Abstract
Aim
To evaluate the sources of fetal haemoglobin (HbF) as an indicator in cancer. An immunohistochemical study was carried out on some of the most common kinds of cancer. All of these cancers had serologically high levels of HbF as evaluated previously.
Methods
Immunoaffinity‐purified anti‐HbF was immunohistochemically used to study F cell distribution in the following cancers: colorectal adenocarcinoma, urinary bladder transitional cell carcinoma, brain tumours, lung carcinoma, breast adenocarcinoma, leukaemia, Burkitt's lymphoma and endometrial carcinoma.
Results
In colorectal adenocarcinoma, HbF‐containing red blood cells (FRBC) were present within thin‐walled vessels or were disposed in dense clusters within the tumour. Some of these cells were nucleated or binucleated HbF‐erythroblasts or HbF‐normoblasts (FNBS). In two cases, high levels of mitoses within HbF‐erythroblasts were observed. In half of the cases with transitional cell carcinoma of the urinary bladder, regional intratumoral blood vessels were found to contain 5–50% FRBC. In the other tumours examined, F cells were not observed. FRBCs, however, were occasionally observed in the regional lymph nodes of some of these cancers.
Conclusions
The evaluation of HbF as a potential plasma marker is suggested by the high concentration of FRBCs in colorectal tumours. The apparent development of FRBCs in colorectal tumour tissues is an interesting observation, as these cells were previously thought to develop in medullary or lymphoid tissues. It is thus suggested that the colonic microenvironment may stimulate extramedullary fetal‐type haematopoiesis.
Fetal haemoglobin (HbF) is recognised as an indicator of haematological disorders,1 as well as of malignant diseases.2,3,4 Unlike most tumour markers, HbF is not a direct product of tumour cells. Its production and accumulation in the blood cells is induced by conditions of malignancy. Increased HbF synthesis in adults is suggested to relate to different pathways in the activation of the γ‐globin gene, as, for instance, DNA demethylation5 or the involvement of cyclic guanosine monophosphate protein kinase.6 In parallel, various growth factors are shown to induce HbF production by adult erythroid progenitors in tissue cultures.7,8,9 To our knowledge, however, the mechanism of growth factors in the activation of the γ‐ globin gene is not yet clear. Concentrations of some HbF‐inducing growth factors are raised in the serum of patients with cancer, providing an explanation for increased concentrations of HbF. For instance, in patients with colorectal cancer, concentrations of two of these growth factors, stem cell growth factor and interleukin‐3, were raised compared with those in healthy controls.10 Other investigators11 have found an inducer (growth factor) for HbF, at a site of fetal haemoglobin‐erythroblast development, in the bone marrow of patients with myelodysplastic syndrome.
HbF is usually measured in erythrocytes of circulating blood. In some malignancies, including germ cell tumours,12 trophoblastic diseases12 and haematological malignancies,12,13 however, large amounts of various blood cells with HbF have been immunohistochemically shown in fine sections of tissue. These cells have been identified as erythrocytes (FRBC), erythroblasts (FNBC) and myeloid cells (FMLC). Such F cells could be the source of HbF detected in the plasma of these patients.4 Here, we examined this hypothesis, extending investigations to some of the most common kinds of cancer previously noted by serological measurement to have raised HbF concentration. The presence of those F cells in the tissues of these patients may help to determine the mechanism of their development in adults during conditions indicative of disease.
Materials and methods
Participants
The East London and City Health Authority Research Ethics Committee approved the programme of research, including studies on archival and stored clinical materials.
Histological specimens from patients were selected from the Archive of the Department of Histopathology, Royal London Hospital, UK. They included specimens of the following diseases: colorectal adenocarcinoma (n = 24), transitional cell carcinoma of the urinary bladder (TCC; n = 17), brain tumours (n = 17), lung cancer (n = 20), breast cancer (n = 14), leukaemia (n = 10), Burkitt's lymphoma (n = 1) and endometrial carcinoma (n = 1). In some of these diseases, local lymph nodes were studied in addition to the tumour tissues. Colorectal specimens from patients with non‐malignant diseases were used as controls.
Immunohistochemical staining
We used the peroxidase‐labelled avidin–biotin method, with an affinity‐purified anti‐HbF.12 All reagents used as well as procedure of staining were as in our previous work.12
Results
Colorectal tumours
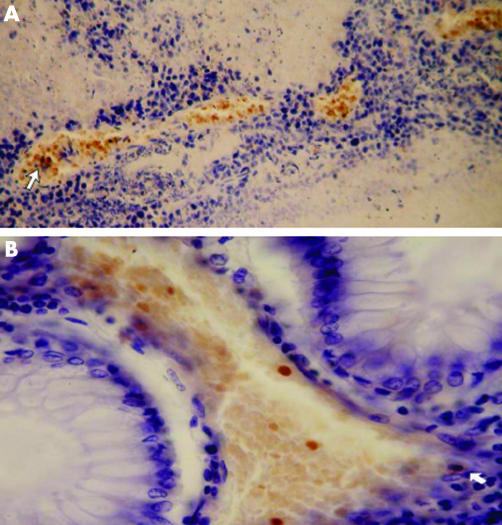
Table 1 summarises the immunohistochemical analyses of the tumour and lymph node specimens in 24 patients. Positive HbF cells were found in the tumour tissues of 10 patients (table 1, patient numbers 1–10). In three other patients HbF cells were found only in the local lymph nodes (table 1, patient numbers 11–13). In the tumour tissues, FRBC were found in groups of 2–4 adjacent capillaries, or small blood vessels, or forming large dense clusters where they generally constituted 100% of the blood cells (figs 1, 2). The bulk of erythrocytes in the large blood vessels, however, was usually without HbF. One group of small blood vessels containing FRBC could be found near another group of small vessels containing only normal RBC, implying that, to some extent, the circulation of FRBC was separated from that of the normal RBC.
Table 1 Distribution of F cells in patients with colorectal tumours.
| Patient no | Sex | Year of birth | Type of tumour | Site | Involvement of the lymph | Vascular invasion | Immunohistochemistry | ||
|---|---|---|---|---|---|---|---|---|---|
| In tumour | FRBC and FMLC in lymph nodes | ||||||||
| FRBC | FNBS | ||||||||
| 1 | Male | 1935 | mdac | Sigmoid colon | (−) | (+) | (+) as large dense concentrations | (+) in mitosis (fig 3A) | Not checked |
| 2 | Male | 1926 | mdac | Sigmoid colon | (+) | (−) | (+) as large dense concentrations(fig 2A) | (+) | (+) |
| 3 | Female | 1931 | mdac | Sigmoid colon | (+) | (−) | (+) as large dense concentrations (fig 1B) | (+) some binucleated | (−) |
| 4 | Female | 1936 | mdac | Sigmoid colon | (?) | (−) | (+) as large dense concentrations | (+) | (−) |
| 5 | Male | 1946 | mdac | Rectum | (+) | (+) | (+) as large dense concentrations (fig 2B) | (+) in mitosis, some binucleated | (−) |
| 6 | Male | 1939 | mdac | Sigmoid colon | (?) | (−) | (+) congestion of mixed | FRBC and FNBS | Not checked |
| 7 | Female | 1924 | mdac | Sigmoid colon | (−) | (−) | (+) as large dense concentrations | (+)some binucleated | (−) |
| 8 | Female | 1924 | wdac | Rectum | (?) | (+) | (+) as large dense concentrations, also in large blood vessels (10%) | (+) | (−) |
| 9 | Female | 1911 | mdac | Rectal colon | (−) | (−) | (+) loosely arranged | (+) | Not checked |
| 10 | Female | 1921 | colonic and ovarian adenocarcinoma | Rectum, colon | (+) | (−) | (+) as large dense concentrations | (+) | (−) |
| 11 | Female | 1934 | mdac | Rectal colon | (+) | (−) | (−) | (−) | (+) |
| 12 | Male | 1934 | mdac | Rectal colon | (+) | (−) | (−) | (−) | (+) |
| 13 | Male | 1930 | mdac | Rectum | (+) | (−) | (−) | (−) | (+) |
| 14 | Female | 1926 | mdac | Rectal colon | (−) | (−) | (−) | (−) | Not checked |
| 15 | Male | 1929 | mdac | Rectal colon | (+) | (−) | (−) | (−) | Not checked |
| 16 | Male | 1973 | mdac | Sigmoid colon | (−) | (+) | (−) | (−) | (−) |
| 17 | Male | 1926 | mdac | Rectal colon | (−) | (−) | (−) | (−) | Not checked |
| 18 | Female | 1926 | mdac | Sigmoid colon | ? | (−) | (−) | (−) | Not checked |
| 19 | Female | 1935 | invac | Rectum | (+) | (−) | (−) | (−) | Not checked |
| 20 | Male | 1926 | invac | Rectum | (+) | (−) | (−) | (−) | Not checked |
| 21 | Male | 1926 | invac | colon | (+) | (−) | (−) | (−) | Not checked |
| 22 | Male | 1917 | mdac | Rectal colon | (−) | (−) | (−) | (−) | (−) |
| 23 | Male | 1939 | wdac | Rectum | (+) | (−) | (−) | (−) | (−) |
| 24 | Male | 1916 | pdac | Rectum | (−) | (−) | (−) | (−) | Not checked |
invac, invasive adenocarcinoma; mdac, mildly differentiated adenocarcinoma; pdac, partially differentiated adenocarcinoma; wdac, widely differentiated adenocarcinoma.
F cell, fetal haemoglobin‐blood cells; FMLC, fetal haemoglobin‐myeloid cells; FNBS, fetal haemoglobin‐normoblasts; FRBC, fetal haemoglobin‐red blood cells.
(+), present, (−), absent, (?), not known.
Figure 1 (A) Four adjacent clusters of immunostained (orange) fetal haemoglobin‐red blood cells (FRBC) in adenocarcinoma of the colon. The arrow indicates the few fetal haemoglobin‐normoblasts (FNBS) among the FRBC‐s. (B) A cluster of immunostained FRBC‐s in adenocarcinoma of the colon. The arrow indicates binucleated FNBS.
Figure 2 (A) Immunostained fetal haemoglobin‐red blood cells (FRBC) as a free cluster and inside blood capillaries in adenocarcinoma of the colon. (B) Immunostained FRBCs within a small blood vessel of adenocarcinoma of the rectum. The arrow indicates two F erythroblasts with small nuclei at a late developmental stage.
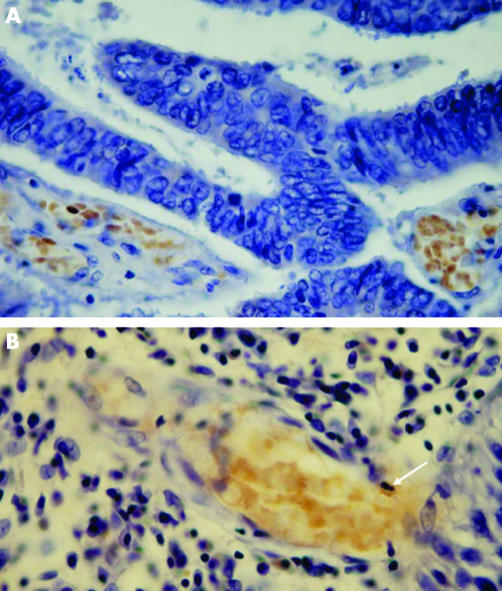
In many cases, FNBS were also found among FRBC (figs 1 and 2) or separately. In two cases (table 1, patient numbers 1 and 5), we observed foci of FNBS, partially in mitosis (fig 3A).
Figure 3 (A) Focus of immunostained fetal haemoglobin‐normoblasts (FNBS) in adenocarcimona of the colon. The three upper FNBS are in mitosis. (B) Immunostained fetal haemoglobin‐myeloid cells (FMLC) as segmented (middle) and juvenile (above and below) cells in a local lymph node of infiltrating ductal carcinoma of the breast. The arrow indicates a normal, unstained segmented myeloid cell.
In the local lymph nodes, there were only small foci of FRBC among the normal lymphocytes.
In 11 of the 24 patients, no cells with HbF were found in the tumour samples. Four of these samples were small biopsy specimens in which the size of the tissue could have been inadequate for detecting F cells. Table 1 shows an inverse correlation between tumours and lymph nodes with regard to the presence of F cells (FRBC or FNBS). Six cases (table 1, patient numbers 3, 4, 5, 7, 8 and 10) were F cell positive in the tumours but F cell negative in the lymph nodes and, inversely, three cases (table 1, patient numbers 11, 12 and 13) were negative in the tumours and positive in the lymph nodes. It should be interesting, in the future, to verify this relationship statistically by using a larger number of samples and to examine its connection with the cancer stage.
We found no difference between an F cell‐positive and an F cell‐negative patient, with regard to sex or age.
Nine control samples comprised three normal colon tissues and six other non‐malignant, neoplastic colon tissues, of which three were dysplastic, non‐invasive rectal tissues and three were non‐invasive tubular adenomas of the colon. All colorectal controls showed no F cells.
TCC
Of the 17 patients in the group with TCC, FRBCs were detected in eight patients in part of the main, large blood vessels comprising 5–50% of the red cells. No F cells were observed in the other nine patients.
Brain tumours
This group comprised 17 patients with various kinds of brain tumours (lymphoma, n = 6; metastatic carcinoma, n = 5; oligoblastoma, n = 2; oligoastrocytoma, n = 2; oligodendroglioma, n = 1; glioma, n = 1), most of whom were without F cells. Only one case of oligodendroglioma showed less than 1% of FRBC in one large vessel (four other cases of oligodendroglioma were F cell negative). We observed a few FRBCs distributed as single cells in the tumour tissue in all five patients with metastatic carcinoma.
Lung cancer
Of the 20 samples of squamous cell carcinoma or small‐cell carcinoma of the lung, only one sample showed 30% of FRBC in the large blood vessels. In all other patients, tumour tissues were without FRBC. Local lymph nodes were examined in five of these patients. Three patients had FRBC distributed within the lymphoid tissue, similar to the patterns seen in lymph nodes of colorectal tumours.
Breast cancer
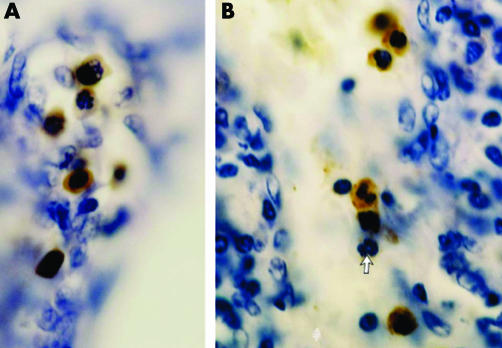
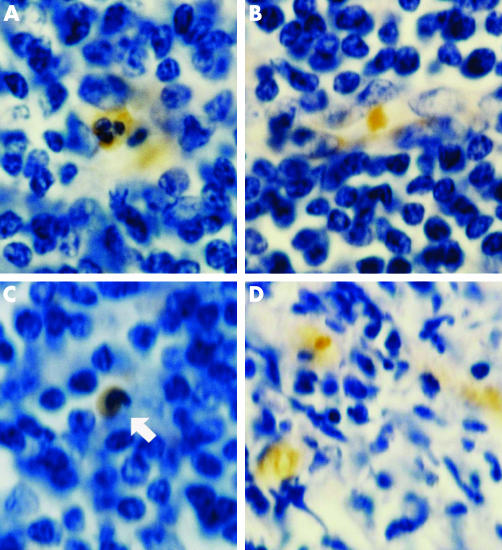
None of the 14 patients with breast cancer had F cells in the tumour tissues. Local lymph nodes were examined in three patients with infiltrating ductal carcinoma, two of which had high concentrations of F cells, including FMLCs (figs 3B, 4A,C) and FRBCs (figs 4A,B). HbF cells were distributed in the lymphoid tissue as single cells or in groups of 2–3 cells. FMLC included all developmental stages (figs 3B, 4A,C), including juvenile, band and segmented cells. In one of these lymph nodes we also observed FRBC in the blood vessels, ranging from 0 to 50% of erythrocytes, and small dense clusters of FRBC in lymphoid tissue similar to those in Burkitt's lymphoma (fig 4D).
Figure 4 (A) Immunostained segmented fetal haemoglobin‐myeloid cells (FMLC) and fetal haemoglobin‐red blood cells (FRBC) in a local lymph node of infiltrating ductal breast carcinoma. (B) Immunostained FRBC in a local lymph node of infiltrating ductal breast carcinoma. (C) the arrow indicates the immunostained band FMLC in a local lymph node of infiltrating ductal breast carcinoma. (D) Foci of immunostained FRBC in a lymph node of Burkitt's lymphoma.
Leukaemia
None of the bone marrow specimens from 10 patients with chronic myeloid leukaemia (n = 6), acute lymphoblastic leukaemia (n = 3) and hairy cell leukaemia (n = 1) had F cells.
In one patient with Burkitt's lymphoma, we observed many foci of densely disposed FRBC in the lymph node (fig 4D), although there were no F cells in the blood vessels. The bone marrow of the same patient had no F cells.
In one patient with endometrial carcinoma, 10–20% FRBC were seen in the blood vessels of the tumour.
Discussion
In most tumours examined here no abnormal concentration of HbF cells was seen in the tumour tissues. Large concentrations of HbF cells, however, were observed in TCC and colorectal carcinoma. In TCC, nearly half the tumours showed 5–50% FRBC in large blood vessels. These FRBC seemed to be an integral part of the general circulation, and no progenitor erythroblasts were evident. The presence of FRBC‐s in TCC would be consistent with induction of HbF production under conditions of malignancy, affecting bone marrow progenitors. In contrast, in tumour tissues of colorectal carcinoma (table 1), FRBCs and FNBSs were not dispersed in the circulation among the normal RBCs. They were, however, separately clustered in small blood vessels or freely in the tumour tissue. This suggests the concomitant existence of two unmixed blood systems: one of FRBCs and another (the principal) of normal RBCs. Together with the finding of FNBS at different stages (figs 1, 2, 4), this supports the hypothesis that development of FRBC has taken place in tumour tissues. Previous works have shown that, under conditions of malignancy, some extramedullary organs such as liver14,15 or spleen12,14,16 may regain their fetal haematopoietic trait, producing HbF‐blood cells. The gut is not generally recognised as an organ of haematopoiesis during mammalian embryo development, as are extraembryonic yolk sac17,18,19,20 and embryonic liver.18,19,20 Developmental studies in the mouse18 identified another important intraembryonic haematopoietic region, designated PAS/AGM, only in the past decade. This region starts functioning at 7.5 days of gestation,20 shortly after the yolk sac and before the liver. At this early developmental stage, the PAS region includes the endoderm of the prospective gut with its associated mesodermal layer.18 In later developmental stages at days 10–13 of gestation, haematopoietic progenitors are found in the embryonic gut,20 which hypothetically may regain that trait in the adult, under conditions of malignancy, consistent with our current data, analogous to hematopoiesis in adult liver and spleen.12,14,15,16
Although most RBCs in the colorectal tumour tissues had only normal adult haemoglobin, a number of large concentrations of FRBCs were seen. In this respect, it is interesting to consider whether these FRBCs are a byproduct induced by the conditions of malignancy or whether they also support tumour tissue for improved oxygen supply, as they do in fetal tissues during gestation.21
With regard to lymphoid tissues, we found clusters of FRBCs in the single case of Burkitt's lymphoma. In patients with lung and breast cancer, F cells were found mainly in the local lymph nodes, which could be the source of the HbF found in the plasma of such patients.4
In the lymph nodes of patients with breast cancer (infiltrating ductal carcinoma), in parallel to the normal myeloid lineage, we showed a lineage of FMLCs at successive stages. We reported the same picture in the deciduos of persistent trophoblastic diseases.12 Although the potential of neoplastic myeloid cells to produce HbF was reported previously,22 we were the first to show its presence in the tissues of patients with non‐haematological malignancy.
Although HbF is an established marker of leukaemia,23 we were surprised that there were no F cells in the bone marrow of any of the 10 patients with leukaemia. Similar negative results were found previously in 10 patients with hairy cell leukaemia in whom F cells were found in the spleen but not in the bone marrow,12 suggesting that the development of F cells in patients with leukaemia takes place in lymphoid tissues rather than in the bone marrow.
In conclusion, our data suggest that an oncofetal genetic reactivation may create a microenvironment with a direct role in the generation of FRBCs in colorectal carcinoma, and that lymphoid tissues draining tumour sites may also act as a similar microenvironment. These studies provide a rationale for the use of HbF as an oncofetal marker and some insight into the mechanism by which HbF may be directly generated by tumours.
Take‐home messages
Firm evidence was obtained suggesting the stimulation of extramedullary fetal‐type haematopoiesis within colorectal tumour tissues.
In analogy to liver and spleen, under malignant conditions, the gut may regain its fetal haematopoietic trait by producing fetal haemoglobin (HbF) as an oncofetal tumour marker.
Foci of HbF‐blood cells were identified in the lymphoid tissues draining tumour sites. As in colorectal carcinoma, these lymphoid tissues may stimulate fetal‐type haematopoiesis.
Acknowledgements
We thank C Nickols and A Brown from the Unit of Experimental Pathology at the Royal London Hospital for their excellent methodological and technical assistance, and S Heather from the Laboratory of Entomology in the Israel Ministry of Health, Jerusalem, for help in preparing the manuscript. This work was partially supported by a travel grant to MW from the Leo Baeck (London) lodge, B'nai‐B'rith.
Abbreviations
F cells - all kinds of blood cells with fetal haemoglobin
FMLC - fetal haemoglobin‐myeloid cells
FNBS - fetal haemoglobin‐normoblasts
FRBC - fetal haemoglobin‐red blood cells
HbF - fetal haemoglobin
RBC - red blood cells
TCC - transitional cell carcinoma of the urinary tract
Footnotes
This work is dedicated to the memory of our colleague and teacher, the late Dr George Brufman from Hadassah University Hospital, Jerusalem, with whom we collaborated in the research on HbF in cancer for more than a decade.
References
- 1.Bertles J F. Human fetal hemoglobin: significance in diseases. Ann N Y Acad Sci 1974241638–652. [DOI] [PubMed] [Google Scholar]
- 2.Chudwin D S, Rucknagel D L, Scholnick A P. Fetal hemoglobin and α‐fetoprotein in various malignancies. Acta Haematol 197758288–293. [DOI] [PubMed] [Google Scholar]
- 3.Wolk M, Kieselstein M, Gera Ben‐Dor C.et al Fetal hemoglobin screening in whole blood and in plasma of cancer patients. Tumor Biol 19911245–51. [DOI] [PubMed] [Google Scholar]
- 4.Wolk M, Newland A C, De la Salle B.et al Refinement of plasma fetal hemoglobin (HbF) measurements, as related to whole blood HbF, in cancer patients. J Tumor Marker Oncol 199914115–126. [Google Scholar]
- 5.Lavelle D E. The molecular mechanism of fetal hemoglobin. Semin Hematol 200441(Suppl 6)3–10. [DOI] [PubMed] [Google Scholar]
- 6.Ikuta T, Ausenda S, Cappellini M D. Mechanism for fetal globin gene expression: role of the soluble guanylate cyclase‐cGMP‐dependent protein kinase pathway. Proc Natl Acad Sci USA 2001981847–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohmer R M, Campbell T A, Bianchi D W. Selectively increased growth of fetal hemoglobin‐expressing adult erythroid progenitors after brief treatment of early progenitors with transforming growth factor beta. Blood 2000952967–2974. [PubMed] [Google Scholar]
- 8.Bhanu N V, Trice T A.et al A signaling mechanism for growth related expression of fetal hemoglobin. Blood 20041031929–1933. [DOI] [PubMed] [Google Scholar]
- 9.Wojda U, Leigh K R, Njorge J M.et al Fetal hemoglobin modulation during human erythropoiesis: stem cell factor has “late” effects related to the expression pattern of CD117. Blood 2003101492–497. [DOI] [PubMed] [Google Scholar]
- 10.Mroczko B, Szmitkowski M, Okulczyk B. Hematopoietic growth factors in colorectal cancer patients. Clin Chem Lab Med 200341646–651. [DOI] [PubMed] [Google Scholar]
- 11.Choi J W, Kim Y, Fujino M.et al F blast production correlates with upregulation of inducible nitric oxide synthase in myelodysplastic syndromes. Ann Hematol 200281548–550. [DOI] [PubMed] [Google Scholar]
- 12.Wolk M, Martin J E, Constantin R. Blood cells with fetal haemoglobin (F‐cells) detected by immunohistochemistry as indicators of solid tumors. J Clin Pathol 200457740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi J W, Kim Y, Fujino M.et al Significance of fetal hemoglobin‐containing erythroblasts (F blasts) and the Fblast/Fcell ratio in myelodisplastic syndromes. Leukemia 2002161478–1483. [DOI] [PubMed] [Google Scholar]
- 14.Choi J W, KIM Y et al Hemoglobin F synthesis is not restricted to fetal erythropoietic organs during extramedullary hematopoiesis. Haematologica 200287323–325. [PubMed] [Google Scholar]
- 15.Von Schweinitz D, Schmidt D, Fuchs K.et al Extramedullary hematopoiesis and intratumoral production of cytokines in childhood hepatoblastoma. Pediatric Res 199538555–563. [DOI] [PubMed] [Google Scholar]
- 16.Underwood J C E, Dangerfield V J M. Immunohistochemical identification of adult and fetal haematopoiesis in the spleen in lymphoma, leukaemia and myeloproliferative diseases. J Pathol 198113471–80. [DOI] [PubMed] [Google Scholar]
- 17.McGrath K E, Koniski A D, Malic J.et al Circulation is established in a stepwise pattern in the mammalian embryo. Blood 20031011669–1675. [DOI] [PubMed] [Google Scholar]
- 18.Dzierzak E, Medvinsky A, de Bruijm M. Qualitative and quantitative aspects of haematopoietic cell development in the mammalian embryo. Immunol Today 199819228–236. [DOI] [PubMed] [Google Scholar]
- 19.Palis J, Robertson S, Kennedy M.et al Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development 19991265073–5084. [DOI] [PubMed] [Google Scholar]
- 20.Medvinsky A L, Gan O I, Semenova M L.et al Development of day‐8 colony‐forming unit‐spleen hematopoietic progenitors during early murine embryogenesis: spatial and temporal mapping. Blood 199687557–568. [PubMed] [Google Scholar]
- 21.Carlson B M. The development of the circulatory system. In: Dolinger E, Maisel J, eds. Patten's foundations of embryology. 5th edn. New York: McGraw‐Hill, 1988594
- 22.Privitera E, Schiro R, Longoni D.et al Constitutive expression of GATA‐1, EPOR, α‐globin and γ‐globin genes in myeloid clonogenic cells from juvenile chronic myelocytic leukemia. Blood 199586323–328. [PubMed] [Google Scholar]
- 23.Rautonen J, Siimes M A. Initial blood fetal hemoglobin concentration is elevated and is associated with prognosis in children with acute lymphoid or myeloid leukemia. Blut 19906117–20. [DOI] [PubMed] [Google Scholar]