Abstract
Background
Crystalline cytoplasmic inclusions are well documented in B cell lymphomas but have rarely been described in reactive plasmacytic infiltrates.
Aim
Three cases of Lelicobacter‐associated gastritis are described in which plasma cells focally contained rhomboid and needle‐shaped crystalline inclusions.
Methods
Crystalline inclusions were identified in the gastric biopsy specimens from three patients undergoing routine upper gastrointestinal endoscopy. The cells were characterised immunohistochemically using the following antisera: cytokeratin, leucocyte common antigen, desmin, CD20, CD68, CD79a, CD138, immunoglobulin (Ig)G, IgA and IgM heavy chains, and κ and λ Ig light chains. Clinical follow‐up data were obtained.
Results
All biopsies showed a Lelicobacter‐associated active chronic gastritis. Variable numbers of plasma cells with intracytoplasmic crystalline inclusions in the superficial lamina propria were seen. The crystals were not stained with any of the antisera tested, but the cells containing the crystals expressed CD79a and CD138 and, in the two assessable cases, showed IgA and λ light chain immunoreactivity. The more numerous morphologically normal plasma cells in each patient were polytypic, and there were no histological features to suggest lymphoma. Crystals were not identified in the plasma cells in mucosal biopsy specimens from other sites in any of the patients.
Conclusions
Crystalline inclusions in plasma cells can occur in association with Lelicobacter gastritis. Although light chain restriction was shown in two patients, the overall histological and clinical findings indicated a reactive process. The presence of plasma cell crystals in isolation should not be considered to be diagnostic of lymphoma.
Crystalline inclusions in the cytoplasm of lymphoid cells are an uncommon but well‐documented finding in B cell lymphoproliferative disorders, including plasmacytoma, multiple myeloma, chronic lymphocytic leukaemia, lympho‐plasmacytic lymphoma, mucosa‐associated lymphoid tissue lymphomas and, rarely, high‐grade lymphomas.1,2,3,4,5 The crystals have variable light microscopic appearances and may exhibit rectangular, elongated, needle‐shaped or rhomboid morphology. Electron microscopy typically shows crystal localisation in the rough endoplasmic reticulum consistent with synthesised but unreleased immunoglobulin. The demonstration of heavy chain or light chain immunoreactivity further supports the origin of the crystals from aggregated immunoglobulin components. However, the crystals may not be immunoreactive, or special techniques such as immunoelectron microscopy may be required to show specific labelling.6 Although the crystals usually accumulate in plasma cells, they can also be seen in extracellular locations or in phagocytic histiocytes. In the second situation (crystal‐storing histiocytosis), the dominant histiocytic component of the cellular infiltrate may mask the underlying lymphoproliferative disorder.7,8
Some authors have noted that intracytoplasmic crystals may also be seen in reactive plasma cell infiltrates,1,9 but this seems to be a very rare event and few such cases have been documented in detail.7,10 To our knowledge, crystalline inclusions have been described only once previously in a patient with gastritis,11 and in that case the plasma cells predominantly showed rounded eosinophilic cytoplasmic inclusions (Russell bodies). In this report, we describe rhomboid and needle‐shaped crystalline plasma cell inclusions in three patients with Lelicobacter‐associated gastritis.
Methods
Intracytoplasmic crystalline inclusions were identified in the gastric biopsy specimens of three patients undergoing routine gastrointestinal endoscopy because of symptomatic dyspepsia, dysphagia and/or diarrhoea. The patients were identified by one of the authors (CJRS) over a 24‐month period in which about 2500 gastric biopsy specimens were examined. At endoscopy, two patients had antral gastritis or erosions, whereas oesophagitis was present in the remaining patient. None of the patients had endoscopic features of malignancy and there was no history of lymphoma or multiple myeloma. Between one and four antral biopsies were performed in each patient and concurrent biopsies were also carried out on the duodenum, colon and rectum (case 1), duodenum, ileum and rectum (case 2) and the oesophagus (case 3).
All biopsy specimens were fixed in 10% buffered formalin and processed in paraffin wax. Sections 4 μm thick were stained initially with haematoxylin and eosin for routine diagnostic purposes and Cresyl Violet for identification of Lelicobacter pylori organisms. After identification of the crystals, additional sections were stained with Sirius Red and periodic acid‐Schiff diastase. Immunohistochemical studies were also carried out using the following antisera (dilutions in parentheses): cytokeratin (AE1/AE3 clone, 1/100), desmin (1/200), CD20 (1/800), CD68 (1/300), CD79a (1/300), κ and λ immunoglobulin (Ig) light chains (both 1/60 000), IgA (1/300), IgG (1/200) and IgM (1/200). All antisera were obtained from Dako (NSW 2019, Australia). All staining batches included appropriate positive and negative controls.
Results
Table 1 summarises the clinical and pathological findings.
Table 1 Clinical, histological and immunohistochemical findings in three patients with gastric plasma cell crystals.
| Age, sex | Symptoms/endoscopy | Gastric biopsy findings | PCC | IHC in PCC | Other findings | Follow‐up | |
|---|---|---|---|---|---|---|---|
| Case 1 | 82, M | Dyspepsia, antral gastritis | Moderate acute and chronic gastritis. HP present | Focal, up to 32/HPF | IgA λ | No PCC in duodenal, colonic or rectal biopsies No paraprotein on serum electrophoresis | Died of unrelated causes 4 months after biopsy |
| Case 2 | 81, M | Dysphagia, oesophagitis | Mild acute and chronic gastritis. HP present | Focal, up to 2/HPF | Insufficient PCC for assessment | No PCC in duodenal, ileal or rectal biopsies | No gastric symptoms 14 months after biopsy |
| Case 3 | 52, F | Diarrhoea, oesophagitis, antral erosion | Moderate acute and chronic gastritis. HP present | Focal, up to 5/HPF | IgA λ | No PCC in oesophageal biopsies | No gastric symptoms 6 months after biopsy History of hypertension and hypothyroidism |
F, female; HP, Lelicobacter pylori; HPF, high‐power field (×400 magnification); IgA, immunoglobulin A; IHC, immunohistochemistry; M, male; PCC, plasma cells with crystals.
All of the gastric biopsy specimens comprised antral‐type mucosa in which there was an acute and chronic gastritis of mild or moderate severity associated with H pylori infection. The mucosal inflammatory component mainly comprised small lymphocytes and morphologically normal plasma cells, but there was also a more focal infiltrate of eosinophil and neutrophil polymorphs. In addition, one of the biopsy fragments from each patient showed plasma cells with abundant pale eosinophilic cytoplasm containing elongated, rectangular or needle‐shaped crystalline inclusions (fig 1). The inclusions varied from approximately 5 to 20 μm in length, were non‐birefringent and were typically arranged in fan‐shaped or parallel arrays. The crystals were not stained with haematoxylin and eosin, Cresyl Violet, Sirius Red or periodic acid‐Schiff diastase and therefore appeared as negative images in the cytoplasm of the plasma cells (fig 2). The crystal‐containing cells were typically distributed in the superficial perifoveolar lamina propria and were present in variable numbers in the three patients, ranging from 2 to 32/high‐power field (×400 magnification, field area 0.19 mm2) maximally. In all of the biopsy specimens, morphologically normal plasma cells lacking crystals were much more numerous. No clear relationship was seen between the distribution of the crystal‐containing cells and the presence of H pylori organisms or any other associated inflammatory cellular component. Also, there was no evidence of crystal deposition in histiocytes or in extracellular locations. Occasional plasma cells with cytoplasmic Russell bodies were also present, but these inclusions were not observed in the cells with crystals. No histological features were present to suggest gastric lymphoma. None of the biopsy specimens from the other mucosal sites showed plasma cells with crystals.
Figure 1 Lelicobacter‐associated active chronic gastritis. The superficial lamina propria includes plasma cells with abundant eosinophilic cytoplasm.
Figure 2 Multiple elongated or needle‐shaped crystals are evident at high magnification in the cytoplasm of the plasma cells.
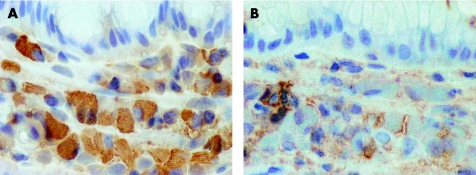
Immunohistochemistry showed that the morphologically normal plasma cells were polytypic in all cases. Sufficiently numerous crystal‐containing plasma cells were present in only two of the biopsy specimens (cases 1 and 3; table 1) to permit immunohistochemical analysis. The crystals themselves were not immunoreactive with any of the antisera tested. However, the cells containing crystals showed CD79a, CD138, IgA and λ light chain expression (figs 3 and 4). The cells with crystals showed no immunoreactivity for cytokeratin, desmin, CD20, CD68, IgG, IgM or κ light chain.
Figure 3 Immunohistochemistry showing CD79a expression by the cells with cytoplasmic crystals. The crystals are unstained, appearing as “negative” images in the cytoplasm.
Figure 4 (A) Plasma cells with crystals, showing immunoreactivity for λ light chain. (B) Occasional morphologically normal plasma cells express κ light chain, but the distended crystal‐containing cells are not reactive.
All patients received standard treatment for Lelicobacter infection with symptomatic improvement and none underwent repeat gastric biopsy. One patient (case 1) died of unrelated causes 4 months after the endoscopy. Serum electrophoresis in this patient did not show an abnormal paraprotein band. The remaining two patients were free from gastrointestinal symptoms 6 and 14 months after biopsy and neither had clinical evidence of lymphoma or myeloma.
Discussion
The accumulation of intracytoplasmic crystalline inclusions is a rare but well‐described feature of B cell lymphoproliferative disorders and is seen most commonly in lymphomas showing plasmacytic differentiation.1 The formation of crystals in such cases is thought to reflect the altered production, storage or secretion of Ig products by the neoplastic cells, and it seems likely that the abnormal proteins exhibit an increased physicochemical tendency to crystallisation. Although the inclusions may be immunoreactive for Ig components, typically there is weak or absent staining. The lack of immunoreactivity may be due to the altered molecular configuration and therefore decreased antigenicity of the stored Ig, or antigen masking resulting from the crystalline structure of the protein.3,4,12
Rare reports are available of crystalline inclusions in gastric lymphoproliferative diseases, including three patients with gastric plasmacytoma.13,14,15 Crystals were present in the cytoplasm of the neoplastic plasma cells and were also identified in histiocytes in one of the cases.13 No consistent pattern of Ig heavy chain or light chain expression was present in these tumours. Fend et al16 described an unusual case of gastric mucosa‐associated lymphoid tissue lymphoma in a patient who initially presented with a secondary nodal immunoblastic lymphoma. IgG and κ positive crystals were identified in plasma cells in the gastric tumour and more rarely in the immunoblastic tumour component. Interestingly, the patient's initial gastric biopsy specimens had been interpreted as reactive, but on review showed occasional plasma cells with cytoplasmic crystals. In retrospect, the authors considered this finding to indicate involvement by the mucosa‐associated lymphoid tissue lymphoma. Other authors have also suggested that the identification of plasma cell crystals can be used to support a diagnosis of lymphoproliferative disorder.13
To our knowledge, there is only one previous report of crystals in plasma cells in a patient with gastritis. Erbersdobler et al11 described the case of an 80‐year‐old woman whose gastric biopsy specimen had a diffuse infiltrate of polytypic plasma cells in the lamina propria, most of which showed prominent eosinophil cytoplasmic inclusions in keeping with “Russell body gastritis”. In addition, the authors noted that some plasma cells contained needle‐shaped crystalline inclusions similar to those described in this report. Lelicobacter infection was absent in this patient. Two additional cases of Russell body gastritis have been reported, both in patients with Lelicobacter‐associated gastritis17,18; crystals were not documented in these cases. Although Russell bodies were present in our biopsy specimens, they were relatively infrequent and not seen in the cells showing crystalline inclusions. We were able to assess the crystal‐containing plasma cells immunohistochemically in two of our patients, and the cells from both showed expression of IgA and λ light chain. The presence of light chain restriction in these patients raises the possibility of a lymphoproliferative process, but there were no histological or endoscopic features to suggest neoplasia, and the more numerous morphologically normal plasma cells in all biopsy specimens were clearly polytypic. Furthermore, no paraprotein was detected on serum electrophoresis in the patient with most numerous crystals, and clinical follow‐up was negative in both other patients. We suggest that the immunohistochemical findings most likely indicate the presence of an expanded but reactive clone of plasma cells whose immunoglobulin product was predisposed to crystal formation. Molecular studies have also previously suggested that monoclonal B cell populations are present in some cases of gastritis,19,20,21 although the data are conflicting.22 It is noteworthy that in the two cases in which immunohistological assessment was possible, the crystals were present in plasma cells synthesising λ light chain. Lymphomas with crystalline inclusions also seem to more often express λ light chain.2 The crystal‐containing plasma cell infiltrates in our cases may have been associated with Lelicobacter infection as crystals were not identified in the biopsy specimens from any of the other mucosal sites. We cannot, however, exclude the possibility that some other localised effect in the gastric mucosa predisposed to crystal deposition.
In conclusion, we described three patients with Lelicobacter‐associated gastritis in whom plasma cells contained intracytoplasmic crystalline Ig inclusions. As crystals were not identified in other mucosal biopsy specimens, it seems that they were possibly related to the Lelicobacter infection. The presence of such crystals, in isolation, should not be considered proof of a lymphoproliferative process.
Take‐home messages
Plasma cells with crystalline cytoplasmic inclusions are described in three patients with helicobacter‐associated gastritis.
Immunoglobulin light chain restriction was demonstrated in two of the cases, but there were no clinical or pathological features to suggest neoplasia.
Crystalline inclusions may be seen within reactive gastric plasmacytic infiltrates and their presence, in isolation, should not be considered indicative of lymphoma.
Footnotes
Competing interests: None declared.
References
- 1.Suarez P, El‐Naggar A K, Batsakis J G. Intracellular crystalline deposits in lymphoplasmacellular disorders. Ann Otol Rhinol Laryngol 1997106170–172. [DOI] [PubMed] [Google Scholar]
- 2.Peters O, Thielmans C, Steenssens L.et al Intracellular inclusion bodies in 14 patients with B cell lymphoproliferative disorders. J Clin Pathol 19843745–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diebold J.Critical commentary to gastric malt lymphoma with crystalline immunoglobulin inclusions and secondary immunoblastic lymphoma in a cervical lymph node. Pathol Res Pract 19951911053–1058. [PubMed] [Google Scholar]
- 4.Spagnolo D V, Papadimitriou J M, Matz L R.et al Nodular lymphomas with intracellular immunoglobulin inclusions: report of three cases and review. Pathology 198214415–427. [DOI] [PubMed] [Google Scholar]
- 5.Wada R, Ebina Y, Kurotaki H.et al Intracytoplasmic immunoglobulin crystals in follicular lymphoma.Hum Pathol 200231141–1144. [DOI] [PubMed] [Google Scholar]
- 6.Gu X, Barrios R, Cartwright J.et al Light chain crystal deposition as a manifestation of plasma cell dyscrasias: the role of immunoelectron microscopy. Hum Pathol 200334270–277. [DOI] [PubMed] [Google Scholar]
- 7.Jones D, Bhatia V K, Krausz T.et al Crystal‐storing histiocytosis: a disorder occurring in plasmacytic tumors expressing immunoglobulin kappa light chain. Hum Pathol 1999301441–1448. [DOI] [PubMed] [Google Scholar]
- 8.Kapadia S B, Enzinger F M, Heffner D K.et al Crystal‐storing histiocytosis associated with lymphoplasmacytic neoplasms: report of three cases mimicking adult rhabdomyoma. Am J Surg Pathol 199317461–467. [DOI] [PubMed] [Google Scholar]
- 9.Cordier A C, Scolari L, Vaerman J ‐ P.et al Ultrastructural aspects of crystal‐like inclusions in a case of IgA plasma cell proliferation. Scand J Haematol 19767143–152. [DOI] [PubMed] [Google Scholar]
- 10.Magalhaes R, Gehrke T, Souto‐Carneiro M M.et al Extensive plasma cell infiltration with crystal IgG inclusions and mutated IgVH gene in an osteoarthritis patient with lymphoplasmacellular synovitis. A case report. Pathol Res Pract 200219845–50. [DOI] [PubMed] [Google Scholar]
- 11.Erbersdobler A, Petri S, Lock G. Russell body gastritis: an unusual, tumor‐like lesion of the gastric mucosa. Arch Pathol Lab Med 2004128915–917. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins R E, Caonje E, Fawcett H.et al Cutaneous crystalline deposits in myeloma. Arch Dermatol 1994130484–488. [PubMed] [Google Scholar]
- 13.Ishido T, Mori N. Primary gastric plasmacytoma: a morphological and immunohistochemical study of five cases. Am J Gastroenterol 199287875–878. [PubMed] [Google Scholar]
- 14.Kobayashi Y, Miyake T, Funakoshi N.et al Gastric plasmacytoma with cylindrical crystalline inclusions. Gastroenterol Jpn 19872281–87. [DOI] [PubMed] [Google Scholar]
- 15.Ferrer‐Rocca O. Primary gastric plasmacytoma with massive intracytoplasmic crystalline inclusions. A case report.Cancer 198250755–759. [DOI] [PubMed] [Google Scholar]
- 16.Fend F, Gabl C, Hittmair A.et al Gastric Malt lymphoma with crystalline immunoglobulin inclusions and secondary immunoblastic lymphoma in a cervical lymph node. Pathol Res Pract 19951911053–1058. [DOI] [PubMed] [Google Scholar]
- 17.Tazawa K, Tsutsumi Y. Localized accumulation of Russell body‐containing plasma cells in gastric mucosa with Lelicobacter pylori infection: ‘Russell body gastritis'. Pathol Int 199848242–244. [DOI] [PubMed] [Google Scholar]
- 18.Ensari A, Savas B, Okcu Heper A.et al An unusual presentation of Lelicobacter pylori infection: so‐called “Russell body gastritis”. Virchows Arch 2005446463–466. [DOI] [PubMed] [Google Scholar]
- 19.Saxena A, Moshynska O, Kanthan R.et al Distinct B‐cell clonal bands in Lelicobacter pylori gastritis with lymphoid hyperplasia. J Pathol 200019047–54. [DOI] [PubMed] [Google Scholar]
- 20.Sorrentino D, Ferraccioli G, De Vitas S.et al B‐cell clonality and infection with Lelicobacter pylori: implications for development of gastric lymphoma.Gut 199638837–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torlakovic E, Cherwitz D, Jessuran J.et al B‐cell gene rearrangement in benign and malignant lymphoid proliferations of mucosa‐associated lymphoid tissue and lymph nodes. Hum Pathol 199728166–173. [DOI] [PubMed] [Google Scholar]
- 22.Genta R M. Le lymphoma imaginaire. Hum Pathol 19988769–770. [DOI] [PubMed] [Google Scholar]