Abstract
Background and objectives
Restenosis is a complication of interventional procedures such as angioplasty and stenting, often limiting the success of these procedures. Knowledge regarding the relative behaviour of different arteries after these procedures is limited, despite the extensive use of different vascular models. Although the results from studies using different vessels are analysed to predict the behaviour of coronary arteries and other vasculature, direct controlled comparisons between different arteries are necessary for a better understanding of the differential response to restenosis.
Methods
This study examines the response to stenting in coronary and internal iliac arteries as characterised by intimal hyperplasia and restenosis. In a swine model of in‐stent stenosis, coronary arteries exhibited higher levels of intimal hyperplasia and per cent stenosis than internal iliac arteries.
Results
After normalisation for injury score, coronary arteries were found to undergo 47% more intimal hyperplasia (p<0.05), whereas per cent stenosis normalised for injury score tended to be higher (p = 0.01). Other measurements reflecting post‐stenting intimal hyperplasia (maximal intimal thickness, medial area) did not exhibit significant differences between the artery groups.
Conclusions
These results show that coronary vessels are more prone to develop significant intimal hyperplasia and subsequent restenosis than internal iliac vessels. A better insight into how different arteries and arterial components behave is important in understanding and developing newer and better therapeutic measures for restenosis.
The vascular response to angioplasty and stenting leads to changes in the vessel wall, which ultimately limit the success of these procedures.1,2 Many investigators have studied the pathology and potential treatments of restenosis in vessels other than coronary arteries.3,4 It has been clearly established, however, that there are inherent morphological differences between arteries, and results from previous studies have shown that coronary and peripheral arteries have unique responses to similar invasive surgical interventions. Some studies show that there is a higher propensity for intimal hyperplasia in coronary arteries than in iliac and carotid arteries after angioplasty.5,6 Other studies, however, have failed to reproduce these results after stenting in a swine model.7
Coronary arteries and peripheral arteries differ in their anatomy. Differences in size, bifurcations, elasticity and curvature, all affect the flow of blood through the artery.8 These differences may alter the shear stress on the vessel, which leads to endothelial cell damage and increased expression of molecules such as intercellular adhesion molecule‐1 and platelet‐derived growth factor.1,10 Some investigators have shown that cellular heterogeneity within the vascular system and vascular smooth muscle cells can differ depending on stage of development,11,12 state of injury13 and location.14,15,16 Coronary arteries also differ from peripheral arteries with respect to blood flow in relation to the cardiac cycle that occurs during the diastolic phase in coronary arteries and during the systolic phase in peripheral vessels.
The differences between the coronary and peripheral arteries highlight the need for a direct comparison of these arteries after identical injurious stimuli. It cannot be assumed that the pathophysiological responses after stenting will be identical in the coronary and iliac vessels, given their unique nature.17,18 In an effort to compare the coronary and iliac arteries, the vascular response in these arteries after stenting was morphometrically studied. We hypothesise that the arterial response to stenting in coronary arteries will be different from that in internal iliac arteries, as reflected by the morphometric indices of intimal hyperplasia, per cent stenosis and maximal intimal thickness.
Materials and methods
Animal protocol
The Institutional Animal Care and Use Committee of Creighton University approved the experimental research protocol for animal studies. All housing and care of the animals followed National Institutes of Health standards. Domestic pigs were obtained from a local supplier. Fourteen pigs weighing 30–40 kg and on a normal diet underwent stent implantation in either the left anterior descending or internal iliac arteries, or both. Three days before stent implantation, the animals were started on aspirin (350 mg/day) and ticlopidine (325 mg/day), which was continued until 1 month after stent placement. Intramuscular ketamine (20 mg/kg) and xylazine (4 mg/ml) were given before intubation. Isoflurane was used for anaesthesia during surgery. After carotid cutdown, a 6F introducer sheath was placed retrogradely into the right or left internal carotid artery. Heparin (5000 U) was given and the coronary vasculature was visualised angiographically using a 6F JR4 catheter. After guide wire placement, Mulitlink (Guidant, Temecula, California, USA) or green rust II (Cook, Indianapolis, Indiana, USA) stents, ranging from 2.5 to 4.0 mm in diameter and 20 mm in length, were placed using balloon dilation at a balloon:artery ratio of 1.1–1.2:1. Post‐stent implantation angiography was performed and the internal carotid artery was ligated. The animals were allowed to recover and were subsequently moved to the animal care facility. Five weeks later angiography was performed, followed by killing of the animals with an overdose of barbiturates.
Tissue harvest and processing
Immediately after the animals were killed, vessels were harvested and fixed in 4% paraformaldehyde in phosphate‐buffered saline overnight at 4°C. The samples were then washed with phosphate‐buffered saline and dehydrated by increasing alcohol gradients and xylene. Tissues were subsequently placed in a 1:1 mixture of methyl methacrylate:xylene at 4°C (1 h), a 1:1 mixture of methyl methacrylate:solution B overnight at 4°C and then a glass phial containing a 2:3 ratio of solution A:solution B (solution A: 2% benzoyl peroxide in methyl methacrylate; solution B: 1% polyethylene glycol‐400 and 0.5% N,N‐dimethyl‐p‐toluidine in butyl methacrylate). Samples were allowed to polymerise overnight at −20°C. Tissue blocks were rough cut and shaped with a band saw and rough grinder, respectively. Thick sections (6 mm) were then cut with a low‐speed diamond wafer saw. Thin sections (4.5 μm) were obtained by microtomy (Leica, Germany) with a tungsten‐carbide blade (Dorn & Hart Microedge, Villa Park, Illinois, USA). These sections were placed on slides and adhered with two applications of Haupt's solution (1% w/v gelatin, 1% w/v phenol crystals and 15% glycerol in phosphate‐buffered saline) each followed with a 24 h incubation at 50°C. They were de‐embedded through two changes of 2‐methoxyethyl acetate and hydrated from 100% ethanol to distilled water. The sections were then stained with haematoxylin and eosin as per standard protocol and mounted for subsequented morphometric analysis.
Morphological analysis
Two observers, blinded to the trial, separately carried out morphometric analysis with Scion Image software for Windows by Scanalytics (Fairfax, Virginia, USA). The following measurements were determined: lumen area, area within the internal elastic lamina (IEL), area within the external elastic lamina and maximal intimal thickness (MIT). Artery sections were assigned an injury score (IS) on the basis of an average of the individual strut injury levels as described previously by Schwartz et al19: 0, strut in intima but not in contact with IEL; 1, strut in contact with but not penetrating IEL; 2, strut penetrating IEL, in contact with media but not contacting external elastic lamina; 3, strut contacting but not penetrating external elastic lamina; and 4, strut penetrating external elastic lamina and in contact with adventitia.
Data analysis
All data analysis was carried out using a two‐sided Student's t test. Significance was defined at the p<0.05 level. All data are presented as mean (SEM).
Results
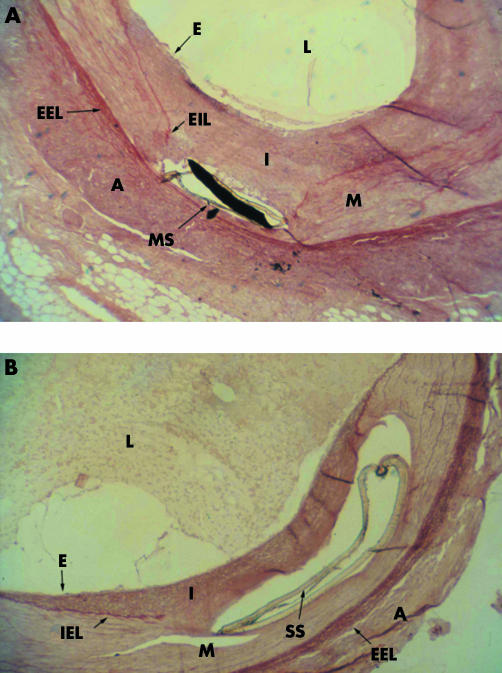
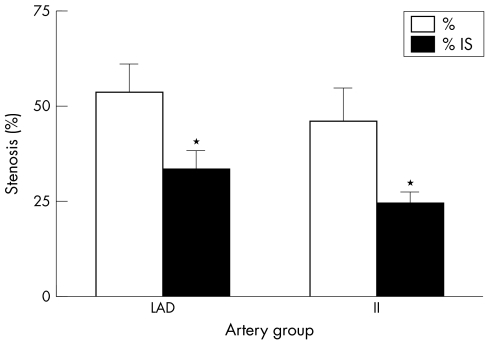
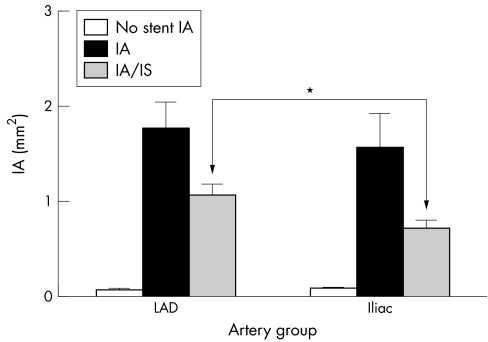
ISR is more pronounced in the coronary arteries than in the internal iliac arteries of the swine model under study. We used green rust II stents in an attempt to induce a significant intimal hyperplasia in the coronary and iliac arteries of the domestic swine model. Figures 1A and B show the photomicrograph of the intimal hyperplasia response in the coronary and the iliac arteries, respectively. Morphometric analysis was performed on the stained sections, with per cent stenosis, intimal area (IA), medial area (MA) and MIT as the parameters of intimal hyperplasia. The per cent stenosis data indicated a trend towards higher levels for the stented coronary arteries than for the stented iliac arteries (fig 2). Per cent stenosis was 53.52% (7.35%; n = 11) for coronary arteries and 46.12% (8.49%; n = 9) for iliac arteries (p = 0.52). Corrected percent stenosis, obtained by dividing per cent stenosis by IS per cent, was 33.29% (5.01%) and 24.3% (2.92%) for the coronary and iliac artery groups, respectively (p = 0.10). No statistical significance, however, seems to exist between the coronary and iliac arteries based on the per cent stenosis and the corrected per cent stenosis. Figure 3 shows the IA and the IA corrected for the injury score (IA/IS). The IA is that between the IEL and the lumen wall. Non‐stented arterial segments from the right coronary artery and the opposite iliac arteries were used as control. Baseline values of IA were between 0.05 and 0.12 mm2 for both the groups and were significantly different from their stented counterparts. The coronary and iliac vessels showed IA values of 1.78 (0.275) mm2 (n = 11) and 1.58 (0.353) mm2 (n = 9), respectively (*p<0.01). These parameters corrected for IS gave the values 1.08 (0.107) mm2 (n = 11) and 0.733 (0.078) mm2 (n = 9) for the coronary and iliac artery groups, respectively (*p<0.05).
Figure 1 Micrographs of (A) green rust II (GRII)‐stented left anterior descending coronary arteries and (B) internal iliac arteries in swine. Methyl methacrylate sections 4.5 μm in thickness were cut, de‐embedded and stained with orcein and haematoxylin to visualise elastin (red or pink; ×4). A, adventitia; E, endothelium; EEL, external elastic lamina; I, intima; IEL, internal elastic lamina; L, lumen; M, media; MS, stent metal in strut site; SS, stent strut site.
Figure 2 Uncorrected per cent stenosis (%) and per cent stenosis after correction for injury score (IS; %/IS). This figure compares the two artery groups with regard to in‐stent stenosis development. After sectioning and staining, artery sections were analysed by morphometric analysis of lumen area, area inside the internal elastic lamina (IEL) and IS. Per cent stenosis was calculated as area within the IEL divided by the lumen area. *p = 0.10 between these two groups (n = 11 and 9 for left anterior descending coronary arteries and internal iliac arteries, respectively).
Figure 3 Uncorrected intimal area (IA) and IA corrected for injury score (IA/IS) for stented and non‐stented coronary and internal iliac arteries. After sectioning of samples, morphometric analysis was carried out using Scion Image software (Scanalytics, Fairfax, Virginia, USA) to determine IA as well as IS. *p<0.05 (n = 11 and 9 for coronary and internal iliac arteries, respectively).
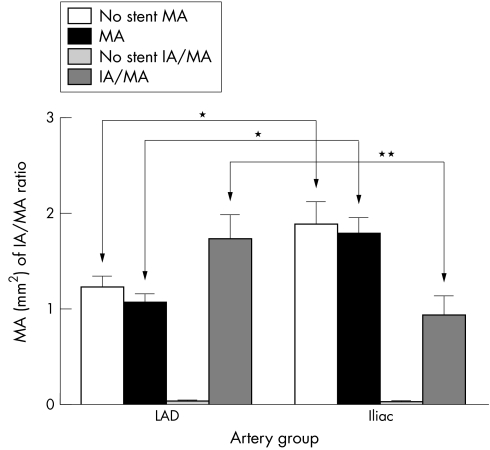
A comparison of the MA and the IA:MA ratio is depicted in fig 4. The larger size of the iliac artery is reflected in the greater MA 1.903 (0.227) mm2 (n = 9) as compared with 1.231 (0.123) mm2 (n = 11) for the coronary artery (*p<0.001). Stented vessel MAs for the iliac and coronary arteries were 1.803 (0.132) mm2 (n = 9) and 1.069 (0.091) mm2 (n = 11), respectively, which were also significant (*p<0.001). Thus, the intergroup MA differences pre‐stenting and post‐stenting were 0.672 and 0.734 mm2, which were significant (Student's t test, p<0.001). Intragroup pre‐stenting and post‐stenting MA differences, however, were similar for the two groups 0.100 and 0.162, respectively. The IA:MA ratios for the stented coronary (LAD) and iliac arteries were 1.736 (0.263) and 0.942 (0.196), respectively (p<0.05).
Figure 4 Medial area (MA) and intimal area/medial area (IA/MA) for non‐stented and stented (left anterior descending coronary arteries (LAD)) and internal iliac arteries (iliac). Samples were embedded, sectioned and stained before morphometric analysis of IA and MA. *p<0.001, **p<0.05 (n = 11 and 9 for coronary and internal iliac arteries, respectively).
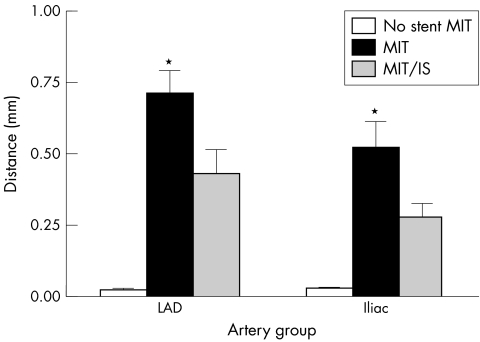
The MIT is the longest direct line measured from the edge of the lumen to the IEL. A comparison of this parameter uncorrected and corrected with the IS of the respective arteries IS is shown in fig 5. Unstented arteries from both locations showed similar levels of intimal thickness. After stenting MIT values were 0.718 (0.077) mm2 and 0.529 (0.088) mm2 whereas MIT/IS values were 0.436 (0.081) mm2 and 0.285 (0.044) mm2 for the coronary and internal iliac arteries, respectively. Although a trend can be seen for lower values in internal iliac arteries for both MIT and MIT/IS, no significance (p>0.05) was demonstrated between artery groups.
Figure 5 Comparison of uncorrected maximal intimal thickness (MIT) and MIT corrected for injury score (MIT/IS) of non‐stented and stented coronary and internal iliac arteries in swine. After embedding, sectioning and staining, morphometric analysis was carried out to determine MIT and IS. MIT is defined as the longest radial distance between the lumen and internal elastic lamina. Stenting increased the MIT significantly for each group compared with the unstented MIT (*p<0.05). No difference was found between the two artery groups for MIT and MIT/IS; p = 0.32 and 0.14, respectively (n = 11 and 9 for coronary and internal iliac arteries, respectively).
Discussion
The pathological response of human arteries to stenting has been well studied and an analysis of this literature can suggest a general trend for restenosis rates of certain human arteries. A series of six coronary stenting studies reported restenosis rates of 22–33% with a mean of 25%.20,21,22,23,24 A comparison of this average with that seen in three studies dealing with human iliac stenting suggests that iliac arteries undergo restenosis 60% less often than coronary arteries.25,26,27 These studies, however, did not directly compare the two arteries and did not report or measure intimal hyperplasia levels.
Few studies have used swine models of stenosis to compare different arteries. Ward et al5 provided information regarding the behaviour of iliac and coronary arteries after angioplasty in swine. They showed a higher level of intimal hyperplasia in coronary arteries; however, end lumen areas were smaller in the iliac arteries secondary to more negative remodelling in these arteries. Characterising the restenotic response after both stenting and angioplasty is important because the arterial reaction is unique after each procedure. The arterial response after stenting and angioplasty is characterised by processes such as intimal hyperplasia and negative remodelling. The relative restenotic contribution of each depends on the intervention performed.17,18 Distinct qualitative and quantitative differences exist between balloon‐injured and stented arteries. Stented arteries experienced a much larger initial lumen gain. Late lumen loss was, however, greater in stented arteries. Intravascular ultrasound has identified intimal hyperplasia as the main cause of ISR and stented arteries incur greater neointimal growth than those with balloon angioplasty, with their net benefit being attributable to their larger initial lumen gain and prevention of remodelling.
Studies comparing peripheral and central arteries after stenting are limited to two investigations. White and colleagues28 measured MIT when comparing left anterior descending coronary arteries and external or internal iliac arteries at various lengths of time after stenting. They showed higher, non‐significantly increased MIT levels in the left anterior descending coronary arteries 42 and 224 days after stenting. Mean intimal thickness levels at 28 days post‐stenting, however, were non‐significantly higher in the iliac vessels. During their study of the effect of tranilast on stenting in swine arteries, Ward et al7 expanded on their previous findings on the comparison of coronary and iliac arteries after angioplasty. As a small part of this publication they showed that coronary arteries produced a non‐significantly higher level of stenosis after stenting than iliac arteries. Data regarding the injury score level or per cent stenosis of these arteries, however, were not presented.
Overall, the trends seen in our study were similar to those published by Ward et al,7 who reported mean intimal hyperplasia values of 1.61 (0.29) and 1.39 (0.21) mm2 for coronary and iliac stented arteries, respectively. In contrast with the study by Ward et al, we show here that after stenting, coronary and internal iliac arteries differ significantly with respect to intimal hyperplasia development when normalised for injury score. Additionally, our studies also showed a trend towards higher per cent stenosis rates in the central arteries. No studies directly comparing per cent stenosis of iliac and coronary arteries after stenting exist. In this respect, this is the first study to directly compare this parameter in peripheral and central arteries after this intervention.
On the basis of intimal area data as well as the trends seen with the other parameters of vascular response, the present comparison of coronary and internal iliac arteries suggests that these arteries have unique responses when subjected to stenting. Previous studies have shown this to be true with regard to both carotid and iliac arteries when they were each compared with coronary arteries after angioplasty.5,6 At the very least, these studies point to the importance of arterial differences when trying to apply findings in one artery to treatment in another. At the most, the data presented in this study suggest a potential target of treatment for restenosis.
The differences between the arteries may be owing to the anatomical differences of the arteries or the actual arterial constituents, which include endothelial, smooth muscle and inflammatory cells. Levels of cellular processes such as proliferation and synthetic state have been shown to depend on vascular smooth muscle cell location or derivation.11,12 An interesting possibility is that these differences may be because of differences in levels of vessel wall apoptosis. Our laboratory has recently shown that human coronary artery vascular smooth muscle cells are more resistant to apoptosis than smooth muscle cells derived from human aortic arteries. Although attempts at quantifying apoptosis in the swine tissue were not successful, a higher resistance of coronary vascular smooth muscle cells to apoptosis could lead to more intimal hyperplasia, as was seen in this study. Irrespective of whether apoptosis, proliferation or another cellular process is responsible for the different responses seen in this study, their modification could lead to more favourable outcomes after coronary artery interventions.
Take home messages
Restenosis is a major complication of vascular interventional procedures such as stenting.
Vascular conduits from different anatomical locations exhibit different response to injury.
Using a porcine model our study demonstrated higher level of intimal hyperplasia in coronary arteries as compared with iliac arteries post‐stenting.
The extent of restenosis was related to the extent of injury (injury score).
Better understanding of the pathophysiology of intimal hyperplasia in different vessels will lead to better therapeutic strategies to combat restenosis.
Abbreviations
IEL - internal elastic lamina
IA - intimal area
IS - injury score
MA - medial area
MIT - maximal intimal thickness
Footnotes
Funding: This work was supported by National Institutes of Health Grants R01HL070885 and R01HL073349 (DKA).
References
- 1.Serruys P W, Strauss B H, Beatt K J.et al Angiographic follow‐up after placement of a self‐expanding coronary‐artery stent. N Engl J Med 199132413–17. [DOI] [PubMed] [Google Scholar]
- 2.Carrozza J P, Jr, Kuntz R E, Levine M J.et al Angiographic and clinical outcome of intracoronary stenting: immediate and long‐term results from a large single‐center experience. J Am Coll Cardiol 199220328–337. [DOI] [PubMed] [Google Scholar]
- 3.Lafont A, Guzman L A, Whitlow P L.et al Restenosis after experimental angioplasty. Intimal, medial, and adventitial changes associated with constrictive remodeling. Circ Res 199576996–1002. [DOI] [PubMed] [Google Scholar]
- 4.Johnson G J, Griggs T R, Badimon L. The utility of animal models in the preclinical study of interventions to prevent human coronary artery restenosis: analysis and recommendations. On behalf of the Subcommittee on Animal, Cellular and Molecular Models of Thrombosis and Haemostasis of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost 199981835–843. [PubMed] [Google Scholar]
- 5.Ward M R, Kanellakis P, Ramsey D.et al Response to balloon injury is vascular bed specific: a consequence of de novo vessel structure? Atherosclerosis 2000151407–414. [DOI] [PubMed] [Google Scholar]
- 6.Badimon J J, Ortiz A F, Meyer B.et al Different response to balloon angioplasty of carotid and coronary arteries: effects on acute platelet deposition and intimal thickening. Atherosclerosis 1998140307–314. [DOI] [PubMed] [Google Scholar]
- 7.Ward M R, Agrotis A, Kanellakis P.et al Tranilast prevents activation of transforming growth factor‐beta system, leukocyte accumulation, and neointimal growth in porcine coronary arteries after stenting. Arterioscler Thromb Vasc Biol 200222940–948. [DOI] [PubMed] [Google Scholar]
- 8.Gotlieb A I, Langille B L. The role of rheology in atherosclerotic coronary artery disease. In: Fuster V, Ross R, Topol EJ, eds. Atherosclerosis and coronary artery disease. Philadelphia: Lippincott‐Raven, 1996595–606.
- 9.Nagel T, Resnick N, Atkinson W J.et al Shear stress selectively upregulates intercellular adhesion molecule‐1 expression in cultured human vascular endothelial cells. J Clin Invest 199494885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Resnick N, Collins T, Atkinson W.et al Platelet‐derived growth factor B chain promoter contains a cis‐acting fluid shear‐stress‐responsive element. Proc Natl Acad Sci USA 1993904591–4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bochaton‐Piallat M L, Gabbiani F, Ropraz P.et al Age influences the replicative activity and the differentiation features of cultured rat aortic smooth muscle cell populations and clones. Arterioscler Thromb 1993131449–1455. [DOI] [PubMed] [Google Scholar]
- 12.Owens G K. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 199575487–517. [DOI] [PubMed] [Google Scholar]
- 13.Majesky M W, Giachelli C M, Reidy M A.et al Rat carotid neointimal smooth muscle cells reexpress a developmentally regulated mRNA phenotype during repair of arterial injury. Circ Res 199271759–768. [DOI] [PubMed] [Google Scholar]
- 14.Le Lievre C S, Le Douarin N M. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol 197534125–154. [PubMed] [Google Scholar]
- 15.Mikawa T, Fischman D A. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc Natl Acad Sci USA 1992899504–9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikawa T, Gourdie R G. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol 1996174221–232. [DOI] [PubMed] [Google Scholar]
- 17.Andersen H R, Maeng M, Thorwest M.et al Remodeling rather than neointimal formation explains luminal narrowing after deep vessel wall injury: insights from a porcine coronary (re)stenosis model. Circulation 1996931716–1724. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann R, Mintz G S, Dussaillant G R.et al Patterns and mechanisms of in‐stent restenosis. A serial intravascular ultrasound study. Circulation 1996941247–1254. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz R S, Huber K C, Murphy J G.et al Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol 199219267–274. [DOI] [PubMed] [Google Scholar]
- 20.Serruys P W, de Jaegere P, Kiemeneij F.et al A comparison of balloon‐expandable‐stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med 1994331489–495. [DOI] [PubMed] [Google Scholar]
- 21.Fischman D L, Leon M B, Baim D S.et al A randomized comparison of coronary‐stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med 1994331496–501. [DOI] [PubMed] [Google Scholar]
- 22.Heuser R, Lopez A, Kuntz R.et al SMART: The microstent's ability to limit restenosis trial. Catheter Cardiovasc Interv 200152269–77 discussion 278. [DOI] [PubMed] [Google Scholar]
- 23.Lau K, Ding Z, Vk V.et al Early and mid‐term angiographic and clinical results after intracoronary duet stent placement. Catheter Cardiovasc Interv 200152173–176. [DOI] [PubMed] [Google Scholar]
- 24.Kastrati A, Schomig A, Elezi S.et al Interlesion dependence of the risk for restenosis in patients with coronary stent placement in in multiple lesions. Circulation 1998972396–2401. [DOI] [PubMed] [Google Scholar]
- 25.Kichikawa K, Uchida H, Yoshioka T.et al Iliac artery stenosis and occlusion: preliminary results of treatment with Gianturco expandable metallic stents. Radiology 1990177799–802. [DOI] [PubMed] [Google Scholar]
- 26.Gunther R W, Vorwerk D, Antonucci F.et al Iliac artery stenosis or obstruction after unsuccessful balloon angioplasty: treatment with a self‐expandable stent. AJR Am J Roentgenol 1991156389–393. [DOI] [PubMed] [Google Scholar]
- 27.Vorwerk D, Gunther R W. Stent placement in iliac arterial lesions: three years of clinical experience with the Wallstent. Cardiovasc Intervent Radiol 199215285–290. [DOI] [PubMed] [Google Scholar]
- 28.White C J, Ramee S R, Banks A K.et al A new balloon‐expandable tantalum coil stent: angiographic patency and histologic findings in an atherogenic swine model. J Am Coll Cardiol 199219870–876. [DOI] [PubMed] [Google Scholar]