induced type (type I).
During revision surgery with reimplantation of a new endoprosthesis, the periprosthetic membrane (fig 2) has to be removed. These membranes are valuable specimens for histomorphological analysis and can be submitted easily by the orthopaedic surgeon to the pathologist, especially in revision centres. This periprosthetic membrane is located at the interface between bone and prosthesis or, in the case of cemented TJA, between bone tissue and bone cement. The periprosthetic membrane must not be mistaken for the neocapsule that forms around the joint space. Despite the fact that the neocapsule is not responsible for loosening, it can give useful diagnostic information. Several studies have shown that the new joint space interacts with the periprosthetic space,21,22,23 and it is generally accepted that the changes in histological appearance are very similar in these different tissue specimens in the same patient.13,17
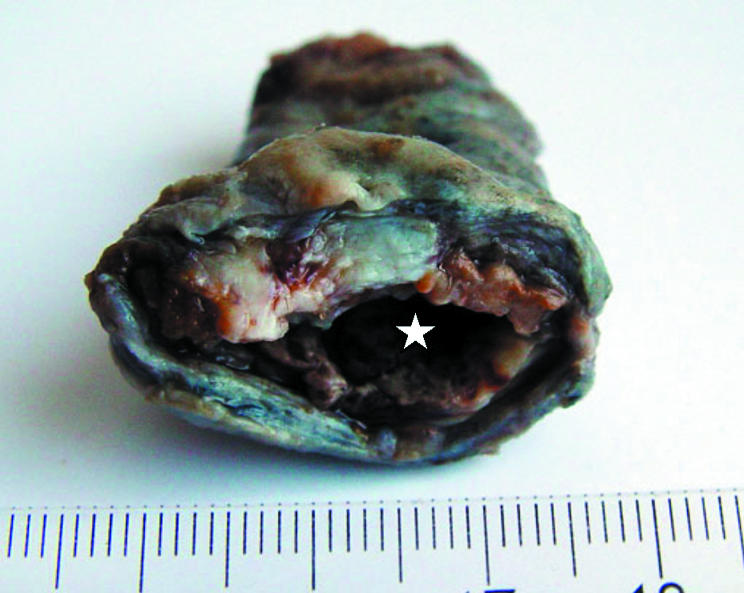
Figure 2 Macroscopic aspect of a bulky periprosthetic membrane (*space of former prosthesis shaft). The histological picture was of the indeterminate type (membrane type IV).
The periprosthetic membrane is marked by a heterogeneous appearance in histopathology. Up to now, histomorphological investigation has usually been carried out in order to confirm or exclude periprosthetic infection. However, in many hospitals specimens are only submitted for investigation if there is suspicion of infection. Because the symptoms of low grade infection are often non‐specific, this condition is often misinterpreted as aseptic loosening. Wear particle disease is often used synonymously for this condition, suggesting that aseptic loosening refers exclusively to wear particles. In our own investigation, we found a group of periprosthetic membranes that showed no signs of either infection or foreign body reaction. Even though this phenomenon was described as long ago as 1983, it has rarely been taken into account.18
As yet there is no histopathological classification system in use that is generally accepted by orthopaedic surgeons and pathologists. The aim of this study was to define histological criteria in order to establish a concise typing of the periprosthetic membrane that would be applicable to the routine investigation of these membranes. The criteria for classification should be non‐ambiguous, simple, and enable a good reproducibility between different pathologists. Apart from facilitating routine histopathological diagnostics, the classification should provide the orthopaedic surgeon with information about the cause of prosthesis loosening, and the classification of tissue samples should serve as a basis for clinical and experimental studies on the aetiology of prosthesis loosening.
MATERIALS AND METHODS
Patients
Informed consent was given by every patient for the usage of surgical specimens in this study. In total, 370 periprosthetic membranes from revision surgery were analysed. Neocapsule tissue was not available for this study. Mean (SD) age of patients was 67.6 (11.0) years (range 22.9 to 92.2), and comprised 58.6% women (n = 217) and 41.4% men (n = 153). The TJA was uncemented in 39.2% of the patients (n = 113) and cemented in 60.8% (n = 175); in 82 cases the status could not be retrospectively identified. The mean (SD) period between primary arthroplasty and revision surgery was 7.4 (6.4) years (range 0.1 to 30.8). Most (79.6%; n = 218) of the patients had undergone total hip replacement, and 20.4% (n = 56) total knee replacement. In 96 cases, no data concerning the location of the prosthesis were available.
The tissue samples were obtained at endoprosthesis revision surgery and immediately fixed in buffered formalin (4%). The samples were embedded in full, so that one paraffin block was obtained from small samples, but from larger samples up to 12 (median 3) paraffin blocks were obtained. Sections of tissue (thickness 5 μm, slide area 30×25 mm) were stained with haematoxylin and eosin. These slides were studied under both normal and polarised light microscopy.
In 65.4% of the cases (n = 242), microbiological investigation including aerobic and anaerobic culture of the tissue specimen was carried out.
Definition of the histological types of periprosthetic membranes
Periprosthetic membrane of the wear particle induced type (type I)
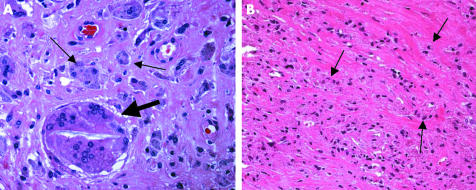
The most important feature of this type of membrane is an infiltration of predominantly macrophages and multinuclear giant cells. PE particles exceeding 5 μm2 of volume are frequently found in multinuclear giant cells (fig 3A), whereas smaller PE particles of ∼2 μm2 are found in macrophages (fig 3B).24 Together, both cell types form >20% of the membrane surface. Lymphocytes may be detected sporadically.
Figure 3 Type I membrane: in (A) a multinucleated giant cell containing a large PE particle (broad arrow) is found together with macrophages (small arrows), whereas in (B) the foreign body reaction is formed exclusively by abundant macrophages (arrows) with tiny black, sharp edged, metal, wear particles. Haematoxylin and eosin; original magnification (A) ×400; (B) ×200.
Depending on particle size, polymethyl methacrylate (PMMA) bone cement fragments often show a basophilic clustered botryoid pattern, or appear slightly birefringent if investigated by polarised light. If, during preparation, this particles are moved away from their original position, the original location is represented by void cavities. In order to show PMMA bone cement on radiographs, zirconium dioxide and barium sulphate is added. These particles measure from 0.5 to 2 μm in diameter. Under light microscopy, they appear grey or yellow brown in colour and are ball‐like to oval in shape. Under polarised light, they show slight white birefringence.17
PE particles are often oblong or irregular in shape, with a slight coloured tinge.25 Depending on size, they are highly birefringent if exposed to polarised light.17 Large particles (up to several mm) are represented by large void spaces because they are usually removed by preparation of the tissue sections. Small PE particles (<1 µm in diameter) are not found by the techniques used,26 but can be detected by oil red staining or electron microscopy.27,28
Metal wear particles (titan, iron, cobalt, chrome, molybdenum) appear as tiny black particles occasionally measuring up to 0.1 mm, round or irregular in shape and with sharp edges.17
Polygonal ceramic particles measure from 0.5 to 10 μm in seize and are yellow white to brown with a distinctive dark edge.17 They are rarely found.
The qualities of the wear particles should be routinely recorded in histopathological reports.
Necroses and infiltrates of lymphocytes vary in extent. Wear particles (most frequently PE) are found in varying amounts concomitant to necrotic areas, thus these areas should be investigated for wear particles using polarised light.
Periprosthetic membrane of the infectious type (type II)
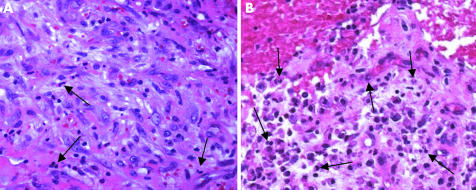
Infection can be either low or high grade.13 Low grade infection is marked by chronic granulation. Activated fibroblasts, proliferation of small blood vessels, oedema, and inflammatory infiltrate of neutrophilic granulocytes characterise this lesion. Plasma cells located close to small lymphocyte aggregates are detected frequently, whereas multinuclear giant cells and macrophages are seen only sparsely (fig 4A). High grade infection is easily identified by an abundance of neutrophilic granulocytes within an oedematous connective tissue (fig 4B).
Figure 4 Type II membrane: in (A), few neutrophilic granulocytes (arrows) are present (low grade infection), whereas in (B) there is an abundance of neutrophils. In both cases, the background contains lymphocytes and plasma cells within granulation tissue with sprouting capillaries and activated fibroblasts (haematoxylin and eosin, original magnification ×400).
Periprosthetic membrane of the combined type (type III)
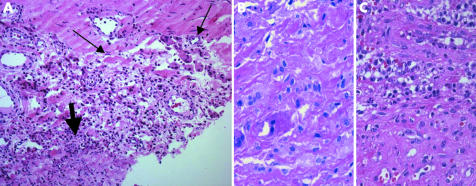
In the combined type, the histological picture is characterised by the combination of the histomorphological changes described for types I and II (fig 5A). These membranes contain areas dominated by wear induced antibody reaction (fig 5B) and areas with inflammatory reaction caused by granulocytes (fig 5C). The distribution of both alterations in the tissue is equal in extent.
Figure 5 Type III membrane. (A) Overview showing the coexistence of wear particle induced (small arrows) and infectious (broad arrow) areas. Details of (B) the wear particle induced area and (C) the infectious area. Haematoxylin and eosin, original magnification (A) ×200; (B, C) ×400.
Periprosthetic membrane of the indeterminate type (type IV)
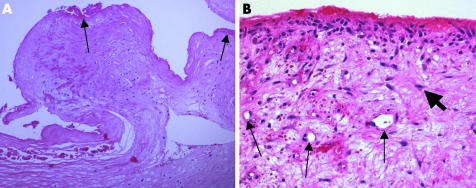
Membranes of the indeterminate type are formed by connective tissue low in cells and rich in collagen fibres. The membrane surface sometimes shows a rim of fibrin (fig 6A) or a synovialis‐like layer of fibroblasts and macrophages (fig 6B). On cursory examination, the latter might be mistaken for a type II membrane, especially if a few neutrophilic granulocytes are present in the surface area. However, granulocytes are lower in number compared with the infectious membrane, and are only found close to the surface.
Figure 6 Type IV membrane. (A) The membrane consists exclusively of a connective tissue with low numbers of fibroblasts/fibrocytes and a fibrin layer on the surface (arrows), whereas in (B) the membrane exhibits granulation tissue with capillaries (small arrows) and activated fibroblasts (broad arrow). In contrast to low grade infections, there are very few neutrophilic granulocytes. Haematoxylin and eosin, original magnification (A) ×200; (B) ×400.
RESULTS
Frequency of histological types
Of 370 samples, 201 periprosthetic membranes (54.3%) were of the wear particle induced type, 19.7% (73 of 370) the infectious type, 5.4% (20 of 370) the combined type, and 15.4% (57 of 370) the indeterminate type. In 5.1% of cases (n = 19), the classification was impossible because the amount of tissue was insufficient, or the origin of the specimen was unclear or definitely incorrect. Some of these samples consisted of organised fibrin only (table 1).
Figure 7 Percentage of histopathological types of samples, n = 370.
Reliability of the classification
Two different researchers, unaware of the clinical diagnosis, examined 294 cases and agreed in 256 cases, thus reaching an inter‐observer reproducibility of 87.1%. A third researcher examined 220 cases, and the inter‐observer reproducibility between these three pathologists was 85.0%. Another two observers examined 50 of these cases, coming to an inter‐observer reproducibility of 86.0%. The higher concordance between five pathologists might be explained by the fact that there were fewer uncharacteristic samples among the 50 cases examined than in the whole study. Dissent occurred most frequently between types I and IV or between types II and III.
Prosthesis lifetime and histological type
The mean time between primary arthroplasty and revision surgery differed among the membrane types, being 10.1 years for type 1, 3.2 years for type II, 4.5 years for type III, and 5.4 years for type IV, respectively (table 2). The differences in lifetime in correlation with the histological type was statistically significant (p<0.001, one way analysis of variance).
Figure 8 Mean prosthesis life time for each membrane type.
Type of fixation of artificial joint replacement and histological classification
Membrane of type I (particle associated) was found in 46.7% of uncemented and 65.1% of cemented prostheses. This results in a statistically significant higher frequency of type I membranes in cemented total arthroplasty (p = <0.001, Pearson's χ2). Membranes of type II (infection associated) occurred in about 20% of both cemented and non‐cemented total joint arthroplasty, while those of the combined type (type III) were found in 3.6% of the cemented and 9.3% of the non‐cemented cases. Membranes of the indeterminate type (type IV) were found in 24.3% of uncemented and 10.1% of cemented prostheses, which was statistically significant (p = <0.001).
Microbiological findings and histological classification
Microbiological records were available for 242 cases, of which 80 cases (33.1%) showed bacterial growth in culture media. In 68 cases, the infection was caused by a single pathogen, and in 12 additional cases, the infection was caused by multiple pathogens. Two pathogens were isolated from 10 of these patients, and the remaining 2 cases had three different bacterial species involved. Coagulase negative Staphylococcus epidermidis (n = 22) and other coagulase negative staphylococci (n = 11)) were the most common groups of pathogens (n = 33) followed by S. aureus (n = 15) (table 3).
Table 3 Bacteria found by microbiological investigation in 242 out of 370 patients listed by rank.
| Bacterial species | Absolute no. of cases (n) | Relative no. of cases (%) | Portion of infectious diseases (%) | |||
|---|---|---|---|---|---|---|
| None found | 162 | 66.9 | N/A | |||
| CNS | 33 | 13.6 | 41.3 | |||
| Staphylococcus epidermidis | 22 | 9.1 | 27.5 | |||
| Other than S. epidermidis | 12 | 4.5 | 13.8 | |||
| Staphylococcus aureus | 15 | 6.2 | 18.8 | |||
| More than one species | 12 | 5.0 | 15.0 | |||
| Propionibacterium acnes | 7 | 2.9 | 8.8 | |||
| Streptococcus sp. | 6 | 2.5 | 7.5 | |||
| Enterococcus sp. | 6 | 2.5 | 7.5 | |||
| Yersinia sp. | 1 | 0.4 | 1.3 | |||
| Total | 242 | 100.0 | 100.2 (rounded) |
128 patients were not available for these investigations. N/A, not applicable; CNS, coagulase‐negative staphylococci; sp., species
In 9 of 242 cases investigated microbiologically, the histological assessment was impossible. Out of the remaining 233 cases, the microbiological findings correlated with the classification proposed in 209 (89.7%), assuming that the correlation with detection of pathogens by bacterial culture methods is correct for histological types II and III. In 13 cases, the histological results were false negative compared with the microbiological results; even though bacteria were detected, histological analysis resulted in type I for 7 cases, and in type IV for 6 cases. In 11 cases, the histology was false positive in comparison to the microbiology: of these 11 cases, histological analysis resulted in type II for 5 cases and type III for 6 cases, even though no pathogen was detected by microbiological methods. Statistical analysis using the nonparametric method of Spearman's ρ resulted in a correlation coefficient of 0.767 between the histological and microbiological diagnoses (p<0.001).
DISCUSSION
Most of the samples (94.9%) were suitable for histological classification. Three independent surgical pathologists investigated identical slide sets of the periprosthetic membranes and resulted in the same typing in 85% of the cases using the proposed classification. In 89.7% of the cases, the histopathology was conclusive for microbiological detection of bacteria. Therefore, we believe that this classification system may be suitable for surgical pathologists to apply a standardised diagnostic tool for typing these lesions.
However, a discrepancy between microbiological and histological findings was found in 10.7% of the cases. This fact raises the question as to whether microbiological culture methods or histological examination are more valid with respect to sensitivity and specificity. In microbiology, false negative findings may result from several factors such as inappropriate incubation time, inappropriate choice of media, and previous antimicrobial therapy given to the patient. False positive detection of bacteria may result from bacterial contamination of the sample from human skin flora. Such problems reduce the level of significance of microbiological culture methods, and have been pointed out in several studies.13,29,30,31 In order to avoid this pitfall, histopathological examination may be helpful. It is important that typical morphology leading to the diagnosis of infection is not overlooked, therefore the pathologist must investigate the sample as completely as possible. This investigation has to be performed meticulously to obtain the full context of the histomorphological changes. The histological diagnosis is clinically relevant, because in the case of periprosthetic infection, the success of revision surgery depends to some extent on the choice of replacement strategy (single stage or two stage reconstruction) and on efficient antibiotic treatment for a sufficient length of time.32 Microbiology alone does not succeed in detecting the pathogen in all cases and false positive results are possible due to contamination. The synopsis of histopathological and microbiological findings verifies the suspicion of periprosthetic infection and gives a rationale for further therapy of the patient.13,33 Furthermore, periprosthetic infection may be proven by fresh frozen sections without delay during revision surgery and the surgical strategy may be changed intra‐operatively if necessary.34
Granulocytes are of special diagnostic relevance for these infections. Pandey and coworkers studied 602 patients undergoing revision surgery and found that histological analysis of a periprosthetic infection correlated best with clinical and microbiological results if at least one neutrophilic granulocyte was detected per high power visual field (×400 magnification, with a minimum of 10 fields examined).13 Sensitivity and specificity were very good, at 100% and 97%, respectively. However, it is not quite clear how many fields per case were examined in that study. In a larger sample, 10 neutrophils may be found, even in non‐infectious tissue. These borderline cases might in fact be periprosthetic membranes of the indeterminate type, especially if the lifetime period was short.
This study is the first to describe the frequency of periprosthetic membranes of the indeterminate type (type IV), which amounted to 15.4% of the cases (n = 57). For cemented prostheses, the frequency of 10.1% was significantly lower (p = <0.001) than in non‐cemented prostheses (24.3%), whereas the opposite was true for membrane type I. This leads to the hypothesis that bone cement may contribute to wear particle induced loosening. On the other hand, cemented fixation may protect TJA from primary instability, a potential reason for the emergence of a type IV membrane. If an implant is not properly fitted to the bone at the very beginning, micromovements will prevent the connection between prosthesis and bone tissue. Instability leads to minimal periprosthetic trauma with microfractures of trabecular bone, bone marrow depression, and development of haematoma. The membrane of the indeterminate type may thus represent scar tissue following initial microtrauma. Moreover, physically inadequate implants can lead to inadequate stress impact and thus concentrate weight bearing stress in the distal part of the prosthesis. In animal experiments, it was shown that osteolysis can result from pressure alone without influence of wear particles or infection.35 This type of osteolysis may lead to a type IV membrane. For the diagnosis of a type IV membrane, the periprosthetic tissue has to be investigated in full. If the tissue sample is too small and not representative, areas of wear particle induced or infectious loosening may be overlooked.
Toxicity of the implant material has also been discussed as a reason for prosthesis loosening. For example, a recent in vitro study demonstrated a size dependent effect of titanium particles on osteoblast proliferation and function.36 However, by definition, no foreign body particles are detected in type IV. The abundance and vitality of osteoclasts and osteoblasts are difficult to assess using haematoxylin and eosin stained periprosthetic membranes only, but toxic effects might be another pathogenetic factor in development of the type I membrane. Furthermore, allergic reactions to implant materials have been the subject of discussion. Two recent publications describe a distinct histomorphological picture with perivascular lymphocytic aggregates in cases of loose metal on metal joint replacements.37,38 The authors of these publications state that the prevalence of these reactions is low, but they may represent a novel mode of failure for some metal on metal endoprostheses by a lymphocyte dominated immunological response. In our study, the described histological picture was rarely found, and such cases were classified as type I or type IV, depending on the amount of foreign body particles found.
Periprosthetic membranes classified according to the system proposed have been subject of gene expression analysis, and several differentially expressed genes have been found, discriminating between types I and II.39 Histological validation of gene expression profiles and clinical studies based on these profiles will clarify the biological background of prosthesis loosening and might establish markers for early diagnosis of prosthesis loosening (unpublished data).
To improve the result of histological diagnosis, the surgical pathologist should be provided with additional clinical data, such as the lifetime of the prosthesis, type of fixation, relevant records on clinical pathology, and microbiological findings by the orthopaedic surgeon. To reduce heterogeneity of the tissue samples, these should be completely processed by the pathologist.
CONCLUSION
A classification of periprosthetic tissue (membrane) with regard to its involvement in loosening of TJA is helpful to standardise histopathological diagnosis and necessary to improve further investigation into the pathogenesis of this condition. The suggestion for classification given here divides this condition into four types, which appear to be correlated with clinical and microbiological features with respect to the type of fixation of the prosthesis (cemented or uncemented) and prosthesis lifetime. The type I membrane is dominated by macrophage related alteration of the tissue and shows a strong correlation with wear particles (PE, PMMA, metal). The type II membrane is characterised by an inflammatory reaction dominated by granulocytes and corresponds to periprosthetic infection. The type III membrane consists of a mixture of features as described in type I and II and is associated predominately with periprosthetic infection, but wear particles are also abundant. The type IV membrane differs completely from the other types and is grossly dominated by connective tissue resembling scarring.
The classification proposed gives surgical pathologists a standardised descriptive tool for these lesions, which should have an impact on both research and diagnostics. In particular, the question of whether loosening is due to bacterial infection or not can be answered in most cases.
Of special interest is the type IV membrane, and the aetiology of this lesion remains unclear and speculative. This condition has to be studied intensively for further understanding of the mechanism of loosening of artificial joint replacement.
ACKNOWLEDGEMENTS
We thank G Fernahl and J Karle for their excellent technical support and C Campisi for proofreading. We would like to point out that this classification system is a consensus result. All authors have substantially contributed to this work by participating in discussions and by providing clinically classified tissue samples. The concept of applying the criteria for the distinction of four types of periprosthetic membranes was devised by V Krenn. Results of the inaugural thesis of R‐A Classen are included in this publication. This work was supported by several non‐profit organisations: Gemeinnütziger Verein ENDO‐Klinik e.V., SFB 421 (protective and pathological consequences of antigen processing, subproject Z3, to V Krenn) and Nationale Genomforschungsnetz (NGFN, SIPAGE, subproject S14T25, to V Krenn).
Abbreviations
PE - polyethylene
PMMA - polymethyl methacrylate
TJA - total joint replacement
References
- 1.Berry D J, Harmsen W S, Cabanela M E.et al Twenty‐five‐year survivorship of two thousand consecutive primary Charnley total hip replacements: factors affecting survivorship of acetabular and femoral components. J Bone Joint Surg Am 200284‐A171–177. [DOI] [PubMed] [Google Scholar]
- 2.Katzer A, Loehr J F. Frühlockerung von Hüftgelenkendoprothesen. Dtsch Arztebl 2003100784–790. [Google Scholar]
- 3.Aldinger P R, Breusch S J, Lukoschek M.et al A ten‐ to 15‐year follow‐up of the cementless spotorno stem. J Bone Joint Surg Br 200385209–214. [DOI] [PubMed] [Google Scholar]
- 4.Grubl A, Chiari C, Gruber M.et al Cementless total hip arthroplasty with a tapered, rectangular titanium stem and a threaded cup: a minimum ten‐year follow‐up. J Bone Joint Surg Am 200284‐A425–431. [DOI] [PubMed] [Google Scholar]
- 5.Wirtz D C, Niethard F U. [Etiology, diagnosis and therapy of aseptic hip prosthesis loosening—a status assessment]. Z Orthop Ihre Grenzgeb 1997135270–280. [DOI] [PubMed] [Google Scholar]
- 6.Gehrke T, Sers C, Morawietz L.et al Receptor activator of nuclear factor kappaB ligand is expressed in resident and inflammatory cells in aseptic and septic prosthesis loosening. Scand J Rheumatol 200332287–294. [DOI] [PubMed] [Google Scholar]
- 7.Moro T, Takatori Y, Ishihara K.et al Surface grafting of artificial joints with a biocompatible polymer for preventing periprosthetic osteolysis. Nat Mater 20043829–836. [DOI] [PubMed] [Google Scholar]
- 8.Pap G, Machner A, Rinnert T.et al Development and characteristics of a synovial‐like interface membrane around cemented tibial hemiarthroplasties in a novel rat model of aseptic prosthesis loosening. Arthritis Rheum 200144956–963. [DOI] [PubMed] [Google Scholar]
- 9.Rader Ch P, Baumann B, Rolf O.et al Detection of differentially expressed genes in particle disease using array‐filter analysis. Biomed Tech (Berl) 200247111–116. [DOI] [PubMed] [Google Scholar]
- 10.Sabokbar A, Fujikawa Y, Neale S.et al Human arthroplasty derived macrophages differentiate into osteoclastic bone resorbing cells. Ann Rheum Dis 199756414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albrektsson T, Albrektsson B. Osseointegration of bone implants. A review of an alternative mode of fixation. Acta Orthop Scand 198758567–577. [DOI] [PubMed] [Google Scholar]
- 12.Krismer M, Stockl B, Fischer M.et al Early migration predicts late aseptic failure of hip sockets. J Bone Joint Surg Br 199678422–426. [PubMed] [Google Scholar]
- 13.Pandey R, Drakoulakis E, Athanasou N A. An assessment of the histological criteria used to diagnose infection in hip revision arthroplasty tissues. J Clin Pathol 199952118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burton D S, Schurman D J. Hematogenous infection in bilateral total hip arthroplasty. Case report. J Bone Joint Surg Am 1975571004–1005. [PubMed] [Google Scholar]
- 15.Jellicoe P A, Cohen A, Campbell P. Haemophilus parainfluenzae complicating total hip arthroplasty: a rapid failure. J Arthroplasty 200217114–116. [DOI] [PubMed] [Google Scholar]
- 16.Ortega‐Andreu M, Rodriguez‐Merchan E C, Aguera‐Gavalda M. Brucellosis as a cause of septic loosening of total hip arthroplasty. J Arthroplasty 200217384–387. [DOI] [PubMed] [Google Scholar]
- 17.Bos I. [Tissue reactions around loosened hip joint endoprostheses. A histological study of secondary capsules and interface membranes]. Orthopade 200130881–889. [DOI] [PubMed] [Google Scholar]
- 18.Goldring S R, Schiller A L, Roelke M.et al The synovial‐like membrane at the bone‐cement interface in loose total hip replacements and its proposed role in bone lysis. J Bone Joint Surg Am 198365575–584. [PubMed] [Google Scholar]
- 19.Hahn M, Vogel M, Schultz C.et al [Histologic reactions of the bone‐implant zone and cortical bone area after long‐term hip replacement]. Chirurg 199263958–963. [PubMed] [Google Scholar]
- 20.Itonaga I, Sabokbar A, Murray D W.et al Effect of osteoprotegerin and osteoprotegerin ligand on osteoclast formation by arthroplasty membrane derived macrophages. Ann Rheum Dis 20005926–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bobyn J D, Jacobs J J, Tanzer M.et al The susceptibility of smooth implant surfaces to periimplant fibrosis and migration of polyethylene wear debris. Clin Orthop 199521–39. [PubMed]
- 22.Surace M F, Berzins A, Urban R M.et al Coventry Award paper. Backsurface wear and deformation in polyethylene tibial inserts retrieved postmortem. Clin Orthop 200214–23. [DOI] [PubMed]
- 23.Urban R M, Jacobs J J, Gilbert J L.et al Migration of corrosion products from modular hip prostheses. Particle microanalysis and histopathological findings. J Bone Joint Surg Am 1994761345–1359. [DOI] [PubMed] [Google Scholar]
- 24.von Knoch M, Buchhorn G, von Knoch F.et al Intracellular measurement of polyethylene particles. A histomorphometric study. Arch Orthop Trauma Surg 2001121399–402. [DOI] [PubMed] [Google Scholar]
- 25.Willert H G, Broback L G, Buchhorn G H.et al Crevice corrosion of cemented titanium alloy stems in total hip replacements. Clin Orthop 199651–75. [PubMed]
- 26.Hirakawa K, Bauer T W, Stulberg B N.et al Comparison and quantitation of wear debris of failed total hip and total knee arthroplasty. J Biomed Mater Res 199631257–263. [DOI] [PubMed] [Google Scholar]
- 27.Hansen T, Otto M, Buchhorn G H.et al New aspects in the histological examination of polyethylene wear particles in failed total joint replacements. Acta Histochem 2002104263–269. [DOI] [PubMed] [Google Scholar]
- 28.Schmalzried T P, Jasty M, Rosenberg A.et al Histologic identification of polyethylene wear debris using Oil Red O stain. J Appl Biomater 19934119–125. [DOI] [PubMed] [Google Scholar]
- 29.Peersman G, Laskin R, Davis J.et al Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop 200115–23. [PubMed]
- 30.Salmela P M, Hirn M Y, Vuento R E. The real contamination of femoral head allografts washed with pulse lavage. Acta Orthop Scand 200273317–320. [DOI] [PubMed] [Google Scholar]
- 31.von Eiff C, Bettin D, Proctor R A.et al Recovery of small colony variants of Staphylococcus aureus following gentamicin bead placement for osteomyelitis. Clin Infect Dis 1997251250–1251. [DOI] [PubMed] [Google Scholar]
- 32.Zimmerli W. [Role of antibiotics in the treatment of infected joint prosthesis]. Orthopade 199524308–313. [PubMed] [Google Scholar]
- 33.Virolainen P, Lahteenmaki H, Hiltunen A.et al The reliability of diagnosis of infection during revision arthroplasties. Scand J Surg 200291178–181. [DOI] [PubMed] [Google Scholar]
- 34.Feldman D S, Lonner J H, Desai P.et al The role of intraoperative frozen sections in revision total joint arthroplasty. J Bone Joint Surg Am 1995771807–1813. [DOI] [PubMed] [Google Scholar]
- 35.Skripitz R, Aspenberg P. Pressure‐induced periprosthetic osteolysis: a rat model. J Orthop Res 200018481–484. [DOI] [PubMed] [Google Scholar]
- 36.O'Connor D T, Choi M G, Kwon S Y.et al New insight into the mechanism of hip prosthesis loosening: effect of titanium debris size on osteoblast function. J Orthop Res 200422229–236. [DOI] [PubMed] [Google Scholar]
- 37.Davies A P, Willert H G, Campbell P A.et al An unusual lymphocytic perivascular infiltration in tissues around contemporary metal‐on‐metal joint replacements. J Bone Joint Surg Am 20058718–27. [DOI] [PubMed] [Google Scholar]
- 38.Willert H G, Buchhorn G H, Fayyazi A.et al Metal‐on‐metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am 20058728–36. [DOI] [PubMed] [Google Scholar]
- 39.Morawietz L, Gehrke T, Frommelt L.et al Differential gene expression in the periprosthetic membrane: lubricin as a new possible pathogenetic factor in prosthesis loosening. Virchows Arch 200344357–66. [DOI] [PubMed] [Google Scholar]