Abstract
Background
Torsion of the testicular appendix is the commonest cause of acute scrotum in children. The histological picture of these cases is variable and many show a heavy acute inflammatory cell infiltrate, unlike the response to pure ischaemic necrosis in other organs. The clinical implications and consequences of this associated inflammation are not clear.
Methods
A retrospective review of all cases presenting with torsion of the testicular appendix between 1987 and 2005 was carried out. In all, 79 cases were reviewed and the degree of acute inflammatory cell infiltrate and other morphological parameters were assessed and compared with the patients' clinical, operative and postoperative findings. Stains for bacteria and fungi were examined.
Results
A positive correlation was found between the severity of acute inflammatory cell infiltrates and the longer duration of symptoms and presence of clinical evidence of torsion of the testicular appendages. No other association was detected between the pattern or degree of acute inflammatory cell infiltrate and any other clinicopathological variable that may indicate pyogenic infection. No bacteria or fungal elements were identified. Marked lymphatic dilation may be the only histological finding to indicate the presence of early torsion in cases of scrotal pain secondary to torsion of the appendix testis.
Conclusion
Heavy acute inflammatory cell infiltrates in the torted testicular appendix can be regarded as marker of progression of this condition. Investigation for infection is therefore not necessary in such cases.
Torsion of the testicular appendix is one of the most frequent causes of acute scrotum; it is the leading cause of acute scrotum in children.1,2 Four testicular appendages have been identified: the appendix testis (a remnant of the paramesonephric duct), the appendix epididymis (a remnant of the mesonephric duct), the paradidymis (organ of Giraldes) and the vas aberrans (organ of Haller). The appendix testis is present in 92% of all testes and is usually located at the superior testicular pole in the groove between the testicle and the epididymis. Histologically, the normal appendix testis is formed of loose gelatinous vascular connective tissue stroma covered by mullerian‐type cuboidal to columnar epithelium. It may be responsible for controlling the amount of serous fluid in the space of the tunica vaginalis.3,4 The appendix testis is particularly susceptible to torsion because it is often pedunculated. It accounts for 95% of cases of appendage torsion.5,6
Although torsion of the testicular appendices is a benign condition and the necrotic tissue is reabsorbed without any sequelae in almost all cases, the clinical presentation is a major challenge to clinicians. The severity of symptoms is often disproportionate to the small size of the organ; many surgeons advocate surgical exploration of the acute scrotum to rule out testicular torsion. Torsion usually leads to the progression from venous congestion and oedema to arterial occlusion, ischaemia and infarction of the appendage. Oedema and necrosis causes pain and local inflammation of adjacent structures (acute hemiscrotum).5,7,8
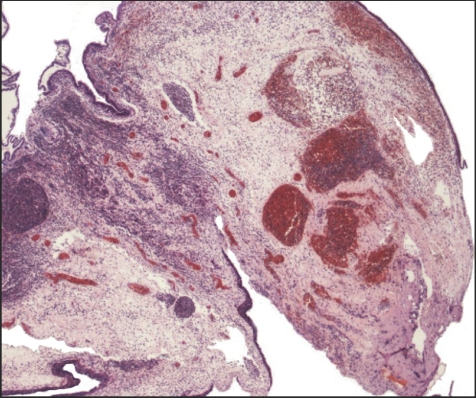
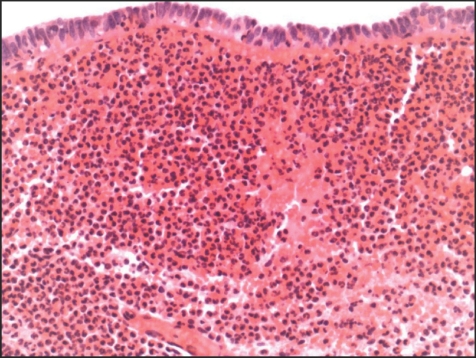
The histological findings in these cases are variable. Some show minimal abnormalities, but many show a massive infiltrate of acute inflammatory cells. This infiltrate is often far heavier than we would associate with a response to venous infarction owing to torsion in other sterile organs (figs 1, 2). Such intense neutrophilic infiltration is more typical of that seen in association with pyogenic bacterial infection in other tissues, and a recommendation of microbiological investigation of these patients is therefore often included in histology reports. No published evaluation has, however, been made of the significance and clinical consequences of this associated acute inflammatory cell infiltrate. This retrospective study was therefore conducted to determine the importance of the associated acute inflammatory cell infiltrates in such cases and to assess the value of further investigation for possible infection in patients.
Figure 1 Appendix testis showing acute inflammatory cell infiltration at a level not associated with aseptic infarction in other organs. This raised a suspicion of pyogenic infection, which we showed to be unfounded (see text). Haematoxylin and eosin; low‐power view of a whole appendix testis (×20).
Figure 2 Appendix testis showing acute inflammatory cell infiltration at a level not associated with aseptic infarction in other organs. This raised a suspicion of pyogenic infection, which we demonstrated to be unfounded (see text). Haematoxylin and eosin; high‐power view of a whole appendix testis (×400).
Materials and methods
A total of 79 consecutive cases of resection of the appendix testis were identified from the archives of the Department of Histopathology, University Hospitals of Leicester, UK, in an 18‐year period from 1 January 1987 to 31 May 2005. Data such as patients' age, tissue size and the histological diagnoses were obtained from the pathology database. Full clinical data, including duration of symptoms, side affected, degree of scrotal redness and swelling, patients' temperature, operative and postoperative findings and urine examination and culture, were collected. The patients had a median age of 10.9 years (range 7 months to 40 years). Haematoxylin and eosin‐stained sections from the diagnostic archive were reassessed for acute inflammatory cell infiltrates, haemorrhage, oedema, vascular and lymphatic dilatation and necrosis. These parameters were semiquantitatively scored with a four‐point scale (0–3) in a blinded fashion and according to set criteria. Each parameter was graded into one of the following: 0, absent; 1, mild; 2, moderate; and 3, severe. In addition, sections stained with Gram stain and periodic‐acid Schiff with diastase were examined for the histological detection of bacteria and fungi, respectively. These special stains were carried out according to routine protocols and positive controls were included.
Statistical analysis
Associations between different pathological variables and clinical findings were analysed with the χ2 and Pearson correlation coefficient tests using SPSS V.10.0 statistical software program. Values of p<0.05 were considered significant.
Results
The mean age of the patients was 11 years, with two patients aged >20 years (29 and 40 years). Of them, 42 (54%) patients were affected on the left side and 36 patients on the right side. The duration of symptoms varied from 6 h to 6 days (median 48 h). Urine examination and culture was carried out in 12 patients and culture of testicular swab in two patients. All the results were negative. No vomiting or postoperative complications were observed in any of the patients. Patients were all afebrile and none of them were given antibiotics before surgery. According to the operative findings, 54 patients were diagnosed with torsion, 11 with possible torsion and four with no clinical evidence of torsion (the appendix testis was either oedematous or an incidental finding that was removed because of the absence of other clinical causes of acute scrotum). The others included three patients were diagnosed with gangrenous testicular appendices, two with epididymo‐orchitis and five with no operative data available. The size of the excised appendices varied from 1.5 to 15 mm (median 6 mm).
We found that the main histological features associated with torsion are stromal oedema, haemorrhage, lymphatic or vascular dilatation, congestion, and sometimes thrombosis with and without acute inflammatory cell infiltrate and necrosis. Foci of calcification were found in three patients. A chronic inflammatory cell infiltrate was found in only one patient. Heavy acute inflammatory cell infiltration (neutrophil polymorphonuclear leucocytes (PNLs)) was found in 42 (54.6%) patients (table 1). Three patterns of infiltration by PNLs were observed:
Table 1 Histological findings in the surgically removed appendix testis.
| Absent | Mild | Moderate | Severe | Not assessed* | |
|---|---|---|---|---|---|
| PNLs | 19 (24%) | 17 (21.5%) | 26 (33%) | 17 (21.5%) | |
| Oedema | 2 (2.5%) | 6 (7.5%) | 16 (20%) | 39 (49%) | 16 (21%) |
| Haemorrhage | 17 (22%) | 17 (22%) | 12 (15%) | 32 (41%) | |
| VI | 4 (5%) | 10 (13%) | 27 (34%) | 38 (48%) | |
| LD | 3 (4%) | 5 (7%) | 13 (18%) | 17 (24%) | 33 (47%) |
| Necrosis | 22 (28%) | 30 (39%) | 26 (33%) |
LD, lymphatic dilation; PNLs, polymorphonuclear leucocytes; VI, vascular congestion.
*Not assessed because of the presence of considerable degree of haemorrhage or other obscuring factors.
Diffuse infiltration of the whole tissue
PNLs found mainly inside blood vessels (either filling the dilated vessels or marginating on the endothelium, which was usually associated with lymphovascular dilatation and oedema)
Presence of PNLs mainly in the subepithelial zone and among epithelial cells (intraepithelial PNLs).
In all patients but one, the presence of PNLs in stromal tissue was associated with intraepithelial PNLs.
Staining with Gram stain and periodic‐acid Schiff for fungi showed no evidence of bacteria or fungi in any case examined.
A positive association was found between the degree of acute inflammatory cell infiltration and the duration of symptoms (Pearson's correlation (CC) = 0.603, p = 0.005), and with operative findings classified as torsion or suspicious of torsion rather than no evidence of torsion (normal; tables 2 and 3).
Table 2 Correlation between degree of inflammatory cell infiltrate and other clinicopathological features.
| Variables | Degree of inflammatory infiltrates | |
|---|---|---|
| χ2 | p Value | |
| Age | 2.76 | 0.43 |
| Side (right or left) | 1.37 | 0.73 |
| Size of appendages | 2.96 | 0.4 |
| Duration of symptoms | 35.1 | 0.02 |
| Haemorrhage | 22.4 | 0.008 |
| Vascular congestion | 27.97 | 0.001 |
| Necrosis | 20.81 | 0.002 |
| Oedema | 17.5 | 0.131 |
Table 3 Correlation between degree of inflammatory cell infiltrate and operative findings (clinical diagnosis).
| Clinical diagnosis | Degree of acute inflammatory cell infiltrate | χ2 | p Value | ||||
|---|---|---|---|---|---|---|---|
| None | Mild | Moderate | Severe | Total | |||
| Torsion appendix | 10 (18.5%) | 11 (20.4%) | 18 (33.3%) | 15 (27.8%) | 54 | 14.2 | 0.028 |
| Suspected torsion | 3 (27.3%) | 3 (27.3%) | 5 (45.5%) | — | 11 | ||
| Normal | 3 (75%) | 1 (25%) | — | — | 4 | ||
Positive associations were found between the degree of acute inflammatory cell infiltration and the presence of haemorrhage (CC = 0.296, p = 0.009), vascular congestion (CC = 0.512, p<0.001), oedema (CC = 0.292, p = 0.009) and necrosis (CC = 0.467, p<0.001) in the excised appendices.
No association was found between the degree of acute inflammatory cell infiltration and other clinicopathological variables, including patients' age, temperature, development of postoperative complication, results of urine examination and culture, affected side of the scrotum, marked scrotal redness and swelling or size of the excised appendices (table 2).
As regards other histological findings, a positive correlation was found between the duration of symptoms and vascular dilatation (CC = 0.689, p = 0.001), and the presence of haemorrhage (CC = 0.477, p = 0.033) and necrosis (CC = 0.565, p = 0.009). Positive associations were found between operative findings (torsion, suspicion of torsion or no evidence of torsion) and the degree of vascular dilatation, haemorrhage, necrosis and oedema (χ2 = 17.75, p = 0.007, in the case of vascular dilatation; χ2 = 22.7, p = 0.001, in haemorrhage; χ2 = 11.5, p = 0.022 in necrosis; and χ2 = 16.56, p = 0.038, in oedema). No other correlation, however, was found between these histological findings and other clinicopathological variables included in our study.
Histological examination of the two testicular appendages that were associated with a clinical evidence of epididymo‐orchitis showed no marked abnormality. In three cases that have been diagnosed clinically as gangrenous, there was necrosis, haemorrhage, vascular dilatation and oedema, but only mild to moderate acute inflammatory cell infiltration.
Some patients were clinically equivocal for torsion or macroscopically normal (n = 7), but their symptoms improved after resection. In these patients, no other causes of acute scrotum were identified on exploration and the testicular appendages were found and thought to be the cause of symptoms and were thus removed. Follow‐up data showed an improvement of patients' conditions after surgical removal of these appendices, with no recurrence of symptoms, suggesting that the cause of symptoms may be an early torsion of these appendices. Histological examination of these cases showed marked lymphovascular dilatation with or without notable stromal oedema and vascular congestion; however, no marked haemorrhage, necrosis or acute inflammatory cell infiltrates were found.
Discussion
In patients with acute scrotum, if the clinical history and physical examination are suspicious or merely equivocal for testicular torsion, the current protocol in our hospitals is that immediate testicular exploration should be carried out, as the rate of successful treatment of testicular torsion correlates inversely with its duration and any delay in diagnosis increases the risk of infarction.1,9,10 The most common cause of acute scrotum in children is torsion of the appendix testis.11,12,13 Consequently, an increasing number of these torted testicular appendages are sent for histopathological examination. We noticed that many of these cases showed massive infiltrates of acute inflammatory cells. The infiltrate is often far heavier than we would expect to be associated with a response to torsion or infarction in other organs and is more typical of that commonly seen in association with pyogenic infection. This raises a suspicion of bacterial infection and further investigation is sometimes advised. We therefore investigated the clinical correlates of this associated inflammatory cell infiltrate and reviewed the histological features commonly found in these excised testicular appendages.
In our study, we found a positive correlation between the degree of acute inflammatory cell infiltration and the length of duration of symptoms, and the presence of a clinical evidence of torsion of the testicular appendages. We did not, however, find any association between the pattern or degree of acute inflammatory cell infiltrates and the various clinicopathological features that may suggest pyogenic infection, such as high temperature, marked scrotal redness and swelling, development of postoperative complications, evidence of urinary infection or the presence of associated epididymo‐orchitis. Moreover, as the presence of considerable acute inflammatory infiltrates in some patients may be secondary to local infection, we examined the case for the presence of organisms that are commonly associated with acute inflammatory cell infiltrates using special stains. No bacteria or fungi were identified, however. We conclude that, despite its histological appearance, the acute inflammation in these cases is not attributable to infection.
Although we could not find the cause of this heavy acute inflammatory cell infiltrate, we are led by exclusion to believe that it is a response to tissue damage owing to the torsion. These results also show that the pattern or degree of acute inflammatory cell infiltrates in these tortuous appendices has no clinical importance.
We also noted that marked lymphatic dilation (without haemorrhage, necrosis or inflammatory cell infiltration) may be the first histological finding in cases of scrotal pain, which are genuinely due to early torsion of the appendix. Thus, the presence of lymphatic dilation with or without stromal oedema can be used as a marker to assess the presence of early torsion of the appendices testis.
In conclusion, our data show that the presence of a heavy acute inflammatory infiltrate in cases of torsion appendix testis is merely a reflection of the duration of the torsion and does not indicate the presence of any other pathological process.
Take‐home messages
Torsion of the testicular appendix is one of the most frequent causes of acute scrotum.
The histological findings in these cases are variable. Some show minimal abnormalities, but many show a massive infiltrate of acute inflammatory cells.
This acute inflammatory cell infiltrates can be regarded as marker of progression of this condition. Investigation for infection is therefore not necessary in such cases.
Marked lymphatic dilation may be the only histological finding to indicate the presence of early torsion in cases of scrotal pain secondary to torsion of the appendix.
Abbreviations
PNLs - polymorphonuclear leucocytes
Footnotes
Competing interests: None declared.
References
- 1.McAndrew H F, Pemberton R, Kikiras C S.et al The incidence and investigation of acute scrotal problems in children. Pediatr Surg Int 200218435–437. [DOI] [PubMed] [Google Scholar]
- 2.Mushtaq I, Fung M, Glasson M J. Retrospective review of paediatric patients with acute scrotum. ANZ J Surg 20037355–58. [DOI] [PubMed] [Google Scholar]
- 3.Ivens U. Morphology and function of the appendix testis. Andrologie 19724245–258. [PubMed] [Google Scholar]
- 4.Jacob M, Barteczko K. Contribution to the origin and development of the appendices of the testis and epididymis in humans. Anat Embryol (Berlin) 2005209287–302. [DOI] [PubMed] [Google Scholar]
- 5.Skoglund R W, McRoberts J W, Ragde H. Torsion of testicular appendages: presentation of 43 new cases and a collective review. J Urol 1970104598–600. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz Montes A M, Jimenez A C, Nunez N R.et al The clinical characteristics of patients with torsion of the testicular and epididymal appendages. Cir Pediatr 19947140–142. [PubMed] [Google Scholar]
- 7.Altaffer LF I I I, Steele S M., Jr Torsion of testicular appendages in men. J Urol 198012456–57. [DOI] [PubMed] [Google Scholar]
- 8.Edelsberg J S, Surh Y S. The acute scrotum. Emerg Med Clin North Am 19886521–546. [PubMed] [Google Scholar]
- 9.Kass E J, Lundak B. The acute scrotum. Pediatr Urol 1997441251–1266. [DOI] [PubMed] [Google Scholar]
- 10.Kadish H A, Bolte R G. A retrospective review of pediatric patients with epididymitis, testicular torsion, and torsion of testicular appendages. Pediatrics 1998102(Pt 1)73–76. [DOI] [PubMed] [Google Scholar]
- 11.Flanigan R C, DeKernion J B, Persky L. Acute scrotal pain and swelling in children: a surgical emergency. Urology 19811751–53. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez R E, Blanco D A, Barbagelata L A.et al Acute scrotum: testicular torsion of Morgagni hydatid. Acta Urol Esp 200428332. [DOI] [PubMed] [Google Scholar]
- 13.Wold J, Edna T H. Torsion of the appendix testis. Tidsskr Nor Laegeforen 1978981693–1694. [PubMed] [Google Scholar]