Abstract
Malignant histiocytosis is a rare invasive proliferation of neoplastic histiocytes. Cases previously reported as malignant histiocytosis were shown to be lymphomas of T or B lineage, especially anaplastic large‐cell lymphomas. A case of malignant histiocytosis is described, in which a patient presenting with symptoms suggestive of pneumonia suddenly deteriorated and died. At autopsy, a large quantity of fresh blood, originating from several ruptured nodules on the enlarged spleen, was seen in the peritoneal cavity. Extensive infiltration by pleomorphic tumour cells and erythrophagocytosis by tumour cells were seen on histological examination of the spleen. Immunohistochemical analysis and staining were carried out. This is the second reported case of malignant histiocytosis presenting with spontaneous splenic rupture.
A 63‐year‐old white woman was admitted with respiratory problems. Some weeks previously she had a synthetic bone graft inserted in her jaw. Because of unusually poor healing after surgery, a decision was made to remove the graft after she returned from a holiday in Cyprus. She returned feeling unwell, complaining of fever and night sweats. Incidentally, her dental surgeon had noticed bleeding gums and some weight loss over recent months. Community‐acquired pneumonia was suspected and she was subsequently started on antibiotics, but her condition deteriorated and she was admitted to the hospital. A computed tomography scan showed bibasal consolidation, bilateral pleural effusions and a small pericardial effusion. The pleural effusions were tapped for symptomatic relief and showed blood only on cytology. Her condition, however, deteriorated further and she was transferred to the intensive therapy unit, where she was put on ventilators and started on inotropes.
On admission, her laboratory findings were as follows: haemoglobin 9.5 mg/dl, platelets 70×109/l, white cell count 6.4×109/l (neutrophils 4.8×109/l, lymphocytes 0.8×109/l, eosinophils 0.01×109/l), C reactive protein 81 mg/l, alkaline phosphatase 220 IU/l, aspartate aminotransferase 108 IU/l, γ glutamyl transpeptidase 113 IU/l, bilirubin 16 mg/l, albumin 17 g/l and sodium 122 mmol/l. A blood film showed a left shift with toxic granulation and thrombocytopenia but no atypical cells. Investigations were negative for Mycoplasma pneumoniae, Mycobacterium tuberculosis and Legionella.
Multiorgan failure secondary to sepsis was suspected. Despite active measures, after 2 days in hospital, the patient's condition deteriorated suddenly and rapidly, with her haemoglobin concentration falling from 8 to 3.6 mg/dl in less than 24 h, and she died.
Postmortem examination
A complete autopsy, including external, gross and microscopic examination of individual internal organs, was carried out. Externally, the body was unremarkable, apart from periorbital oedema and general yellow discolouration of the skin with petechial haemorrhages and purpura on the chest and legs.
In the peritoneal cavity there was 2.5 l of fresh blood, which accounted for the sudden fall in haemoglobin in the intensive therapy unit. The free intraperitoneal blood was seen to originate from several ruptured haemorrhagic nodules on the surface of the enlarged spleen, which weighed 440 g. The heart appeared pale and showed mild dilatation of the right ventricle. Both lungs were pale, markedly oedematous and had a slightly firm texture. The liver was enlarged but otherwise normal. No lymphadenopathy was observed.
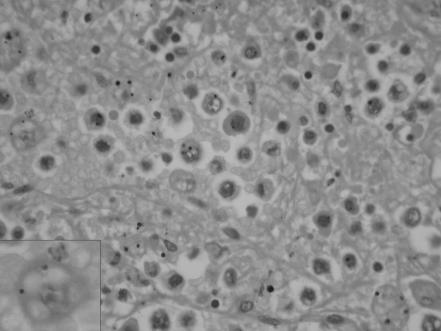
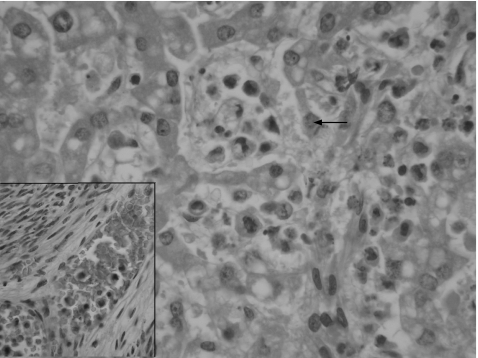
Histologically, there was extensive infiltration of the red pulp of the spleen by tumour cells, with polylobated, pleomorphic nuclei and prominent nucleoli. These discohesive cells had well‐defined cell borders and abundant eosinophilic cytoplasm that was occasionally foamy or clear (fig 1). Many binucleate and some multinucleate forms were present. Phagocytosed erythrocytes and leucocytes were seen in several tumour cells, predominantly those in the spleen and liver (fig 2). Scattered mitoses were also noted. Spindled cells were not seen.
Figure 1 High‐power photomicrograph showing extensive infiltration of the spleen by tumour cells with resultant effacement of normal architecture. The inset shows malignant histiocytes with well‐defined cell borders and abundant eosinophilic foamy cytoplasm (haematoxylin–eosin staining, 400×).
Figure 2 High‐power photomicrograph showing malignant histiocytes infiltrating and distending the sinusoids of the liver. Cytophagocytosis by a tumour cell can be seen just above the centre of the field (arrow). The inset shows blood vessel permeation in the myometrium by tumour cells (haematoxylin–eosin staining, 400×).
Tumour cells were seen infiltrating the parenchyma of the lungs, myocardium and liver. In the lungs, the neoplastic cells infiltrated alveolar spaces and blood vessels. In the myocardium, these cells were also seen in blood vessels. Numerous tumour cells were also seen spilling into the substance of the liver from sinusoids. Similar cells were seen in the small blood vessels of the hilar lymph nodes, kidney, adrenal glands, intestines, pancreas and endometrium.
Immunohistochemical analysis was carried out, with the tumour cells staining strongly with CD68 and CD43. They were negative for the T cell marker CD3 and the B cell markers CD79a and CD20. The cells were also negative for CD30, epithelial membrane antigen (EMA), CD23, CD21, S100 protein and myeloperoxidase.
Discussion
Malignant histiocytosis is a neoplasm composed exclusively of cells showing morphological and immunophenotypic features similar to those of mature tissue histiocytes.1 It is a rare tumour, accounting for less than 1% of adult non‐Hodgkin's lymphomas in the US and Europe.2,3,4 Few bona fide examples of malignant histiocytosis are found in the literature.5,6,7,8
Much confusion exists in the literature regarding the terminology of the entity, spanning various appellations such as atypical Hodgkin's disease, histiocytic lymphoma, histiocytic medullary reticulosis and malignant histiocytosis. Originally described in 1939 by Scott and Robb Smith as histiocytic medullary reticulosis, the entity was designated by Rappaport1 in 1966 as malignant histiocytosis. Malignant histiocytosis and true histiocytic lymphoma are now generally considered to be neoplasms of similar cell types, differing only in their presentation as disseminated (malignant histiocytosis) or localised (true histiocytic lymphoma).2,8 The term histiocytic sarcoma was introduced in 1970 by Mathe et al9 to include the whole spectrum of disseminated and localised forms from true histiocytic lymphomas to malignant histiocytosis. Other authors, however, serve to confuse the picture by limiting the term “histiocytic sarcoma” to the localised lesion previously called true histiocytic lymphoma, while classifying cases with multiple sites of involvement as “malignant histiocytosis”.
The advent and widespread availability of ancillary techniques enabled definitive lineage determination, and many cases originally regarded as malignant histiocytosis or true histiocytic lymphoma were later shown to be CD30 (Ki1)‐positive anaplastic large‐cell lymphoma or other types of lymphoid neoplasms.2,10,11,12
The disease is more prevalent in men (male/female ratio approximately 2:1).2,4 There is a wide age range, with a predilection for patients in their second and third decades.8 Constitutional symptoms are common, with most patients presenting with fever, night sweats and weight loss, as seen in our case. The primary sites commonly associated are the lymph nodes (in as many as a third of cases3), gastrointestinal tract and skin.6,7,13
Most patients have anaemia, thrombocytopenia and leukopenia at diagnosis. Occasionally, thrombocytosis is present. Neoplastic cells, if scanty, may not always be evident in blood films.13 As bone marrow and organs can sometimes be only focally affected, most diagnoses are made at postmortem examination.13 We reviewed the blood film in our patient several times, but did not find any neoplastic cells. Bone marrow biopsies before and after death were not undertaken, as a haematological malignancy was not suspected at that time.
Microscopically, malignant histiocytosis is composed of highly cellular, non‐cohesive proliferations of round, oval or polygonal cells. These neoplastic histiocytes contain abundant, eosinophilic cytoplasm that is occasionally foamy because of a high lipid content.6 The multilobed, occasionally bizarre nuclei have prominent nucleoli and frequent mitotic figures. Phagocytic activity may be present but is not pathognomonic.2,6 Earlier entities erroneously diagnosed as malignant histiocytosis because of prominent erythrophagocytosis by purportedly malignant histiocytes have now been shown to be reactive histiocytes or erythrophagocytic neoplasms of non‐histiocytic derivation.2 Characteristically, there is widespread infiltration of the liver, spleen and lymph nodes with a sinusoidal growth pattern, eventually resulting in diffuse replacement of the concerned viscera. Ultrastructurally, numerous lysosomes are seen within the cytoplasm. Birbeck granules and cellular junctions, seen in Langerhans cells and interstitial dendritic cells, are not present.3,7 Unlike combined tumours, such as malignant fibrous histiocytoma, there is no differentiation along other cell lines.8
Immunophenotypically, there is consistent expression of one or more histiocytic markers, including CD68, lysozyme, CD11c and CD14. S100 protein may be expressed, occasionally by activated normal macrophages.2 The absence of myeloid markers—for example, myeloperoxidase, CD33 and CD34—is essential, as certain histiocytic markers (eg, CD68) overlap with cells of myeloid lineage.7 Dendritic cell markers (CD1a, CD21 and CD35) and CD30, HMB45, EMA and keratin expression are also absent.3,7,8,14 Specific B cell and T cell markers are negative. The presence of CD4 (expression of T helper antigen by histiocytes) is a potential diagnostic pitfall in cases in which malignant lymphoma is suspected.3,7 The Ki67 index varies from 10% to 90%, with a mean of around 20%.3,8
The differential diagnosis of malignant histiocytosis includes large‐cell non‐Hodgkin's lymphomas and metastatic carcinoma. The most important differential diagnosis to consider is anaplastic large‐cell lymphoma, which may be morphologically indistinguishable from malignant histiocytosis, but with expression of CD30 and EMA. Langerhans cell histiocytosis also forms part of the differential diagnosis, but this tumour lacks appreciable nuclear atypia and has a characteristic milieu of eosinophils, neutrophils and lymphocytes. Langerhans cell histiocytosis also expresses both CD1a and S100 protein, whereas malignant histiocytosis does not show CD1a expression.3,4,7 Another differential to be borne in mind is the M5 monocytic subtype of acute myeloid leukaemia that may present with soft‐tissue disease15 and may be consequently misdiagnosed as malignant lymphoma or anaplastic carcinoma. The detection of tumour cells in peripheral blood and bone marrow is therefore important in alerting us to the diagnosis, especially as the abundant cytoplasm and occasional reniform nuclei of the malignant monocytes are features also shared by tumour cells of malignant histiocytosis. Immunohistochemically, this sarcomatous leukaemic subtype stains for lysosyme and myeloid antigens.15,16 Metastatic carcinoma can be distinguished by straightforward immunohistochemical analysis.
Take‐home messages
A case of malignant histiocytosis, a rare aggressive neoplasm of histiocytes presenting with spontaneous splenic rupture, is described.
The tumour consists of pleomorphic mitotically active tumour cells that may show erythrophagocytosis. Anaplastic large cell lymphoma is sometimes morphologically indistinguishable, but malignant histiocytosis stains strongly with CD68.
In the present case, the patient presented clinically with symptoms suggesting pneumonia. Neoplastic infiltration of the lungs and heart accounted for the breathlessness and effusions. The hepatomegaly and deranged liver function tests were due to infiltration of the liver by neoplastic cells. The mechanical pressure exerted by neoplastic cells infiltrating extensively would have led to capsular weakening and splenic rupture.
Spontaneous rupture of the spleen is a rare but well‐recognised complication of haematological malignancies, which may also occur in infective and metabolic conditions. From 1966 to 2002, 79 cases of leukaemia and 23 cases of lymphoma, in which pathological rupture of the spleen occurred, have been described worldwide.17 To our knowledge, this is the second case of malignant histiocytosis presenting with splenic rupture reported in the literature.
Regardless of the site of origin, this tumour has an aggressive behaviour and often presents with B symptoms, lymphadenopathy and mediastinal involvement. In a study by Pileri et al,18 72% of patients succumbed rapidly despite chemotherapy. Mean survival time was 12 months. B symptoms and bulky disease seemed to influence prognosis, both suggesting a more aggressive course of the tumour. Although the tumour is aggressive and has a poor prognosis, it may be potentially curable19 and a definitive diagnosis by immunohistological means is thus necessary to guide treatment.
Abbreviations
EMA - epithelial membrane antigen
Footnotes
Competing interests: None declared.
Informed consent was received for publication of this case report.
References
- 1.Rappaport H. Histiocytosis. In: Atlas of tumour pathology: tumours of the haematopoietic system. Section 3, fascicle 8. Washington, DC: Armed Forces Institute of Pathology, 196648–63.
- 2.Warnke R A, Weiss L M, Chan J K C.et alAtlas of tumour pathology: tumours of the lymph nodes and spleen. Series 3, fascicle 14. Washington, DC: Armed Forces Institute of Pathology, 1995
- 3.Weiss L M, Grogan T M, Muller‐Hermelink H K.et al Histiocytic and dendritic cell neoplasms: histiocytic sarcoma. In: Jaffe ES, ed. WHO classification of tumours: pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press, 2001278–319.
- 4.Gatter K. True histiocytic lymphoma (histiocytic sarcoma). In: Gatter K, Delsol G, eds. Atlas: the diagnosis of lymphoproliferative diseases. Oxford: Oxford University Press, 2002238–241.
- 5.Cheuk W, Walford N, Lou J.et al Primary histiocytic lymphoma of the central nervous system: a neoplasm frequently overshadowed by a prominent inflammatory component. Am J Surg Pathol 2001251372–1379. [DOI] [PubMed] [Google Scholar]
- 6.Copie‐Bergman C, Wotherspoon A C, Norton A J.et al True histiocytic lymphoma: a morphologic, immunohistochemical and molecular genetic study of 13 cases. Am J Surg Pathol 1998221386–1392. [DOI] [PubMed] [Google Scholar]
- 7.Pileri S A, Grogan T M, Harris N L.et al Tumours of histiocytes and accessory dendritic cells: an immunohistochemical approach to classification from the International Lymphoma Study Group based on 61 cases. Histopathology 2002411–29. [DOI] [PubMed] [Google Scholar]
- 8.Sun W, Nordberg M L, Fowler M R. Histiocytic sarcoma involving the central nervous system. Am J Surg Pathol 200327258–265. [DOI] [PubMed] [Google Scholar]
- 9.Mathe G, Gerard‐Marchant R, Texier J L.et al The two varieties of lymphoid tissue: ‘reticulosarcomas', histiocytic and histioblastic types. Br J Cancer 197024687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egeler R M, Schmitz L, Sonneveld P.et al Malignant histiocytosis: a reassessment of cases formerly classified as histiocyte neoplasms and review of the literature. Med Pediatr Oncol 1995251–7. [DOI] [PubMed] [Google Scholar]
- 11.Van der Valk V D, Meijer C J. Histiocytic sarcoma: clinical picture, morphology, markers, differential diagnosis. Pathol Annu 1985201–28. [PubMed] [Google Scholar]
- 12.Wilson M S, Weiss L M, Gatter K C.et al Malignant histiocytosis: a reassessment of cases previously reported in 1975 based on paraffin section immunophenotyping studies. Cancer 199066530–536. [DOI] [PubMed] [Google Scholar]
- 13.Lichtman M A, Komp D M. Inflammatory and malignant histiocytoses. In: Beutler E, ed. Williams haematology. 5th edn. New York: McGraw‐Hill, 1995885–894.
- 14.Ralfkiaer E, Delsol G, O'Connor N.et al Malignant lymphomas of true histiocytic origin: a clinical, histologic, immunophenotypic and genotypic study. J Pathol 19901609–17. [DOI] [PubMed] [Google Scholar]
- 15.Bain B, Manoharan A, Lampert I.et al Lymphoma‐like presentation of acute monocytic leukaemia. J Clin Pathol 198336559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunning R D, Matutes E, Flandrin G.et al Acute myeloid leukaemia not otherwise categorised. In: Jaffe ES, ed. WHO classification of tumours: pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press, 200191–105.
- 17.D'Valerio D. A traumatic rupture of the spleen in adults. J R Coll Surg Edinb 200247437–445. [PubMed] [Google Scholar]
- 18.Pileri S, Mazza P, Rivano M T.et al Malignant histiocytosis (true histiocytic lymphoma) clinicopathological study of 25 cases. Histopathology 19859905–920. [DOI] [PubMed] [Google Scholar]
- 19.Tseng A, Jr, Coleman C N, Cox R S.et al The treatment of malignant histiocytosis. Blood 19846448–53. [PubMed] [Google Scholar]