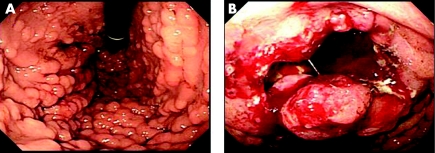
We report on a 51‐year‐old female patient with familial adenomatous polyposis (FAP) that developed into advanced gastric cancer after long‐term observation without any treatment (>12 years). We observed gastric carcinogenesis using immunohistochemistry. The patient had a FAP pedigree, and onset of FAP is reported in 11 members of her family. During clinic visits, a total of 12 endoscopies of the large bowel were carried out and tubular adenoma of group 2–3 was seen during all examinations. In a recent detailed examination of the upper gastrointestinal tract, Borr type III gastric cancer was found, with many hepatic metastasis lesions and many polyps in the fundic gland (fig 1A,B).
Figure 1 (A) Many polyps in the fundic gland region and (B) Borr type III advanced gastric cancer with deep ulcer in the upper gastric body, observed by endoscopic examination.
Systemic chemotherapy was not effective and the patient died on 3 June 2005. A pathological autopsy was carried out with the consent of her family.
Materials and methods
All specimens obtained during autopsy were stored in 10% formalin. Haematoxylin and eosin staining was carried out using a routine technique. Immunohistochemical methods were used on paraffin wax‐embedded specimens after formalin fixation. Monoclonal antibodies were used for immunostaining of p53 and cytokeratin 7, the dilution ratios of which were as follows: anti‐p53 antibody (1/50, DO‐7, Novocastra Laboratories Ltd, Newcastle, UK) and anti‐cytokeratin 7 antibody (1/100, OV‐TL 12/30, DakoCytomation, Ely, UK). A portion of the specimens was used to perform double staining to detect p53 protein and cytokeratin 7 expressions by using EnVision/AP reagent (Dakocytomation, Code noK4017/4018).
Discussion
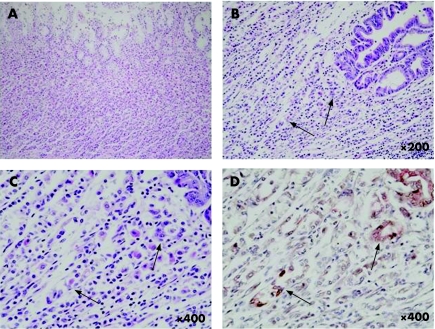
Few reports exist on the long‐term observation of the natural medical history (without any treatment) of patients with FAP who have an obvious family history. Our patient was extremely rare; she developed concomitant advanced gastric cancer during the clinical course of the disease. Gastric cancer complications in patients with FAP are not highly reported in the literature.1,2,3,4,5 Most patients, however, had gastric cancer in the gastric antrum and adenoma derived from intestinal metaplastic lesions in the gastric antrum. Because in our patients gastric cancer occurred in the upper gastric body without surrounding adenoma, there is the possibility that a factor other than the generally speculated carcinogenesis from adenoma was associated. Figure 2A shows gastric mucosa in the vicinity of gastric cancer. No chronic gastritis accompanied by atrophy was observed. Almost normal gastric mucosa was seen, with little atrophy or inflammation. When dissection of the mucosa was conducted, however, haematoxylin and eosin staining showed sporadic atypical cells in a few areas (fig 2B). Two atypical lesions displaying low‐grade dysplasia were noted at higher magnification (fig 2C). Focusing on this region, sequential sections were prepared for double staining for p53 protein and cytokeratin 7. The region with two protein expressions in atypical cells was found consistent with this area. It was confirmed that these cells express both p53 and cytokeratin 7 (fig 2D). Fundic gland polyps around the cancer tissue were concomitantly subjected to the two immunostaining techniques. Expression of protein was found and it was not considered possible that carcinogenesis resulted from atypia of the fundic gland polyp. Thus, in this case, as a mechanism of gastric carcinogenesis rather than conventional carcinogenesis from adenoma or atypical transformation of fundic gland polyp in the foci of atypical cells in normal gastric mucosa, malignancy was induced via expression of p53 or cytokeratin 7. Adenocarcinoma developed and gradually extended deeper, to grow into a highly malignant adenocarcinoma with poorly differentiated type.
Figure 2 An almost normal gastric mucosa is seen, with little atrophy or inflammatory findings at the gastric mucosa in the vicinity of the gastric cancer (A). Sporadic atypical regions are seen developing in a few areas (arrows) around the gastric carcinogenesis at low power magnification (B) and at higher magnification (C). Double staining of p53 protein and cytokeratin 7 showed apparently positive (arrows), consistent with the region noted to be atypical by haematoxylin and eosin staining (D).
Footnotes
Competing interests: None declared.
References
- 1.Hoffmann D C, Goligher J C. Polyposis of the stomach and small intestine in association with familial polyposis coli. Br J Surg 197158126–128. [DOI] [PubMed] [Google Scholar]
- 2.Jagelman D G, DeCosse J J, Bussey H J. Upper gastrointestinal cancer in familial adenomatous polyposis. Lancet 198811149–1151. [DOI] [PubMed] [Google Scholar]
- 3.Goodman A J, Dundas S A, Scholefield J H.et al Gastric carcinoma and familial adenomatous polyposis (FAP). Int J Colorectal Dis 19883201–203. [DOI] [PubMed] [Google Scholar]
- 4.Schlosseberg D, Weber W, Stoffel U.et al Periampullary, colorectal and gastric cancer in two siblings. Int J Cancer 198842839–841. [DOI] [PubMed] [Google Scholar]
- 5.Iwama T, Mishima Y, Utsunomiya J. The impact of familial adenomatous polyposis on the tumorigenesis and mortality at the several organs. Its rational treatment. Ann Surg 1993217101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]