Abstract
Background and aim
The production of prostaglandins is regulated by cyclo‐oxygenases (COXs), which also have a role in tumour development and progression in various malignancies, including breast cancer. The mechanisms by which COX‐2 contributes to unfavourable prognosis are still poorly understood. The association between expression of COX‐2 and possible linked signalling pathways—namely, Akt, extracellular regulated kinases (ERK1/2), the stress‐activated kinase p38 or Her‐2/neu—is assessed in a series of 113 node‐negative breast cancers.
Results
COX‐2 was identified as an independent prognostic factor (p = 0.034) in node‐negative breast cancer by survival analysis. The lack of a relationship between COX‐2 expression and activated Akt, Erk1/2, p38 and Her‐2/neu was indicated by statistical analysis.
Conclusions
The prognostic effect of COX‐2 expression on lymph node‐negative breast cancer is confirmed—COX‐2 is probably not regulated by HER‐2, Akt, Erk1/2 or p38. Further studies are necessary for the elucidation of the signalling pathways responsible for the modification of COX‐2 expression and the increased aggressiveness of breast cancers overexpressing COX‐2.
Breast carcinoma is the most common malignancy in women. In the US, in 2005, about 211 000 cases of breast cancers were diagnosed, with 40 800 patients dying from the disease.1 Besides commonly accepted prognostic markers such as lymph node status, tumour size and grade,2 numerous other potential prognostic parameters have emerged. Among the potential prognostic parameters, cyclo‐oxgenase (COX)‐2 is of particular interest, as it may also offer the option of treatment with non‐steroidal anti‐inflammatory drugs (NSAIDs).3 Cyclo‐oxygenases regulate the synthesis of prostaglandins and are thus the major target of NSAIDs. Its two isoforms (COX‐1 and COX‐2) have different expression patterns, with COX‐1 being expressed in a broad variety of tissues. COX‐2 and its main product, prostaglandin E2 (PGE2), are inducible by growth factors and inflammatory stimuli. Additionally, COX‐2 has been shown to participate in tumour development and progression.4,5 Prostaglandins exert their effects locally in both autocrine and paracrine patterns. The effects of PGE2 are mediated by a family of G protein‐coupled receptors—namely, EP1, EP2, EP3 and EP4. The relevance of prostaglandins in breast cancer was studied by Bennett and Rolland more than 20 years ago—they found raised prostaglandin levels in breast cancer tissue and indicated that prostaglandin production may serve as a prognostic marker.6,7,8
Both COX‐2 mRNA and protein levels were found to be raised in human breast cancer, 9,10 which was associated with decreased survival.11,12 The application of NSAIDs was markedly associated with reduced development and progression of colonic tumours in patients with familial adenomatous polyposis (FAP),13,14 whereas the data obtained on breast cancer are conflicting.15,16
The mechanisms by which COX‐2 contributes to the poor prognosis have not been completely elucidated yet. COX‐2 has been shown to contribute to both tumorigenesis and the malignant phenotype of tumour cells by different mechanisms, including (1) the inhibition of apoptosis by increased production of PGE2, leading to increased expression of Bcl‐2 and attenuation of nitric oxide (NO) signalling; (2) increased angiogenesis by increased PGE2 production with subsequent vascular endothelial growth factor production; (3) increased invasiveness by overexpression of CD44; and (4) increased cell growth by activation of EP receptors.17 The growth factor‐mediated activation of the PI3‐kinase/PKB (Akt) pathway is a key element of cellular survival and proliferation. Four phosphorylation sites (serine 124, threonine 308, threonine 450 and serine 473) have been identified at the PH domain of Akt. Activation of the Akt kinase requires phosphorylation at the serine 473 and threonine 308 sites. So far, the studies evaluating the potential prognostic value of the Akt kinase in breast cancer have yielded conflicting results.18,19 Activation of the Akt kinase is markedly associated with reduced overall disease‐specific survival.20 St Germain and coworkers21,22 suggested that the PI3‐kinase–Akt pathway participates in the regulation of COX expression. In addition to the serine–threonine kinase Akt, two members of mitogen‐activated protein kinase (MAPK) signalling pathways are implicated in the expression of COX‐2—namely, extracellular regulated kinases (ERK1/2) and stress‐activated protein kinase p38.23 A recent study identified stress‐activated kinase p38 as an upstream regulator of COX‐2 expression and showed overexpression of both phospho‐p38 (pp38) and COX‐2 in ductal carcinoma in situ.24 Besides the Akt and MAPK signalling pathways, Her‐2/neu has also been implicated in the regulation of COX‐2.25
This immunohistochemical study assesses the possible association between COX‐2 expression and the activation of putative signalling pathways, namely phospho‐Akt (pAkt; serine 473 and threonine 308), phospho‐ERK (pERK)1/2, pp38 or Her‐2/neu, in a series of 113 node‐negative breast cancers.
Materials and methods
Patients
This study comprised 117 female patients with breast cancer (mean age 61.9 years), who underwent surgery and further adjuvant treatment at the Clinic of Gynaecology (University of Duisburg‐Essen, Essen, Germany) between 1989 and 1996. Clinical records and follow‐up information (overall disease‐specific survival) were available in all cases. Only patients with a negative lymph node status, which was confirmed on histological examination after standard axillary dissection, were included. All surgical material was fixed in 4% formalin and routinely processed. The tumours were classified according to the pathological tumour‐node‐metastasis system (6th edition) and graded according to Ellis and Elston.26 During follow up, 17 of 117 patients died: 13 patients died from breast cancer, the remaining 4 patients died from either benign disease (n = 2) or concurrent cancer (n = 2) and were thus excluded from survival analysis. Statistical analysis was based on a median follow‐up period of 7 years.
Immunohistochemistry (IHC)
Cyclo‐oxygenase‐2 and pAkt immunostaining
In this study, a monoclonal rabbit‐anti‐human COX‐2 antibody (DCS, Hamburg, Germany) was used. Immunostaining was carried out on 5‐μm‐thick paraffin‐wax sections with an automated staining device (DAKO Autostainer, Glostrup, Denmark). Antigen retrieval was carried out with 0.01 M citrate buffer at pH 6.1 for 20 min in a hot water bath (95°C). The primary antibody was incubated for 30 min at 1:250 dilution. Antibody was demonstrated with a commercially available antibody anti‐mouse IgG detection kit (EnVision, DakoCytomation, Carpenteria, California, USA). Positive controls (colorectal carcinoma) were included in each staining series. Mouse immunoglobin replacing the primary antibody was used as a negative control.
In invasive carcinoma COX‐2 was scored as follows: no immunoreactivity (−); positive staining independent of the amount of tumour cells (+). Immunostaining with pAkt (1/2/3) serine 473 and pAkt (threonine 308) was carried out with polyclonal rabbit anti‐human antibodies (Santa Cruz Biotechnology, Santa Cruz, California, USA). Subsequent to antibody retrieval (as described earlier), the primary antibodies were incubated overnight in a humidified chamber at 4°C at a 1:2000 dilution (pAkt serine 473) and 1:5000 dilution (pAkt threonine 308). The alkaline phosphatase anti‐alkaline phosphatase method was used for demonstration of the antibodies.
pAkt immunostaining was scored as the percentage of positively immunostained tumour nuclei by assessing 300 tumour cells at the invasive tumour front (as tumour aggressiveness is considered to be highest here). The percentage of pAkt serine 473‐positive and threonine 308‐positive tumour cell nuclei ranged from 7% to 87% and from 10% to 95%, respectively. Tumours with ⩾64% positive nuclei (mean value pAkt 473) and 61% (mean value pAkt 308) were classified as pAkt positive, respectively.
pERK1/2 IHC
Monoclonal phospho‐p44/42 MAPK antibody (threonine 202/tyrosine 204; Cell Signalling Technology, Beverly, Massachusetts, USA) was used at a 1:100 dilution. Staining procedures were carried out as previously described.20,27 Tumour cells with strong specific immunostaining, independent of the amount of stained cells, were scored as strongly positive (2+). Tumours exhibiting a detectable but faint immunostaining were scored as weak (1+), whereas tumours with a minimal, hardly detectable or missing staining pattern were classified as negative (0).
pp38 IHC
Monoclonal pp38 MAPK antibody (threonine 180/tyrosine 182; Cell Signalling Technology) was used at 1:1000 dilution. Antigen retrieval was carried out with 0.01 M citrate buffer at pH 6.1 for 45 min in a hot water bath (95°C). Antibody was demonstrated with a commercially available detection kit (ChemMate Detectin Kit, DakoCytomation). Positive controls (colorectal carcinoma) were included in each staining series. Mouse immunoglobin replacing the primary antibody was used as a negative control. Cytoplasmatic intensity of pp38 staining (1, absent; 2, low, moderate and strong) and nuclear pp38 staining (1, absent to low; 2, ⩽50% nuclear positivity; 3, >50% nuclear positivity) was evaluated. Addition of the intensity and proportion scores provided the final category score (negative, intermediate and strong) used for further statistical analysis.
Oestrogen receptor status
The oestrogen receptor status was determined with an anti‐human oestrogen receptor α antibody (clone 1D5, DAKO, Glostrup, Denmark) as described previously.20 Briefly, a tumour was regarded as receptor negative if none or <10% of the tumour cells showed weak or no nuclear immunostaining.
Fluorescent in situ hybridisation (FISH)
The HER‐2/neu gene copy number was determined with the PathVysion HER‐2/neu kit (Abbot GmbH, Diagnostica, Wiesbaden‐Delkenheim, Germany) containing a mixture of SpectrumOrange‐labelled HER‐2/neu gene region and SpectrumGreen‐labelled centromere region for chromosome 17. The HER‐2/neu gene copy level was assessed as the ratio of HER‐2/neu gene copies to chromosome 17 centromere copies. Sections embedded in paraffin wax (2 μm thick) were dewaxed for 3×5 min in xylene, dehydrated in graded ethanol series and washed in 2× sodium salt citrate (SSC; 1× SSC is 150 mM sodium chloride and 15 mM sodium citrate, pH 7). The sections were air dried, pretreated (80°C, 30 min) and digested with protease (37°C, 90 min) before hybridising with the PathVysion probe. Slide and probes were denatured simultaneously by incubation at 72°C for 1 min on a HYBrite hybridisation system (Vysis, Downers Grove, Illinois, USA). After incubating overnight in a dark humidity chamber at 37°C, the slides were washed for 2 min in 0.4×SSC/0.3% nitroprusside‐40 at 72°C. The slides were then mounted in p‐phenylenediamine dihydrochloride (Vysis), which contained p‐phenylenediamine dihydrochloride as counterstain. The FISH analysis was carried out with an Nikon Eclipse 80i (Nikon GmbH, Düsseldorf, Germany). In every tumour cell nucleus that was considered for analysis, the number of red and green signals was counted according to the manufacturer's guidelines, and the ratio of c‐erbB‐2 gene signals relative to chromosome 17 centromere signals per nucleus was calculated. Amplification of the c‐erbB‐2 gene was assumed at a ratio of >2.
HER‐2 protein expression by IHC
The DAKO Hercept Test was used to detect the HER‐2/neu protein expression (DAKO, number K 5204). Staining procedures were carried out according to the manufacturer's protocol. All IHC 2+ tumours were analysed with FISH to determine the level of the HER‐2/neu gene copy. For statistical analysis, negative (DAKO score 0 and 1+; IHC 2+ cases lacking amplification) and positive (IHC 2+ cases with amplification and DAKO score 3+) groups were created.
Mitotic count
Mitotic count was calculated taking into consideration the actual area of 10 high‐power fields (HPF) of the Nikon Eclipse E600 microscope. Mitotic count results were grouped as 0–8, 9–16 and >16 mitotic figures per HPF (field diameter 0.55 mm).28
Statistical analysis
All immunostaining was assessed in a blind‐trial manner by two of the authors, who had no knowledge of the clinical outcome. All data were statistically analysed with SPSS V.12 for Windows. COX‐2 and pAkt (serine 473) immunostains were evaluated by KJS and JB; pERK1/2 by KJS and JW; and pAkt (threonine 308) and pp38 by KJS and H‐SL. In case the evaluators disagreed, slides were re‐evaluated by both and a final decision was made. Interobserver agreement of categorical data (COX, pERK1/2 and pp38) was calculated with the κ coefficient; the κ value was interpreted using the commonly cited scale of Landis et al.29 Interobserver agreement on COX‐2, pERK1/2 and pp38 staining evaluation was substantial (κ = 0.618, 0.702 and 0.654, respectively). Interobserver agreement on continuous data was evaluated by using Pearson's correlation coefficient. Staining scores of pAkt serine 473 and threonine 308 yielded a significant interobserver concordance (Pearson's r = 0.605 and 0.677, respectively; p<0.001). The mean percentage of both pAkt stains was used for statistical analysis. Relationships between ordinal parameters were investigated by two‐tailed χ2 analysis. Pearson's correlation coefficient was used for detecting significant correlations among numerical parameters. Overall survival curves were estimated by the Kaplan–Meier method, and any differences in the survival curves were compared by the log rank test. For multivariate analysis, the Cox regression model was used. Overall, 95% confidence intervals were used throughout.
Results
Analysis of HER‐2 status
A total of 110 cases were analysed for HER‐2/neu protein expression: 44 tumours were classified as negative (0), 38 as negative (1+), 15 as weakly positive (2+) and 13 as strongly positive (3+). Of the 15 cases scored as weakly positive (2+), two showed a Her‐2/neu gene amplification.
Immunohistochemical analysis of COX‐2
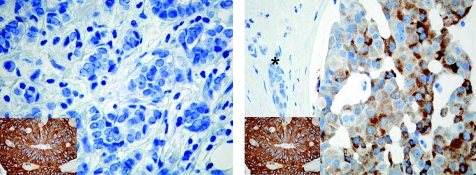
In tumour cells COX‐2 immunostaining was exclusively observed in the cytoplasm, with a granular staining pattern. Normal breast tissue constantly lacked COX‐2 immunoreactivity. In contrast with the homogeneous positive staining pattern in colorectal cancer, a rather scattered distribution of COX‐2 protein expression was observed in breast cancer specimens. Table 1 summarises the statistical correlation of the COX‐2 status with clinicopathological parameters, as well as the other immunostaining results of this study. In χ2‐analysis COX‐2 overexpression was significantly associated with a poorer differentiation grade (p = 0.004), whereas all other parameters investigated lacked significance. Figure 1 shows representative immunohistochemical COX‐2 staining.
Table 1 Clinicopathological data and cyclo‐oxygenase‐2 expression in node‐negative breast cancers.
| Parameter investigated | COX‐2 negative, n = 64 (56.68%) | COX‐2 positive, n = 49 (43.4%) | p Value (χ2 analysis) |
|---|---|---|---|
| Tumour type (n = 113) | NS | ||
| Invasive ductal | 49 (43.4) | 37 (32.7) | |
| Invasive lobular | 12 (10.6) | 3 (2.7) | |
| Mucinous/tubulo‐lobular | 2 (1.8) | 6 (5.4) | |
| Others | 1(0.9) | 3 (2.7) | |
| Grading (n = 113) | 0.004 | ||
| Well | 21 (18.6) | 4 (3.5) | |
| Moderate | 28 (24.8) | 34 (30.1) | |
| Poor | 15 (13.3) | 11 (9.7) | |
| Oestrogen receptor status (n = 107) | NS | ||
| Positive | 36 (33.6) | 30 (28) | |
| Negative | 25 (23.42) | 16 (15) | |
| HerceptTest/FISH* (n = 110) | NS | ||
| Negative | 52 (47.3) | 39 (35.5) | |
| Positive | 10 (9.1) | 9 (8.2) | |
| Mitotic rate (n = 113) | NS | ||
| 0–8 | 35 (31) | 18 (15.9) | |
| 9–16 | 15 (13.3) | 21 (18.6) | |
| >16 | 14 (12.4) | 10 (8.8) | |
| pAkt (308) (n = 95) | NS | ||
| Negative | 20 (28.4) | 30 (21.1) | |
| Positive | 21 (22.1) | 24 (25.3) | |
| pAkt (473) (n = 113) | NS | ||
| Negative | 21 (21.1) | 19 (31.6) | |
| Positive | 43 (38.12) | 30 (26.5) | |
| Tumour size (n = 113) | NS | ||
| pT1(a,b,c) | 40 (35.4) | 24 (21.2) | |
| pT2 | 23 (20.4) | 25(22.1) | |
| pT3 | 1 (0.9) | 0 (0) | |
| pERK1/2 (n = 110) | NS | ||
| Negative | 8 (7.3) | 6 (5.5) | |
| Intermediate | 15 (13.6) | 20 (18.2) | |
| Strong | 38 (34.5) | 23 (20.9) | |
| pp38 (n = 92) | |||
| Negative | 9 (9.8) | 7 (7.6) | NS |
| Intermediate | 25 (27.2) | 22 (23.9) | |
| Strong | 16 (17.4) | 13 (14.1) |
COX, cyclo‐oxygenase; ERK, extracellular regulated kinase; FISH, fluorescent in situ hybridisation; NS, not significant; pAkt, phospho‐Akt; pERK, phospho‐ERK; pp38, phospho‐p38.
*FISH of all immunohistochemistry 2+ tumours was carried out. Cases without amplification were classified as negative.
Figure 1 Representative immunohistochemical staining of cyclo‐oxygenase‐2 (COX‐2) in node‐negative breast cancers: breast carcinoma completely lacking COX‐2 protein expression (left). Specific cytoplasmatic COX‐2 expression (right). The complete lack of immunostaining in an adjacent normal breast duct (asterisk) is seen. Inset: strong COX‐2 expression in a colorectal carcinoma serving as positive control. Original magnification ×400.
Immunohistochemical analysis of pAkt (threonine 308 and serine 473)
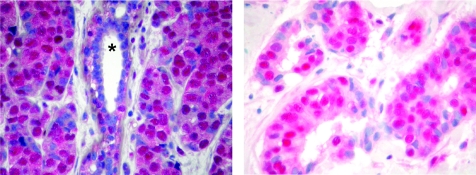
Immunostaining for pAkt (serine 473) was predominantly localised to tumour nuclei. Occasionally a specific faint cytoplasmatic pAkt staining was noticed, but was disregarded for calculation of immunohistochemical scores. Immunostaining for pAkt (threonine 308) was mainly located to the nucleus, with more intense cytoplasmatic staining than for pAkt (serine 473). Table 1 summarises the results of χ2 analysis of pAkt expression and relevant clinicopathological parameters. Significant concordance between pAkt (serine 473) and pAkt (threonine 308) staining scores (Pearson's r = 0.420; p<0.001) was observed. Figure 2 shows representative immunohistochemical pAkt staining.
Figure 2 Representative immunohistochemical staining of two samples of breast cancer for phospho‐Akt (pAkt; threonine 308; left) and pAkt (serine 473; right). Tumour cell nuclei mainly showed strong pAkt immunoreactivity; weak immunostaining was observed in the cytoplasm of tumour cells. The scattered positively stained nuclei are seen in a normal breast duct (asterisk). Original magnification ×400.
Immunohistochemical analysis of pERK1/2 and pp38
Both pERK1/2 and pp38 immunostaining exhibited a specific nuclear and cytoplasmatic staining pattern. Whereas normal breast tissue did not show any pERK staining, pp38 immunostaining was occasionally detected in normal breast tissue adjacent to invasive breast cancer. Table 1 summarises the results of pERK1/2 and pp38 immunostaining. No relationship was found between pERK1/2 or pp38 staining results and COX‐2 expression.
Relationship of COX‐2 and pAkt expression
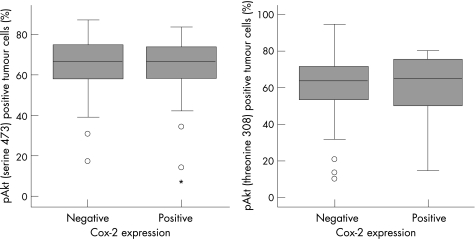
χ2 analysis showed no correlation between COX‐2 expression and pAkt (threonine 308) and pAkt (serine 473) expression. No relationship was found between the number of positively stained tumour nuclei for pAkt (serine 473 and threonine 308) and COX‐2 protein expression (fig 3), either.
Figure 3 Box plots showing the relationship between cyclo‐oxygenase‐2 (COX‐2) immunoexpression and the percentage of phospho‐Akt (pAkt; serine 473 and threonine 308) positively stained tumour nuclei. Tumours with or without COX‐2 expression exhibited equal amounts of pAkt tumour cells independent of the phosphorylation site. Open circles indicate outliers; asterisks indicate extreme values. Data were available for 95 cases (pAkt threonine 308) and for 113 cases (pAkt serine 473).
Prognosis
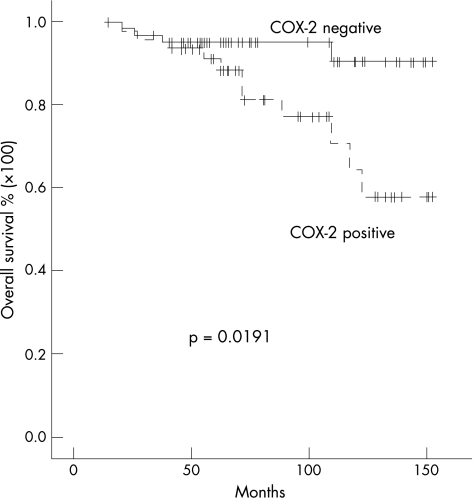
COX‐2 overexpression of the complete series was significantly associated with decreased overall survival (p = 0.0191; fig 4). Parallel analysis showed that histological grading (p = 0.0010), mitotic count (p = 0.0393) and the pAkt (serine 473) status (p = 0.0190) were significantly related to a decreased overall survival. A negative oestrogen receptor status tended to be associated with (p = 0.076) a worse prognosis (table 2). Increased tumour size (cut‐off point 1.7 cm) was significantly associated with reduced overall survival in univariate analysis (p = 0.0462). If the cut‐off point was, however, selected according to the tumour‐node‐metastasis classification, tumour size failed to reach statistical significance.
Figure 4 Kaplan–Meier survival plot of 113 node‐negative breast cancers in relation to cyclo‐oxygenase‐2 (COX‐2) protein expression. Log rank test: p = 0.0191.
Table 2 Univariate analysis (Kaplan–Meier, log‐rank test) for prognostic significance of cyclo‐oxygenase‐2, histological grading, phospho‐Akt (serine 473 and threonine 308), receptor status, HER‐2/neu, mitotic count, tumour size and phospho‐p38.
| Parameter | p |
|---|---|
| COX‐2 (negative/positive) | 0.0191 |
| Histological grade | 0.0010 |
| pAkt (473) (negative/positive) | 0.0190 |
| pAkt (308) (negative/positive) | NS |
| ER status | NS |
| HER‐2 status | NS |
| MC (0–8, 9–16, >16) | 0.0393* |
| Tumour size | NS |
| pp38 (negative/intermediate/positive) | NS |
COX, cyclo‐oxygenase; ER, oestrogen receptor; MC, mitotic count; NS, not significant; pAkt, phospho‐Akt; pp38, phospho‐p38.
COX‐2 and the relevant clinicopathological parameters (histological grading mitotic count and pAkt) were subject to multivariate analysis; only COX‐2 expression (p = 0.034) and histological grading (p = 0.010) were statistically associated with the overall survival of patients (table 3).
Table 3 Multivariate Cox regression analysis of disease‐specific overall survival in 113 cases of node‐negative breast cancers.
| Variable* | Relative risk | 95% CI | p Value |
|---|---|---|---|
| COX‐2 | 3.509 | 1.099 to 11.208 | 0.034† |
| Grading | 5.537 | 1.244 to 24.532 | 0.025† |
| pAkt (473) | 3.976 | 0.861 to 18.366 | 0.077 |
| Mitotic count | 0.774 | 0.278 to 2.156 | 0.624 |
COX, cyclo‐oxygenase; pAkt, phospho‐Akt.
*Coding of variables: pAkt was coded as 1, negative; and 2, positive. COX‐2 was coded as 1, negative; and 2, positive. Mitotic count was coded as 1, 0–18; 2, 9–16; and 3, >16.
†Significant.
Discussion
Novel markers defining node‐negative high‐risk patients who benefit from adjuvant treatment would be desirable, particularly in patients with node‐negative breast cancer. This study on a series of 113 node‐negative breast cancers showed an independent correlation of COX‐2 expression with disease‐specific overall survival in multivariate analysis, thus supporting previous reports describing a prognostic impact of COX‐2 expression in node‐negative breast cancer.11,12 Against the background of the well‐known COX‐2 inhibiting effect of NSAIDs, recent studies have investigated the association of NSAID use and breast cancer risk. In fact, a reduction in risk with aspirin use was seen among patients with breast cancer with hormone receptor‐positive tumours. These findings indicate that NSAIDs may serve as effective chemopreventive agents for breast cancer.30
Previous studies showed that COX‐2 is upregulated in a variety of cancers—for example, colon, breast, lung, pancreas and oesophagus.31,32,33,34,35 In cell culture and animal models, induction of COX‐2 has been shown to promote cell growth, inhibit apoptosis and improve cell motility and adhesion.36 The mechanisms underlying these multiple actions of COX‐2 are, however, still largely unknown. The serine/threonine kinase Akt, which may function as an upstream activator or downstream effector of the COX‐2 gene, was found to regulate COX‐2 transcription in human endometrial cancer cells by increased transcriptional activity of nuclear factor‐κB. This resulted in an increased COX‐2 gene expression, suggesting that Akt was an upstream regulator.21,22 Additionally, inactivation of glycogen synthase kinase‐3β by Akt seems to play an important part in the ultraviolet‐β induction of COX‐2 transcription in human keratinocytes.37 In contrast, a recently published study on hepatocellular cancer cells and human hepatocellular carcinoma tissue described the activation of Akt as a downstream signalling event induced by COX‐2 overexpression.38 Transfection of COX‐2 expression vector in human hepatocellular cells resulted in improved phosphorylation of Akt, particularly at the phosphorylation site threonine 308.
To our knowledge, this is the first study to analyse the association of COX‐2 protein expression and activation (phosphorylation) of Akt in human breast cancers. Our immunohistochemical approach, however, failed to provide any evidence of a direct link between Akt phosporylation (both at serine 473 and threonine 308) and COX‐2 overexpression.
Additional signalling pathways, including ERKs, the stress‐activated kinase p38 and Her‐2/neu, are thought to interact with COX‐2. Cell line studies have shown that COX‐2 expression can be stimulated by ERKs23 and p38,24 putting the ERK pathway and p38 pathway upstream of COX‐2. A recent study on cells generated from human breast tissue explants found p38 to be responsible for COX‐2 expression and established p38 as an upstream regulator of COX‐2 in vitro.24 Moreover, the same research group showed that activation of p38 and overexpression of COX‐2 characterises ductal carcinoma in situ lesions and the nearby morphologically normal epithelium, whereas normal epithelium from disease‐free breast tissue exhibited low pp38 levels. Interestingly, no direct correlation of COX‐2 expression and pp38 levels in invasive breast cancers could be established in our study. As we did not analyse premalignant lesions of the breast, this is not surprising. Gauthier et al suggest that p38 may participate in an early event that contributes to a malignant phenotype in mammary epithelial cells. It is imaginable, however, that once a tumour has acquired a highly aggressive phenotype reflected by an invasive growth pattern, activation of p38 is not required anymore. This may explain the partly missing phospho‐p38 (pp38) staining in COX‐2‐positive breast cancers. As for Akt, however, no relationship between activated Erk1/2 or p38 and COX‐2 expression could be established in our series of node‐negative breast cancers.
Furthermore, we showed that there was no correlation between Her‐2/neu protein overexpression and COX‐2. Recent studies on colon carcinoma and breast cancer cells provide evidence that Her‐2/neu is implicated in the regulation of COX‐2 overexpression.25,39 In a clinical trial, a decreased HER‐2 expression was observed in the rectal mucosa of patients with FAP after NSAID chemoprevention.40 This suggests a regulatory effect of drug‐related COX‐2 expression on the HER‐2 tyrosine kinase activity at least in patients with FAP. For breast carcinoma, a relevant regulatory role of Her‐2/neu in COX‐2 protein expression remains controversial. One study found a significant correlation between COX‐2 and Her‐2/neu,11 whereas most other studies, including this study, did not find such a relationship.12,34,41,42 We are aware that detection of changes in HER‐2/neu by IHC is not the gold standard, especially in tumours scored 2+ by IHC (HerceptTest). In this subgroup, gene amplification was found in as many as 48% of cases.43 To increase the significance of the statistical analysis of a potential correlation between HER‐2 and COX‐2 proteins, we determined the HER‐2/neu gene copy level of all 2+ cases by FISH. We refrained from applying the FISH analysis to the complete series, as studies report an excellent correlation between FISH and IHC in 0, 1+ and 3+ scored tumours.43 The lack of correlation with Her‐2/neu, however, suggests that, unlike in colorectal cancer cells, type 1 receptor tyrosine kinase does not stimulate COX‐2 expression in human breast cancer.
Take‐home messages
Cyclo‐oxygenase‐2 (COX‐2) protein expression is an independent prognostic predictor in node‐negative breast cancer.
COX‐2 protein expression is probably not influenced by HER2, Akt, extracellular regulated kinase‐1/2 or phospho‐38.
Further studies are necessary to elucidate the signalling pathways responsible for the modification of COX‐2 expression in breast cancers.
In conclusion, this study confirms the independent prognostic value of immunohistochemical COX‐2 protein expression in node‐negative breast cancer, thus offering an additional adjuvant therapeutic approach with COX‐2 inhibitors. As we were unable to provide evidence for the regulation of COX‐2 expression by HER‐2, pAkt, pERK1/2 or pp38, further studies will be necessary to disclose the detailed signalling pathways responsible for the modification of COX‐2 expression and the increased biological aggressiveness of breast cancers overexpressing COX‐2.
Acknowledgements
The technical assistance of Dorothe Möllmann is acknowledged.
Abbreviations
COX - cyclo‐oxygenase
ERK - extracellular regulated kinases
FAP - familial adenomatous polyposis
FISH - fluorescent in situ hybridisation
IHC - immunohistochemistry
MAPK - mitogen‐activated protein kinase
NSAID - non‐steroidal anti‐inflammatory drug
pAkt - phospho‐Akt
pERK - phospho‐ERK
PGE2 - prostaglandin E2
pp38 - phospho‐p38
SSC - sodium salt citrate
Footnotes
Competing interests: RK, FO AND KWS are members of the West German Cancer Centre Essen (WTZE), Essen, Germany. KJS, RC, JW, RK, FO, KWS and HAB are members of the University Breast Cancer Centre Essen, Essen, Germany.
References
- 1.Jemal A, Murray T, Ward E.et al Cancer statistics, 2005. CA Cancer J Clin 20055510–30. [DOI] [PubMed] [Google Scholar]
- 2.Goldhirsch A, Glick J H, Gelber R D.et al Meeting highlights: International Consensus Panel on the Treatment of Primary Breast Cancer. Seventh international conference on adjuvant therapy of primary breast cancer. J Clin Oncol 2001193817–3827. [DOI] [PubMed] [Google Scholar]
- 3.Khuder S A, Herial N A, Mutgi A B.et al Nonsteroidal antiinflammatory drug use and lung cancer: a metaanalysis. Chest 2005127748–754. [DOI] [PubMed] [Google Scholar]
- 4.Howe L R, Subbaramaiah K, Brown A M.et al Cyclooxygenase‐2: a target for the prevention and treatment of breast cancer. Endocr Relat Cancer 2001897–114. [DOI] [PubMed] [Google Scholar]
- 5.Liu C H, Chang S H, Narko K.et al Overexpression of cyclooxygenase‐2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem 200127618563–18569. [DOI] [PubMed] [Google Scholar]
- 6.Bennett A, Stamford I F, Cooper D. Human cancer prostaglandins and patient survival. Adv Prostagland Thromb Leukotr Res 199121B875–878. [PubMed] [Google Scholar]
- 7.Bennett A, Charlier E M, McDonald A M.et al Prostaglandins and breast cancer. Lancet 19772624–626. [DOI] [PubMed] [Google Scholar]
- 8.Rolland P H, Martin P M, Jacquemier J.et al Prostaglandin in human breast cancer: evidence suggesting that an elevated prostaglandin production is a marker of high metastatic potential for neoplastic cells. J Natl Cancer Inst 1980641061–1070. [PubMed] [Google Scholar]
- 9.Hwang D, Scollard D, Byrne J.et al Expression of cyclooxygenase‐1 and cyclooxygenase‐2 in human breast cancer. J Natl Cancer Inst 199890455–460. [DOI] [PubMed] [Google Scholar]
- 10.Brueggemeier R W, Quinn A L, Parrett M L.et al Correlation of aromatase and cyclooxygenase gene expression in human breast cancer specimens. Cancer Lett 199914027–35. [DOI] [PubMed] [Google Scholar]
- 11.Ristimaki A, Sivula A, Lundin J.et al Prognostic significance of elevated cyclooxygenase‐2 expression in breast cancer. Cancer Res 200262632–635. [PubMed] [Google Scholar]
- 12.Denkert C, Winzer K J, Muller B M.et al Elevated expression of cyclooxygenase‐2 is a negative prognostic factor for disease free survival and overall survival in patients with breast carcinoma. Cancer 2003972978–2987. [DOI] [PubMed] [Google Scholar]
- 13.Hawk E, Lubet R, Limburg P. Chemoprevention in hereditary colorectal cancer syndromes. Cancer 1999862551–2563. [DOI] [PubMed] [Google Scholar]
- 14.Winde G, Schmid K W, Schlegel W.et al Complete reversion and prevention of rectal adenomas in colectomized patients with familial adenomatous polyposis by rectal low‐dose sulindac maintenance treatment. Advantages of a low‐dose nonsteroidal anti‐inflammatory drug regimen in reversing adenomas exceeding 33 months. Dis Colon Rectum 199538813–830. [DOI] [PubMed] [Google Scholar]
- 15.Harris R E, Namboodiri K K, Farrar W B. Nonsteroidal antiinflammatory drugs and breast cancer. Epidemiology 19967203–205. [DOI] [PubMed] [Google Scholar]
- 16.Egan K M, Stampfer M J, Giovannucci E.et al Prospective study of regular aspirin use and the risk of breast cancer. J Natl Cancer Inst 199688988–993. [DOI] [PubMed] [Google Scholar]
- 17.Wendum D, Masliah J, Trugnan G.et al Cyclooxygenase‐2 and its role in colorectal cancer development. Virchows Arch 2004445327–333. [DOI] [PubMed] [Google Scholar]
- 18.Panigrahi A R, Pinder S E, Chan S Y.et al The role of PTEN and its signalling pathways, including AKT, in breast cancer; an assessment of relationships with other prognostic factors and with outcome. J Pathol 200420493–100. [DOI] [PubMed] [Google Scholar]
- 19.Perez‐Tenorio G, Stal O. Activation of AKT/PKB in breast cancer predicts a worse outcome among endocrine treated patients. Br J Cancer 200286540–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitz K J, Otterbach F, Callies R.et al Prognostic relevance of activated Akt kinase in node‐negative breast cancer: a clinicopathological study of 99 cases. Mod Pathol 20041715–21. [DOI] [PubMed] [Google Scholar]
- 21.St Germain M E, Gagnon V, Mathieu I.et al Akt regulates COX‐2 mRNA and protein expression in mutated‐PTEN human endometrial cancer cells. Int J Oncol 2004241311–1324. [PubMed] [Google Scholar]
- 22.St Germain M E, Gagnon V, Parent S.et al Regulation of COX‐2 protein expression by Akt in endometrial cancer cells is mediated through NF‐kappaB/IkappaB pathway. Mol Cancer 200437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subbaramaiah K, Chung W J, Dannenberg A J. Ceramide regulates the transcription of cyclooxygenase‐2. Evidence for involvement of extracellular signal‐regulated kinase/c‐Jun N‐terminal kinase and p38 mitogen‐activated protein kinase pathways. J Biol Chem 199827332943–32949. [DOI] [PubMed] [Google Scholar]
- 24.Gauthier M L, Pickering C R, Miller C J.et al p38 regulates cyclooxygenase‐2 in human mammary epithelial cells and is activated in premalignant tissue. Cancer Res 2005651792–1799. [DOI] [PubMed] [Google Scholar]
- 25.Simeone A M, Li Y J, Broemeling L D.et al Cyclooxygenase‐2 is essential for HER2/neu to suppress N‐(4‐hydroxyphenyl)retinamide apoptotic effects in breast cancer cells. Cancer Res 2004641224–1228. [DOI] [PubMed] [Google Scholar]
- 26.Elston C W, Ellis I O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long term follow up. Histopathology 199119403–410. [DOI] [PubMed] [Google Scholar]
- 27.Schmitz K J, Grabellus F, Callies R.et al High expression of focal adhesion kinase (p125FAK) in node‐negative breast cancer is related to overexpression of HER‐2/neu and activated Akt kinase but does not predict outcome. Breast Cancer Res 20057R194–R203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellis P S, Whitehead R. Mitosis counting—a need for reappraisal. Hum Pathol 1981123–4. [DOI] [PubMed] [Google Scholar]
- 29.Landis J R, Koch G G. The measurement of observer agreement for categorical data. Biometrics 197733159–174. [PubMed] [Google Scholar]
- 30.Terry M B, Gammon M D, Zhang F F.et al Association of frequency and duration of aspirin use and hormone receptor status with breast cancer risk. JAMA 20042912433–2440. [DOI] [PubMed] [Google Scholar]
- 31.Nozoe T, Ezaki T, Kabashima A.et al Significance of immunohistochemical expression of cyclooxygenase‐2 in squamous cell carcinoma of the esophagus. Am J Surg 2005189110–115. [DOI] [PubMed] [Google Scholar]
- 32.Okami J, Yamamoto H, Fujiwara Y.et al Overexpression of cyclooxygenase‐2 in carcinoma of the pancreas. Clin Cancer Res 199952018–2024. [PubMed] [Google Scholar]
- 33.Sano H, Kawahito Y, Wilder R L.et al Expression of cyclooxygenase‐1 and ‐2 in human colorectal cancer. Cancer Res 1995553785–3789. [PubMed] [Google Scholar]
- 34.Half E, Tang X M, Gwyn K.et al Cyclooxygenase‐2 expression in human breast cancers and adjacent ductal carcinoma in situ. Cancer Res 2002621676–1681. [PubMed] [Google Scholar]
- 35.Wolff H, Saukkonen K, Anttila S.et al Expression of cyclooxygenase‐2 in human lung carcinoma. Cancer Res 1998584997–5001. [PubMed] [Google Scholar]
- 36.Cao Y, Prescott S M. Many actions of cyclooxygenase‐2 in cellular dynamics and in cancer. J Cell Physiol 2002190279–286. [DOI] [PubMed] [Google Scholar]
- 37.Tang Q, Gonzales M, Inoue H.et al Roles of Akt and glycogen synthase kinase 3beta in the ultraviolet B induction of cyclooxygenase‐2 transcription in human keratinocytes. Cancer Res 2001614329–4332. [PubMed] [Google Scholar]
- 38.Leng J, Han C, Demetris A J.et al Cyclooxygenase‐2 promotes hepatocellular carcinoma cell growth through Akt activation: evidence for Akt inhibition in celecoxib‐induced apoptosis. Hepatology 200338756–768. [DOI] [PubMed] [Google Scholar]
- 39.Vadlamudi R, Mandal M, Adam L.et al Regulation of cyclooxygenase‐2 pathway by HER2 receptor. Oncogene 199918305–314. [DOI] [PubMed] [Google Scholar]
- 40.Winde G, Lugering N, Glodny B.et al Decreased HER‐2 tyrosine kinase expression in rectal mucosa of FAP patients following low‐dose sulindac chemoprevention. Cancer Lett 1998134201–207. [DOI] [PubMed] [Google Scholar]
- 41.Witton C J, Hawe S J, Cooke T G.et al Cyclooxygenase 2 (COX2) expression is associated with poor outcome in ER‐negative, but not ER‐positive, breast cancer. Histopathology 20044547–54. [DOI] [PubMed] [Google Scholar]
- 42.Costa C, Soares R, Reis‐Filho J S.et al Cyclo‐oxygenase 2 expression is associated with angiogenesis and lymph node metastasis in human breast cancer. J Clin Pathol 200255429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dowsett M, Bartlett J, Ellis I O.et al Correlation between immunohistochemistry (HercepTest) and fluorescence in situ hybridization (FISH) for HER‐2 in 426 breast carcinomas from 37 centres. J Pathol 2003199418–423. [DOI] [PubMed] [Google Scholar]