Abstract
Background
The p16 and retinoblastoma (Rb) gene products are part of the retinoblastoma pathway controlling the G1–S transition of the cell cycle. Few studies on the expression of p16 and retinoblastoma proteins in oral cavity squamous carcinomas have been conducted.
Aim
To correlate the expression of p16 and retinoblastoma proteins to clinicopathological characteristics in these tumours.
Methods
45 patients with resected oral cavity squamous carcinoma were selected, for whom this was the initial treatment and who were followed up for 5 years or until death. Immunohistochemical stains with antibodies to the Rb and p16 gene products were carried out on paraffin wax‐embedded tissue. Data on clinicopathological features such as tumour differentiation, nodal status, stage and survival outcome were collected.
Results
Retinoblastoma expression was seen in 39 of 45 (87%) patients and p16 expression in 6 of 45 (13%) patients. A significant inverse correlation was observed between retinoblastoma and p16 expression as nearly all retinoblastoma negative cases were p16 positive, and vice versa. When examined for clinicopathological correlates, it was found that all 39 tumours that expressed retinoblastoma displayed marked keratinisation and were of low–moderate histological grade. Conversely, five of the six tumours that expressed p16 were found to be poorly differentiated, with minimal keratin expression.
Conclusions
Salient relationships were seen between expression of retinoblastoma and p16 and keratinisation. A marked loss of keratin production was evident in the tumours that expressed p16. Tumours expressing retinoblastoma were seen to exhibit more widespread keratinisation. In addition, an inverse staining pattern was found for retinoblastoma and p16 as retinoblastoma‐expressing tumours were nearly universally p16 negative and vice versa. No correlation of expression of either p16 or retinoblastoma was found with survival or stage. A link between the histologically observable morphology and expression of cell cycle regulatory protein with the expression of p16 and retinoblastoma has been suggested with keratinisation and differentiation of status.
Retinoblastoma (Rb) and p16 gene products are part of the retinoblastoma pathway that controls the cell cycle. Cell cycle regulation is critical in tumorigenesis and Rb gene inactivation has been reported in many cancers. The Rb gene is located on the long arm of chromosome 13. The retinoblastoma protein is a nuclear phosphoprotein that is expressed in most normal cells. Rb functions during the G1–S transition within the cell cycle. The hypophosphorylated form of the retinoblastoma protein mediates G1 arrest. The phosphorylation of retinoblastoma protein at the G1–S transition is driven by the cyclin‐dependent kinases (CDKs), in protein complexes with cyclin D1. Strong inhibitors control these complexes, including the tumour suppressor gene, p16. The p16 gene is located on chromosome 9p21. p16 protein blocks cellular proliferation at the G1–S phase of the cell cycle by binding to the CDKs, primarily CDK4 and CDK6. This prevents formation of the catalytically active cyclin D–CDK4 or CDK6 complex, which phosphorylates the retinoblastoma protein that drives the cell cycle.1 The inactivation of p16 and retinoblastoma leads to an imbalance of cell cycle regulation and therefore, unrestricted proliferation and tumorigenesis.
We studied the expression of p16 and retinoblastoma proteins in patients with oral cavity squamous carcinomas.
Design
Patient selection and clinical findings
In all, 45 patients with resected (primary therapy) oral cavity squamous carcinomas followed up for 5 years or until death, were selected. The age range of the patients was 25–84 years. Of them, 32 patients were men and 13 were women. In all, 6 patients had stage I disease, 3 stage II disease, 9 stage III disease and 25 stage IV disease. The sites that were associated were tongue (17 patients); floor of the mouth (13); gum (8); retromolar trigone (3); and buccal mucosa (2). By definition, the tumours arose in the oral cavity and none were base‐of‐tongue. All tumours were squamous cell carcinomas. None of them were classified as verrucous, papillary or basaloid squamous carcinomas on review. Five tumours exhibited poorly differentiated histological patterns. These cases exhibited areas of cells with hyperchromatic nuclear features that grew in an aggregated or nested pattern and had high nuclear:cytoplasmic ratios. No areas of central necrosis, mucinous change, hyalinisation or cystic space formation, characteristic of basaloid squamous carcinomas, were seen. The remaining 40 tumours were well to moderately differentiated and all exhibited eosinophilic cytoplasm and keratin production.
Immunohistochemical staining for retinoblastoma and p16
The tumours were immunohistochemically stained using antibodies to the Rb and p16 gene products and by immunohistochemical methods. Tissue blocks containing tumour and morphologically normal tissue samples were selected to provide an internal control of staining. In brief, 5‐μm sections of formalin‐fixed, paraffin wax‐embedded tumour tissue were deparaffinised with xylene and alcohol. Immunohistochemical staining for p16 was carried out using p16 ink4a kit (DakoCytomation, Carpinteria, California, USA) with a final antibody dilution of 1:25 (by following the directions in the kit). Antigen retrieval was accomplished by immersing the slides in solution (DakoCytomation Target Retrieval Solution, DakoCytomation) for 10 min in a steamer set at 95°C.
For retinoblastoma, endogenous peroxide was quenched with H2O or H2O2. The slides for retinoblastoma were antigen retrieved by immersion into Tris or HCl buffer, pH 10 and microwaved in a 500 W microwave oven four times for 5 min each. Retinoblastoma antibody (DakoCytomation) was used at a 1:25 concentration in diluent (DakoCytomation) for 30 min at room temperature. The slides were rinsed with DAKO 1X Wash Buffer (DakoCytomation) twice for 5 min. DakoCytomation Rabbit Envision HRP System reagent (DakoCytomation) was then applied to the slides for 30 min at room temperature. Slides for both antibodies were developed by applying DAKO DAB Plus (DakoCytomation) for 3–5 min until colour developed, followed by counterstaining with haematoxylin.
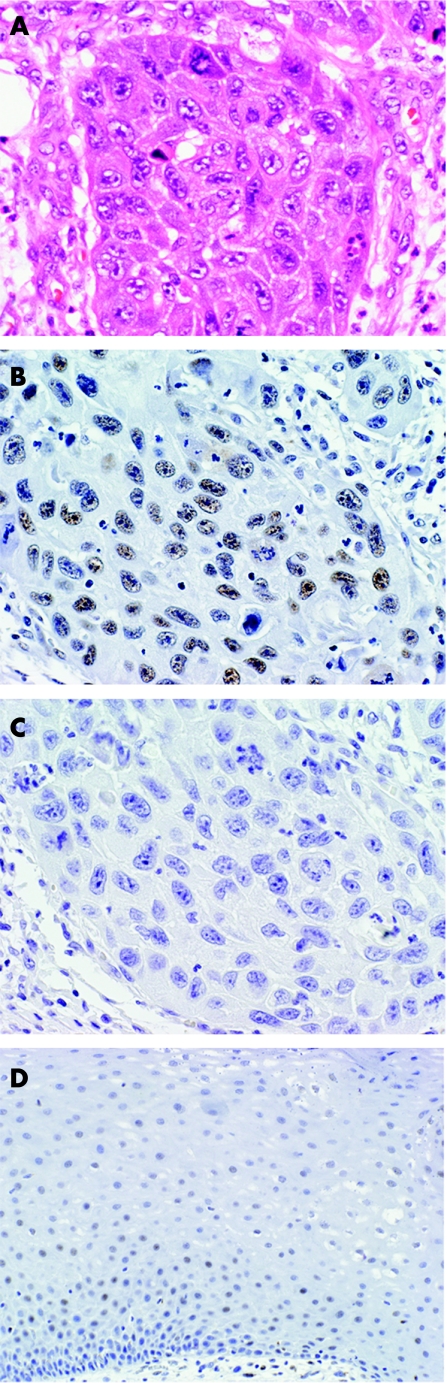
Mucosa that appeared morphologically normal in the sections stained was used as a control. For retinoblastoma, faint and scattered nuclear staining was present in the normal epithelium. Near areas of inflammation and in some mucosa adjacent to the tumour, increased intensity and a greater number of nuclei staining for retinoblastoma were seen (fig 1A). p16 expression was not observed in normal mucosa (fig 1B). Tumours were evaluated semiquantitatively for the amount of staining of both antibodies on a scale of 0–4 (0, no staining; 1, staining of up to 24% of tumour cell nuclei; 2, staining of 25–49% of nuclei; 3, staining of 50–74% of nuclei; and 4, staining of >75% of nuclei). Similarly, the amount of keratin production (as determined by visual inspection with haematoxylin and eosin stain) by the tumour was semiquantitated on a scale of 0–4, with 0, no keratin production and 4, to > 75% of the tumour producing keratin. Tumours were graded as 1, well differentiated; 2, moderately differentiated; and 3, poorly differentiated. The disease status of the patients was staged according to the American Joint Committee on Cancer.2 Fisher's exact test was used for categorical variables and log rank analysis was used for survival differences (Stata V.6).
Figure 1 Moderately differentiated oral cavity squamous cell carcinoma (A) showing positive reaction with retinoblastoma antibody (B) but no reaction with p16 antibody (C). Panel D illustrates normal mucosa stained with retinoblastoma antibody.
Results
The age range of the patients whose tumours were p16 negative or retinoblastoma positive was 25–84 (median = 58) years and the age range of the patients whose tumours were p16 positive or retinoblastoma negative was 39–78 (median = 58) years. Of the tumours expressing retinoblastoma, 5 of 6 were stage I, 3 of 3 stage II, 8 of 9 stage III and 23 of 27 stage IV (table 1).
Table 1 Clinicopathological correlates.
| ID | Site | Age/sex | Stage/node status | Histological grade | Keratin | p16 | Retinoblastoma |
|---|---|---|---|---|---|---|---|
| 1 | Gum | 67/F | I/N0 | 1 | 4 | 0 | 2 |
| 2 | Buccal | 84/F | IV/N0 | 2 | 4 | 0 | 3 |
| 3 | Buccal | 59/M | I/N0 | 3 | 0 | 3 | 0 |
| 4 | Fom | 59/M | IV/N0 | 2 | 4 | 0 | 2 |
| 5 | Fom | 70/M | III/N0 | 2 | 4 | 0 | 3 |
| 6 | Fom | 69/M | IV/N0 | 2 | 4 | 0 | 1 |
| 7 | Gum | 59/M | IV/N0 | 2 | 4 | 0 | 4 |
| 8 | Gum | 77/F | IV/N0 | 2 | 4 | 0 | 3 |
| 9 | Rmt | 59/M | IV/N1 | 2 | 4 | 0 | 3 |
| 10 | Tongue | 39/M | IV/N2 | 3 | 1 | 4 | 0 |
| 11 | Tongue | 69/M | IV/N2 | 3 | 1 | 4 | 0 |
| 12 | Tongue | 25/F | I/N0 | 1 | 4 | 0 | 3 |
| 13 | Tongue | 25/M | IV/N2 | 2 | 4 | 0 | 2 |
| 14 | Tongue | 69/F | I/N0 | 2 | 4 | 0 | 2 |
| 15 | Tongue | 57/F | IV/N2 | 1 | 4 | 0 | 3 |
| 16 | Fom | 52/M | IV/N2 | 2 | 4 | 0 | 3 |
| 17 | Gum | 57/M | IV/N1 | 2 | 4 | 0 | 2 |
| 18 | Tongue | 67/M | IV/N1 | 2 | 4 | 0 | 4 |
| 19 | Tongue | 52/M | III/N1 | 1 | 4 | 0 | 2 |
| 20 | Tongue | 25/M | IV/N2 | 2 | 4 | 0 | 3 |
| 21 | Tongue | 61/M | IV/N1 | 2 | 4 | 0 | 0 |
| 22 | Tongue | 46/M | I/N0 | 2 | 4 | 0 | 1 |
| 23 | Gum | 75/F | IV/N1 | 2 | 4 | 0 | 2 |
| 24 | Gum | 71/F | IV/N2 | 2 | 4 | 0 | 3 |
| 25 | Gum | 67/M | IV/N1 | 2 | 4 | 0 | 4 |
| 26 | Tongue | 45/F | II/N0 | 1 | 4 | 0 | 4 |
| 27 | Tongue | 52/M | IV/N2 | 2 | 4 | 3 | 1 |
| 28 | Gum | 67/M | II/N0 | 2 | 4 | 0 | 2 |
| 29 | Tongue | 72/F | III/N0 | 1 | 4 | 0 | 3 |
| 30 | Tongue | 51/F | IV/N0 | 2 | 4 | 0 | 3 |
| 31 | Fom | 68/M | IV/N1 | 2 | 4 | 0 | 2 |
| 32 | Fom | 54/M | III/N0 | 2 | 4 | 0 | 2 |
| 33 | Fom | 52/M | III/N1 | 2 | 4 | 0 | 3 |
| 34 | Fom | 61/M | IV/N1 | 2 | 4 | 0 | 3 |
| 35 | Fom | 48/M | IV/N0 | 1 | 4 | 0 | 4 |
| 36 | Fom | 42/M | II/N0 | 2 | 4 | 0 | 2 |
| 37 | Rmt | 68/F | IV/N 2 | 2 | 4 | 0 | 2 |
| 38 | Tongue | 58M | III/N1 | 1 | 4 | 0 | 3 |
| 39 | Tongue | 58/M | III/N0 | 1 | 4 | 0 | 2 |
| 40 | Rmt | 45/M | IV/N0 | 2 | 4 | 0 | 2 |
| 41 | Tongue | 43/M | III/N1 | 2 | 4 | 0 | 3 |
| 42 | Tongue | 47/M | I/N0 | 2 | 4 | 0 | 2 |
| 43 | Fom | 58/M | III/N0 | 3 | 0 | 3 | 0 |
| 44 | Fom | 78/M | IV/N1 | 3 | 1 | 4 | 0 |
| 45 | Fom | 57/F | I/N0 | 1 | 4 | 0 | 3 |
Fom, floor of the mouth; I–IV: stage of tumour; N0, no regional lymph node metastasis; N1, metastasis in a single ipsilateral lymph node <3 cm; N2, metastasis in a single node >3 cm or metastasis to multiple lymph nodes; Rmt, retromolar trigone.
Expression of retinoblastoma proteins was seen in 39 of 45 (87%) patients and p16 proteins in 6 of 45 (13%) patients. A significant inverse correlation was noted between retinoblastoma and p16 expression (p<0.001, Fisher's exact test) in the tumours as almost all retinoblastoma negative cases were p16 positive or vice versa (table 2).
Table 2 Comparison of p16 and retinoblastoma expression (keratinisation and differentiation status also noted).
| p16 negative (n = 39) | p16 positive (n = 6) | p Value | |
|---|---|---|---|
| Retinoblastoma negative (n = 6) | Keratinising and moderately differentiated (n = 1) | All minimally keratinising and poorly differentiated (n = 5) | <0.001 |
| Retinoblastoma positive (n = 39) | All keratinising and well to moderately differentiated (n = 38) | Keratinising and moderately differentiated (n = 1) |
Tumours expressing retinoblastoma showed low to moderate differentiation and about 75% or more of the cells exhibited keratinisation. Figure 1A shows a moderately differentiated squamous carcinoma that is retinoblastoma positive (fig 1B) but p16 negative (fig 1C).
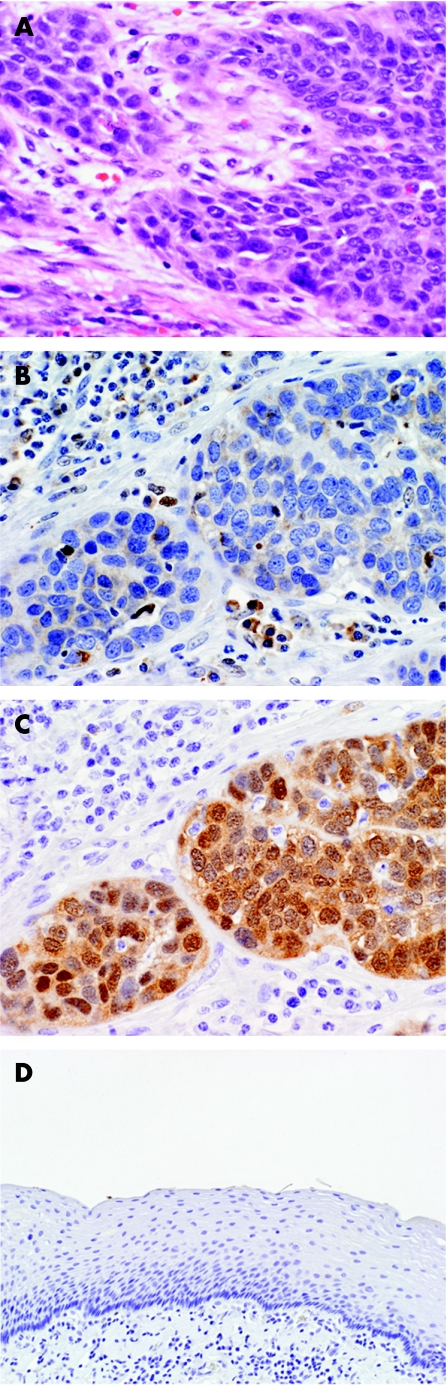
Unexpectedly, five of the six tumours expressing p16 were poorly differentiated. These tumours had extensive areas (> 50% of the tumour) that showed non‐keratinising patterns. The tumour cells in these cases were characterised by high nuclear:cytoplasmic ratios, minimal eosinophilic cytoplasm and a growth pattern that was nested (fig 2A). Figure 2B shows one of these poorly differentiated carcinomas, showing lack of retinoblastoma expression. The same tumour is seen expressing p16, however (fig 2C). These tumours lacked necrosis, central cystic formation, mucinous change or other features of basaloid squamous cell carcinoma. In one case, both p16 and retinoblastoma were not expressed and in another case both p16 and retinoblastoma were expressed. In both these instances the tumours were moderately differentiated.
Figure 2 Poorly differentiated oral cavity squamous cell carcinoma (A) showing no reaction with retinoblastoma antibody (B) but a positive reaction with p16 antibody (C). p16 antibody staining of normal mucosa is shown in panel D.
In all, 21 of the 25 patients alive at the 5‐year follow‐up had tumours expressing retinoblastoma, whereas 18 of 20 patients with tumours expressing retinoblastoma were dead at 5 years. Neither stage of disease nor survival outcome was significantly associated with retinoblastoma expression (p = 0.89 and p = 0.56, respectively, Fisher's exact test). Of the 22 patients with regional nodal disease 18 had neoplasms that expressed retinoblastoma, whereas 21 of the 23 patients without nodal disease had tumours that expressed retinoblastoma (p = 0.35, Fisher's exact test). Metastatic deposits were not examined for expression of p16 or retinoblastoma.
Of the six tumours that were p16 positive five had perineural invasion and three had vascular invasion, whereas of the 39 p16 negative tumours 32 had perineural invasion and 16 had vascular invasion (p = 1.0 and p = 0.69, perineural and vascular invasion, respectively, Fisher's exact test). Similarly, of the 39 retinoblastoma positive tumours, 32 exhibited perineural invasion and 16 exhibited vascular invasion.
Four of the p16‐expressing tumours were stage IV at the time of presentation, but only two of these patients were dead at the 5‐year follow‐up. Four of the six p16‐positive tumours also had regional lymph node metastases. By log rank analysis, neither expression of retinoblastoma nor that of p16 correlated with survival (p = 0.71 and p = 0.50, respectively).
Discussion
Control of the cell cycle is a tightly regulated process, one in which an imbalance can lead to unrestricted proliferation. Rb gene inactivation, reflected by the absence of retinoblastoma protein expression, has been reported in various solid cancers.3,4,5 The hypophosphorylated form of retinoblastoma protein prevents the cells from transitioning across the G1 checkpoint by sequestering transcription factors, such as E2F, which are necessary to activate the S‐phase genes.6 Phosphorylation of retinoblastoma protein is driven by the CDKs or cyclinD1 complex that propagates the transition from G1–S phase. The tumour suppressor gene, p16, inhibits many of these protein complexes that drive the G1–S transition. The p16 protein exerts its tumour suppressor function by binding to the cyclin D1–CDK4 or CDK6 complex, which prevents the phosphorylation of the retinoblastoma protein. The lack of retinoblastoma protein phosphorylation ultimately results in G1 arrest. Inactivation of p16 may occur through several different mechanisms including homozygous deletion, single base‐pair mutation, and methylation of the gene promoter, leading to subsequent transcriptional silencing.7 In the squamous cell carcinomas of head and neck, inactivation of p16 was by homozygous deletion in 33%,8 by methylation in 20%9 and by mutation in 10%10 of primary cancers. Loss of p16 activity allows the CDKs to trigger phosphorylation of retinoblastoma protein during the mid‐G1 to the late‐G1 phase of the cell cycle, thereby eliminating the function of growth suppression.
Previous studies11,12,13 have shown that in cell lines lacking functional retinoblastoma protein, high levels of p16 are often observed. In this study, retinoblastoma expression was seen in 39 of 45 (87%) patients and p16 expression in 6 of 45 (13%) patients. We discovered a significant inverse relationship between retinoblastoma and p16 expression (p<0.001) in our tumours, which confirms previous observations of retinoblastoma and p16 inactivation to be inversely correlated in some tumours, including head and neck squamous cell carcinomas.14,15 Almost all of our retinoblastoma‐positive cases were p16 negative and vice versa.
Retinoblastoma and p16 positive staining is observed in normal mucosal epithelium, primarily around the basal cell layer. Oral premalignant lesions often lack p16 expression, supporting the fact that p16 is inactivated at an early stage in the process of head and neck tumorigenesis.13,16 Papadimitrakopoulou et al16 attempted to define the role of p16 in head and neck tumorigenesis by analysing oral premalignant lesions. They found that 47% of patients with oral premalignant lesions showed lack of p16 protein expression by immunohistochemistry, which correlated with loss of heterozygosity in roughly 80% of the cases.
Previous reports17 have suggested that retinoblastoma protein function is absent in malignant oral epithelium and others have shown raised retinoblastoma protein levels during oral cancer progression. Although the alteration in retinoblastoma protein expression tumorigenesis has not been completely elucidated, there is increasing evidence that the retinoblastoma protein pathway is rendered dysfunctional in oral cavity squamous cell carcinomas.
Aside from the tight regulatory interaction of retinoblastoma and p16, the inverse relationship indicates that a mutation in either Rb or p16 is sufficient to disrupt the cell cycle at the G1‐checkpoint pathway, leading to cell proliferation. Our data support the finding that loss of p16 is common in oral cavity squamous cell carcinomas. The evidence that oral cavity premalignant lesions show loss of heterozygosity at 9p21 and loss of p16 staining by immunohistochemistry suggests that the loss of p16 protein may be a greater factor in the development of oral cavity squamous cell carcinomas.16
Take‐home messages
A statistically significant inverse relationship was seen between expression of Rb and p16 in this series of oral cavity squamous cell carcinomas.
Expression of p16 and loss of Rb expression was primarily seen in the oral cavity squamous cell carcinomas that exhibited extensive basal and morphology, suggesting a possible link between morphology and cell cycle regulatory protein expression.
No statistically significant correlation of expression of either p16 or Rb was found with survival or stage.
Although no correlation was found in our study between clinical status and retinoblastoma or p16 expression, Tanaka et al18 found that loss of pRb2 or p130, a suppressor oncogene in the retinoblastoma family, correlated with reduced survival rate in oral squamous cell carcinomas. Tsai et al19 found no relationship between p16 gene changes and location or clinical stage of the tumour. They did discover a close relationship between p16 changes and cancer metastasis to neck lymph nodes, and the p16 changes gradually increased with poorly differentiated grades of tumour. They concluded that changes in p16 may contribute to the metastatic potential or histological grade of the tumour cells.19 We found no correlation of p16 or retinoblastoma protein expression and clinicopathological parameters, including stage of disease, survival status and perineural or vascular invasion.
This study aimed to examine immunohistochemically the expression of retinoblastoma and p16 proteins in patients with oral cavity squamous cell carcinomas. Five of the six tumours positive for p16 or negative for retinoblastoma expression showed a marked non‐keratinising appearance and poor differentiation. Keratinising differentiation was seen in the other 39 tumours that did not stain for p16. This suggests a possible link between morphology and cell cycle regulatory protein expression in oral cavity squamous carcinoma. The correlation of p16 to a morphological finding was unexpected. The tumours that exhibited p16 had significant portions consisting of cells with a predominantly non‐keratinised, poorly differentiated morphology. These tumours did not meet the criteria for basaloid squamous carcinoma as they lacked extensive hyalinisation, central necrosis, mucinous change and cyst formation. The tumours did, however, show areas in which the tumour cells had reduced cytoplasm, showed nuclear hyperchromatism and nuclear chromatin clumping. These poorly differentiated cells were arranged in clusters or groups, often with a rounded border. We hypothesise that p16 protein or retinoblastoma protein has an effect on cell differentiation. The cells are probably arrested at a stage within the process of differentiation, leading to the tumours comprised predominantly of poorly differentiated non‐keratinised areas.
The number of cases in our series is limited, but the correlation of the markers studied and the degree of keratinisation and differentiation were striking. Clearly, the study should be expanded to increase the number of tumours studied in the oral cavity as well as in other sites. Nevertheless, an important inverse relationship was detected for retinoblastoma and p16 protein expression, as reported by others, but, importantly, a correlation to morphology was also found. The finding of neoplasms with extensive areas of keratinisation expressing only retinoblastoma but not p16 or, conversely, neoplasms with extensive areas of poorly differentiated tumour cells expressing only p16 suggests that these oncoproteins are related to the morphological findings seen on routine haematoxylin and eosin sections.
Acknowledgements
This work was supported in part by the FW Stamler Professorship to Dr Robinson.
Abbreviations
CDKs - cyclin‐dependent kinases
Rb - retinoblastoma gene
Footnotes
Competing interests: None.
Ethical approval: The University of Iowa institutional review board approved this work.
References
- 1.Wing Yuen P, Man M, Yin Lam K.et al Clinicopathological significance of p16 gene expression in the surgical treatment of head and neck squamous cell carcinomas. J Clin Pathol 20025558–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Joint Committee on Cancer AJCC cancer staging manual. 6th edn. New York: Springer, 200023–32.
- 3.Meling G I, Lothe R A, Borresen A L.et al Genetic alterations within the retinoblastoma locus in colorectal carcinoma. Relation to DNA ploidy pattern studies by flow cytometric analysis. Br J Cancer 199164475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyton R F, Huang Y, Blount P L.et al Frequent loss of heterozygosity at the retinoblastoma locus in human esophageal cancers. Cancer Res 1991515766–5769. [PubMed] [Google Scholar]
- 5.Jiang W, Zhang Y J, Kahn S M.et al Altered expression of the cyclin D1 and retinoblastoma genes in human esophageal cancer. Proc Natl Acad Sci USA 1993909026–9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pande P, Mathur M, Shukla N K.et al pRb and p16 protein alterations in human oral tumorigenesis. Oral Oncol 199834396–403. [DOI] [PubMed] [Google Scholar]
- 7.Geisler S A, Olshan A F, Weissler M C.et al p16 and p53 protein expression as prognostic indicators of survival and disease recurrence from head and neck cancer. Clin Cancer Res 200283445–3453. [PubMed] [Google Scholar]
- 8.Cairns P, Ploascik T J, Eby Y.et al, Frequency of homozygous deletion at p16/cdkn2 in primary human tumors. Nat Genetics 199511210–212. [DOI] [PubMed] [Google Scholar]
- 9.Merlo A, Herman J G, Mao L.et al 5' CpG island methylation is associated with transcriptional silencing of the tumor suppressor p16/CDKN2/MTs1 in human cancers. Nat Med 19954686–692. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S Y, Klein‐Szanto A J P, Sauter E R.et al Higher frequency alterations in the p16/CDKN2 gene in squamous cell carcinoma cell lines than in primary tumors of the head and neck. Cancer Res 1994545050–5053. [PubMed] [Google Scholar]
- 11.Andl T, Kahn T, Pfuhl A.et al Etiological involvement of oncogenic human papilloma virus in tonsillar squamous cell carcinomas lacking retinoblastoma cell cycle control. Cancer Res 1998585–13. [PubMed] [Google Scholar]
- 12.Lai S, El‐Nagger A K. Differential expression of key cell cycle genes (p16/cyclin D1/pRb) in head and neck squamous carcinomas. Lab Invest 199979255–260. [PubMed] [Google Scholar]
- 13.Nakahara Y, Shintani S, Mihara M.et al Alterations of Rb, p16 INK4A and cyclin D1 in tumorigenesis of oral squamous cell carcinomas. Cancer Lett 20001603–8. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Nichols M A, Shay J W.et al Transcriptional repression of the D‐type cyclin dependent kinase inhibitor p16 by retinoblastoma susceptibility gene product pRb. Cancer Res 1994546078–6082. [PubMed] [Google Scholar]
- 15.Okami K, Demetrick D J, Spillare E A.et al Mutations and altered expression of p16INK4 in human cancer. Proc Natl Acad Sci USA 19949111045–11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papadimitrakopoulou V, Izzo J, Lippman S.et al Frequent inactivation of p16 INK4a in oral premalignant lesions. Oncogene 1997141799–1803. [DOI] [PubMed] [Google Scholar]
- 17.Todd R, Hinds P W, Munger K.et al Cell cycle dysregulation in oral cancer. Crit Rev Oral Biol Med 20021351–61. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka N, Ogi K, Odajima T.et al pRb/p130 protein expression is correlated with clinicopathologic findings in patients with oral squamous cell carcinoma. Cancer 2001922117–2125. [DOI] [PubMed] [Google Scholar]
- 19.Tsai C, Yang C, Chou L.et al The correlation between alteration of p16 gene and clinical status in oral squamous cell carcinoma. J Oral Pathol Med 200130527–531. [DOI] [PubMed] [Google Scholar]