Abstract
A major proportion of the workload in many histopathology laboratories is accounted for by endometrial biopsies, either curettage specimens or outpatient biopsy specimens. The increasing use of pipelle and other methods of biopsy not necessitating general anaesthesia has resulted in greater numbers of specimens with scant tissue, resulting in problems in assessing adequacy and in interpreting artefactual changes, some of which appear more common with outpatient biopsies. In this review, the criteria for adequacy and common artefacts in endometrial biopsies, as well as the interpretation of endometrial biopsies in general, are discussed, concentrating on areas that cause problems for pathologists. An adequate clinical history, including knowledge of the age, menstrual history and menopausal status, and information on the use of exogenous hormones and tamoxifen, is necessary for the pathologist to critically evaluate endometrial biopsies. Topics such as endometritis, endometrial polyps, changes that are induced by hormones and tamoxifen within the endometrium, endometrial metaplasias and hyperplasias, atypical polypoid adenomyoma, adenofibroma, adenosarcoma, histological types of endometrial carcinoma and grading of endometrial carcinomas are discussed with regard to endometrial biopsy specimens rather than hysterectomy specimens. The value of ancillary techniques, especially immunohistochemistry, is discussed where appropriate.
In many histopathology laboratories, endometrial specimens account for a major proportion of the workload. Most specimens are taken because of abnormal uterine bleeding or other related symptoms, and the pathologist is expected to exclude an endometrial cancer or a precancerous lesion. In some cases, a benign cause for abnormal uterine bleeding is identified, such as endometritis or endometrial polyp. In this review, I will outline my approach to the interpretation of endometrial biopsy specimens, especially concentrating on areas which, in my experience, create difficulties for pathologists. Endometrial biopsy specimens are now rarely taken to date the endometrium and to assess whether ovulation has occurred, as serum measurements of various hormones give equivalent or more information. In this review, dating of the endometrium will not be discussed, as this has been dealt with in detail recently.1 Similarly, pure mesenchymal lesions, benign or malignant, may be identified on endometrial biopsy and these will not be discussed further.
Clinical history
In evaluating an endometrial biopsy specimen, an adequate clinical history is important, including the age of the patient and the reason for the biopsy. The menopausal status as well as the date of onset of the last menstrual period and the length of the menstrual cycle in premenopausal women should be provided. In many cases of postmenopausal bleeding, the patient is not actually postmenopausal but rather is perimenopausal, with a prolonged interval between periods. This results in the clinician and the patient assuming that the woman is postmenopausal. Before biopsy, many women with abnormal uterine bleeding are already taking exogenous hormones, especially progestogenic compounds, to control the bleeding, and this information is not always conveyed to the pathologist. Other women may be taking hormone replacement therapy or contraceptives. These hormonal compounds may alter the morphological appearance of the endometrium and a knowledge that these, and other relevant drugs such as tamoxifen, are being taken is of paramount importance to the pathologist.
Criteria for adequacy of endometrial biopsy specimens
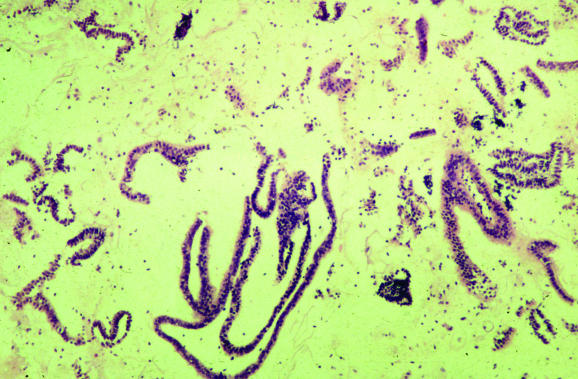
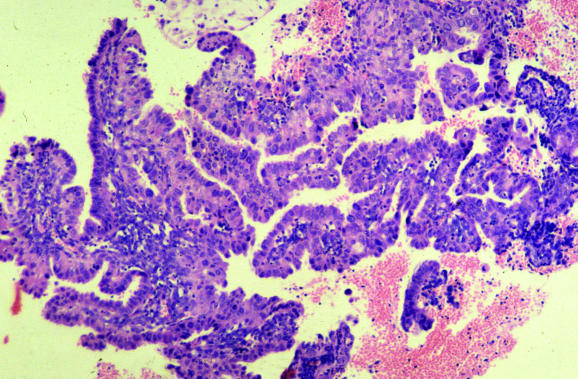
Previously, endometrial biopsy specimens were largely obtained by dilatation and curettage carried out under general anaesthesia. Most endometrial specimens are now taken at outpatients by pipelle or other techniques, with the result that many biopsy specimens contain scant, or even no, endometrial tissue. Paradoxically, superficial endometrial biopsy specimens with scant tissue often take longer to assess than intact biopsy specimens with an appreciable amount of tissue. The pathologist is faced with making a decision on whether the biopsy specimen is adequate. A recent study showed that there is considerable disagreement among specialist gynaecological pathologists about what constitutes an adequate endometrial biopsy specimen.2 It has been shown that in a postmenopausal woman with an atrophic endometrium and no focal lesion on ultrasound scan, the presence of scant endometrial tissue in biopsies from outpatients is the norm and there is little chance of missing relevant pathology.3 Furthermore, the classification of a biopsy specimen as inadequate may have medicolegal and clinical implications. For example, some clinicians routinely conduct a repeat biopsy when an endometrial specimen has been classified as inadequate. It is my policy in reporting endometrial specimens that a biopsy specimen (from either outpatient clinic or curettage) is classified as inadequate only if no endometrial tissue is present. If there is any endometrial tissue, no matter how little, I do not categorise the specimen as inadequate. Instead, I use the term “unassessable” for those biopsies where minimal endometrial tissue is present and state that, although there is no hyperplasia, malignancy or any other specific diagnostic lesion, the tissue cannot be assessed. The presence of even a minimal amount of endometrial tissue provides presumptive evidence that the endometrial cavity has been entered, although theoretically endometrial‐type glands with or without stroma can be derived from tuboendometrial metaplasia or endometriosis within the cervix. It is emphasised that biopsy specimens with scant tissue, which usually comprise only superficial strips of endometrial glands (fig 1), should be examined carefully under high power to look for mitotic activity (abnormal in truly postmenopausal women) and atypia. If intact tissue, comprising glands and stroma, is present then this can be typed, although with a comment that only a limited amount of tissue is available for examination.
Figure 1 Typical endometrial biopsy specimen from an outpatient, which is scant and is composed of only superficial strips of endometrial glands with a pseudopapillary architecture.
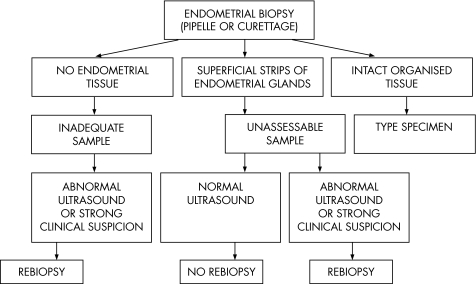
Figure 2 is a suggested algorithm for assessing the adequacy of an endometrial biopsy specimen. The interpretation of the relevance of an unassessable specimen or scant specimen rests with the clinician. As discussed, this is the norm with an atrophic endometrium and no focal lesion on ultrasound scan, but not a reason for repeating the biopsy. With a thickened endometrium, a focal lesion or a strong clinical suspicion of major pathology, however, a scant specimen may be an indication of the need for repeat biopsy. Generally, this should comprise a formal curetting rather than an outpatient biopsy.
Figure 2 Algorithm for assessment of the adequacy of an endometrial biopsy specimen.
Diagnostic algorithm for examination of endometrial biopsies
Tissues not derived from the endometrium are commonly represented in endometrial biopsies. These should be identified, evaluated and mentioned in the pathology report. Cervical tissue, either cervical glandular or squamous epithelium with or without stroma, may be present and should be examined to exclude major pathology. The presence of adipose tissue or even intestinal mucosa may indicate uterine perforation, which should be conveyed to the clinician. In cases of uterine perforation, mesothelial cells or inflammatory cells may be mixed in with the adipose tissue. This may reflect the presence of underlying pelvic or peritoneal abnormalities, which has resulted in a reactive mesothelial proliferation that has “fixed” the uterus and made perforation more likely. Adipose tissue in an endometrial biopsy specimen may rarely be derived from a uterine lipoleiomyoma or lipoma. Other tissues that may be present in an endometrial biopsy specimen include myometrial smooth muscle and tissue derived from the lower uterine segment or isthmus. Tissue from the lower uterine segment may morphologically be confused with an endometrial polyp, as the stroma has a fibrous appearance and the glands are often few in number. The absence of thick‐walled stromal blood vessels and the characteristic admixture of mucinous endocervical epithelium (usually present on the surface) and ciliated tubal‐type epithelium (usually in the deeper glands) should, however, suggest an origin from the lower uterine segment.
After assessing tissue that is not derived from the endometrium, the endometrial tissue present (if any) should be examined. It is useful to give some indication of the amount of endometrial tissue represented and the criteria for adequacy have already been discussed. Next, the endometrium should be typed with reference to the age of the patient, the date of onset of the last menstrual period and any hormonal treatment. The morphological features of a cyclical and atrophic endometrium will not be detailed, but a few salient points are mentioned in the following sentences. Caution should be exercised in diagnosing a proliferative endometrium in the absence of mitoses, although these may be few in number. Stromal and glandular mitoses are commonly found in a proliferative endometrium. An atrophic endometrium, which may or may not be an indication of the postmenopausal state (atrophy is also characteristic of some hormonal agents), may be confused with a proliferative endometrium, as the glands commonly have a tubular appearance and there may be apparent nuclear stratification. A further point of confusion is that not all areas of the endometrium respond at the same rate to endogenous or exogenous hormones—for example, some areas may show proliferative features but others exhibit early secretory activity. Difficulty arises when an endometrium from a truly postmenopausal woman (as stated previously, some women with postmenopausal bleeding are not truly postmenopausal, but rather are perimenopausal, with irregular cycles) shows proliferative activity without features of hyperplasia. My approach is to state in the report that there is continuing proliferative activity suggesting ongoing oestrogenic stimulation, although there are no features of hyperplasia or malignancy. Endometrial proliferative activity may occur with uterine prolapse and in endometrial polyps in postmenopausal women.
Artefacts in endometrial biopsy specimens
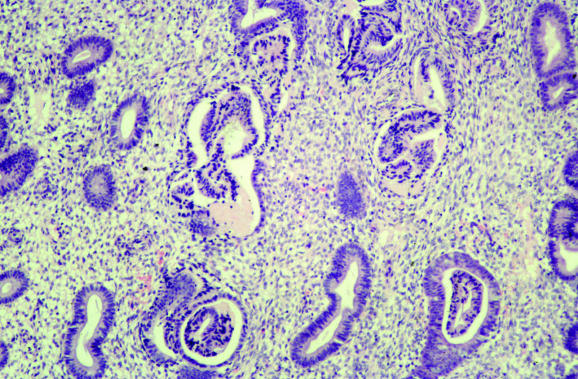
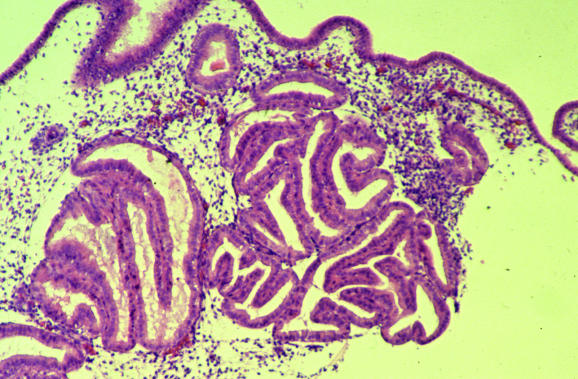
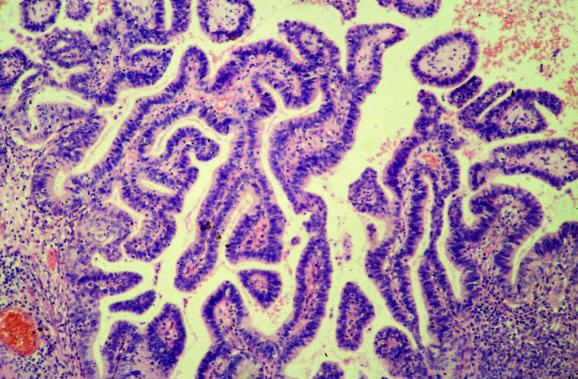
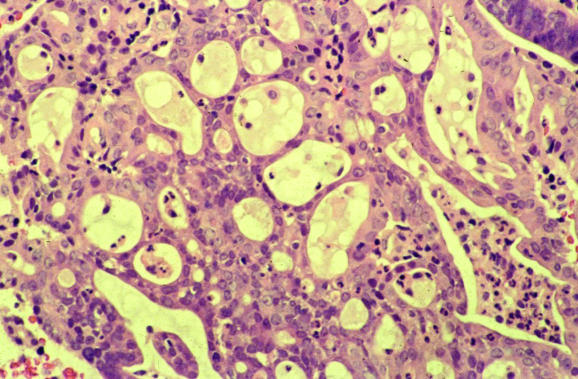
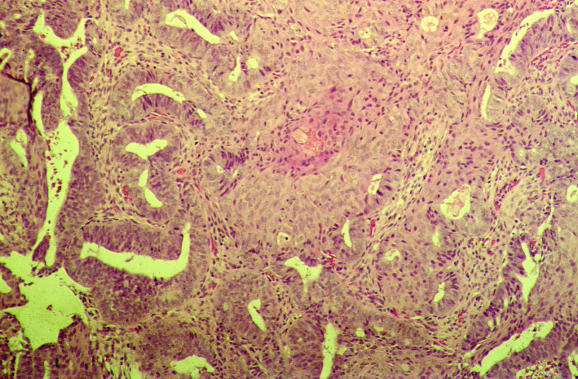
Several common artefacts are observed in endometrial biopsy specimens, which have received scant attention in the literature. Some of these may be misinterpreted as endometrial hyperplasia or even as carcinoma if not appreciated to be artefactual. Telescoping refers to glands within glands (fig 3) and is commonly seen. Artefactual crowding and compression of glands may result in consideration of a complex endometrial hyperplasia (fig 4). With this artefact, the glands become moulded together and often there is tearing of the tissue around the glands, which is a clue to the artefactual nature. An artefact that is especially common with, but not exclusive to, biopsy specimens from outpatients is the presence of superficial strips of endometrium with a pseudopapillary architecture (fig 1). This may result in consideration of a wide range of papillary lesions, benign and malignant, which occur in the endometrium. Such superficial strips of papillary endometrium, which are generally atrophic, should be examined under high power to look for proliferative activity and nuclear atypia. Crushed endometrial glands and stroma may be extremely cellular and can cause concern. As with other tissues, crushed areas should not be viewed in isolation.
Figure 3 Telescoping or gland‐within‐gland appearance, a common artefact in endometrial biopsy specimens.
Figure 4 Compression artefacts with moulding of glands. Tearing of the tissue is seen around the glands.
Endometritis
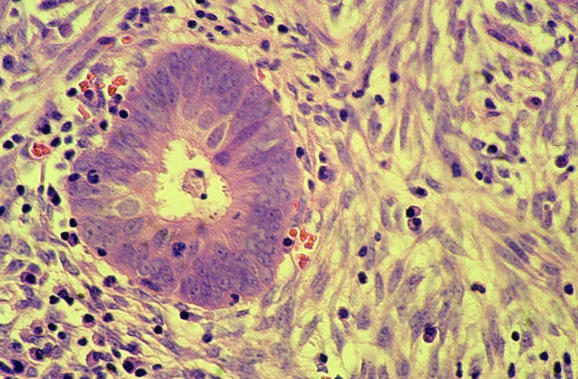
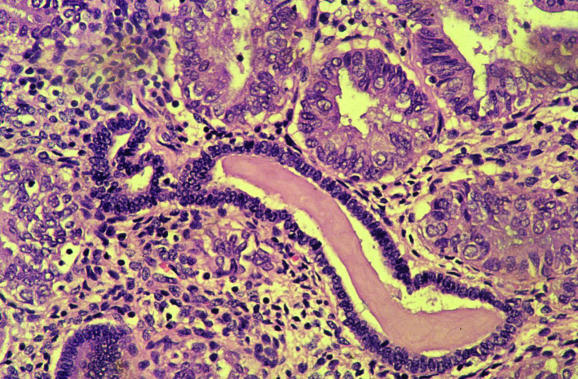
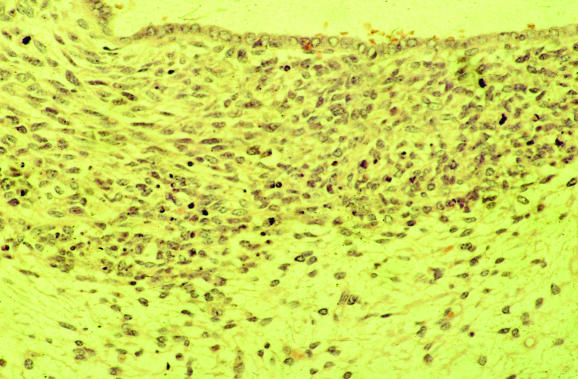
Endometritis may result in symptoms of abnormal uterine bleeding and the pathologist should always exclude this. Lymphocytes, including natural killer cells and lymphoid aggregates, are a normal component of the endometrium, and polymorphs are characteristic of the premenstrual and menstrual phases. The presence of plasma cells is widely regarded as the most useful criterion for a diagnosis of endometritis, although these are often admixed with other inflammatory cells, both acute and chronic, and may be a minor component of the inflammatory cell infiltrate.4,5,6 In endometritis, the inflammatory cell infiltrate may be focal and plasma cells just beneath the surface glands are usually most easily identified. Other morphological features that alert the pathologist to a possible endometritis may also exist. These include an endometrium that exhibits a disturbance in maturation—for example, focal areas that are out of cycle with other areas, stromal oedema and, characteristically, a spindle‐cell alteration of the stroma, especially around glands (fig 5). A degree of architectural complexity and cytological atypia may also be seen if the inflammatory cell infiltrate is marked, resulting in potential overdiagnosis of a hyperplasia or carcinoma. Problems in recognising plasma cells may occur, especially on histological sections that are less than optimal. Endometrial stromal cells may have a plasmacytoid appearance with eccentric nuclei, and the pathologist should be certain that classic plasma cells are present. It is emphasised that in the absence of the other morphological features of endometritis described earlier, an exhaustive search for plasma cells is not justified. Occasional stromal plasma cells may be identified in an otherwise normal endometrium, and in these circumstances a diagnosis of endometritis should not be made. Plasma cells may be present in the stroma of endometrial polyps and also in association with an endometrial malignancy. A variant of endometritis, termed “focal necrotising endometritis”, has been described and is characterised by an inflammatory cell infiltrate comprising lymphocytes, neutrophils and histiocytes without plasma cells.7 Granulomatous endometritis raises the possibility of sarcoidosis, tuberculosis and other granulomatous diseases.
Figure 5 Endometritis with plasma cells in the stroma and a spindle‐cell appearance.
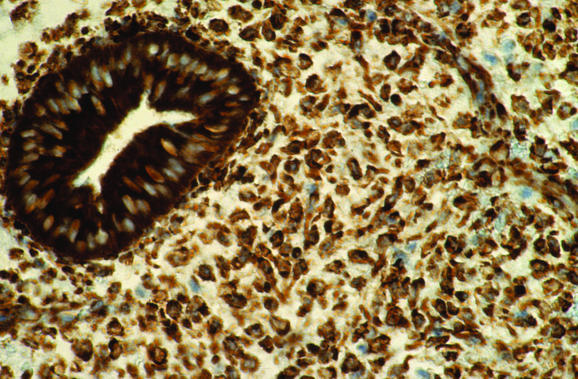
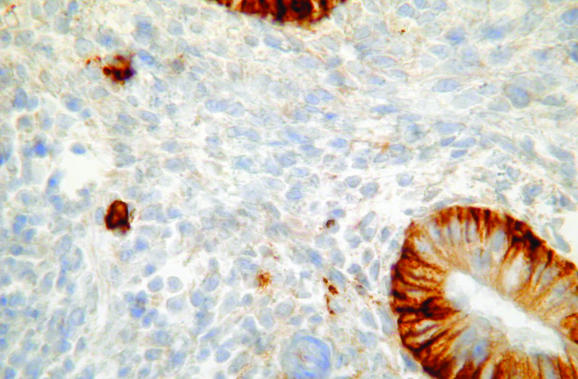
Ancillary studies may aid in the diagnosis of endometritis, although they are not generally necessary. Histochemical staining with methyl green pyronin may be used to identify plasma cells, as can immunohistochemical analysis or in situ hybridisation for κ and λ light chains.8 Plasma cell markers VS38 (CD38) and syndecan (CD138) have also been used to identify plasma cells,9,10,11 although in my experience endometrial glandular and stromal cells are diffusely positive with VS38 (fig 6), making identification of plasma cells impossible. By contrast, syndecan is more useful, as most stromal cells are negative, although endometrial glands are positive (fig 7). Antibodies against B and T lymphoid cells may also be of value.12,13,14,15,16 In the normal endometrium, most lymphocytes are T cells and natural killer cells (granulated lymphocytes).12,13,14,15,16 B lymphoid cells are rare, accounting for fewer than 1% of all endometrial leucocytes,12,13,14,15,16 and are found mainly in lymphoid aggregates in the basal cell layer as well as in scattered individual cells more superficially. A recent study showed that in cases of endometritis, the number of T lymphocytes and natural killer cells did not differ from controls.12 A substantially increased number of B cells, however, was identified in cases of endometritis and these were observed in unusual locations, such as beneath the surface epithelium, intraepithelially and within glandular lumina. This may be of value in diagnosing endometritis on small biopsy specimens in which the superficial endometrium is preferentially sampled.
Figure 6 Immunohistochemical staining for VS38, with postive endometrial glands and stroma, making identification of plasma cells difficult.
Figure 7 Immunohistochemical staining for syndecan, which stains plasma cells and helps in confirming endometritis. The endometrial glands are positive but the stroma is negative.
Endometrial polyps
Polyps are a common cause of abnormal bleeding in premenopausal and postmenopausal women. The pathological diagnosis is generally straightforward if the gynaecologist is aware of the presence of a polyp, has conveyed this information to the pathologist and has removed the polyp intact. The gynaecologist may believe that a polyp is present, but histological examination shows a cyclical endometrium, often secretory in type, reflecting the fact that an abundant secretory endometrium may have a polypoid appearance. Often, the gynaecologist is not aware of the presence of a polyp. In such instances, fragments of polyp are often admixed with fragments of non‐polypoid endometrium, making the diagnosis difficult, as the features can be subtle. When a biopsy is carried out for abnormal uterine bleeding, the pathologist should always consider the possibility of a polyp. On examination under low power, the initial clue to the presence of a polyp is often the admixture of fragments of a normal cyclical endometrium and fragments that are morphologically different.17 The stroma is generally more fibrous than in the surrounding endometrium, although this is not invariable. Other morphological features commonly found in polyps include collections of thick‐walled stromal blood vessels, glandular architectural abnormality (often in the form of dilated glands with unusual shapes and focal crowding) and various epithelial metaplasias. The glands within a polyp often show proliferative activity, even when the surrounding endometrium does not. The following points on endometrial polyps are worthy of mention:
Proliferative activity is common in endometrial polyps, even in postmenopausal women. This is of no relevance, although I generally include a comment to this effect in the pathology report. In such circumstances, it is useful if non‐polypoid endometrium is also present, and whether this also shows proliferative activity needs to be mentioned. If no non‐polypoid endometrium is represented, I comment, stating that proliferative activity may occur in, and be confined to, endometrial polyps in postmenopausal women, but that non‐polypoid endometrium is not represented.
A diagnosis of simple hyperplasia should not be made in the case of an endometrial polyp, as a glandular architecture reminiscent of simple hyperplasia is a normal feature within a polyp. Focal mild glandular crowding is not uncommon and in my opinion does not warrant a diagnosis of complex endometrial hyperplasia. With considerable glandular crowding, however, a diagnosis of complex hyperplasia should be made. This may be confined to the polyp or also include the non‐polypoid endometrium. As discussed earlier, the histological features of any non‐polypoid endometrium represented should be described and, if none is present, this should be mentioned, and the report should state that complex hyperplasia may be confined to the polyp or also include the non‐polypoid endometrium. In such circumstances, the gynaecologist should probably conduct a repeat biopsy of the endometrium. If atypical hyperplasia is present in an endometrial polyp, this should be reported and the surrounding non‐polypoid endometrium thoroughly evaluated; this often necessitates repeat biopsy.
Carcinomas, so‐called “polyp cancers”, may arise in endometrial polyps. Obviously, if a cancer is identified in an endometrial polyp removed by biopsy, it is impossible to ascertain, without full evaluation of the surrounding endometrium, whether the cancer has arisen in or has secondarily involved the polyp. Polyp cancers may be endometrioid in type, but serous proliferations, serous carcinoma or its precursor lesion endometrial intraepithelial carcinoma (EIC), have a particular propensity to arise in or be associated with otherwise benign endometrial polyps.18,19 Endometrial polyps should be carefully examined to look for small foci of serous carcinoma or EIC. This is discussed more fully later.
Endometrial polyps are particularly common in association with tamoxifen. This is also discussed later.
Other benign endometrial lesions
It is useful to have a checklist of benign lesions other than those listed earlier, including granulomas, placental site nodules and the various forms of epithelial and stromal metaplasias. These metaplasias will not be discussed in detail, as they have been reviewed recently.20 The following are a few salient points regarding endometrial epithelial metaplasias:
Epithelial metaplasias, especially squamous or mucinous in type, may coexist with hyperplasia or a carcinoma. In case of florid epithelial metaplasia, a hyperplastic or malignant process should be looked for and excluded. This may be problematic when there is florid squamous metaplasia of the keratinising or morular type (fig 8). Assessing the glandular architecture and cytology may be difficult, as the squamous elements can be so extensive that the underlying glandular component is almost totally obliterated. Such florid examples of squamous metaplasia, which may occur in association with obstruction, so‐called ichthyosis uteri, may also result in consideration of a well‐differentiated squamous carcinoma. Indeed, in the setting of chronic obstruction, squamous carcinoma of the endometrium may develop from florid squamous metaplasia.
Ciliated cells are common in a normal cyclical endometrium.21 I would reserve a diagnosis of ciliated metaplasia for cases characterised by extensive ciliation and where the cells have abundant eosinophilic cytoplasm, resulting in an appearance similar to the epithelium of the normal fallopian tube. Ciliated cells are normal in the lower uterine segment and should not be interpreted as ciliated metaplasia.
Papillary syncytial metaplasia, although termed metaplasia, is actually a reparative response occurring after breakdown22 (fig 9). When florid, this may be suggestive of a serous carcinoma or an EIC. In this circumstance, attention should be paid to the background endometrium, which, in papillary syncytial metaplasia, usually shows features of breakdown. In problematic cases, p53 immunohistochemistry (discussed below) may be of value.
Many endometrial epithelial metaplasias occur without obvious causation. Epithelial metaplasias are, however, especially likely to be seen in endometrial polyps and in association with hormonal preparations.21
With florid mucinous metaplasia, exclusion of a well‐differentiated mucinous adenocarcinoma can be extremely difficult, if not impossible, on endometrial biopsy, as some mucinous adenocarcinomas of the endometrium are cytologically bland, even those which show myometrial infiltration. Mucinous proliferations of the endometrium, which are identified on biopsy, can be subdivided into categories based on the degree of architectural complexity, which correspond to an increasing risk of the presence of mucinous adenocarcinoma in subsequent hysterectomy.23
Benign papillary proliferations (not strictly metaplasias) with fibrovascular cores are rarely seen, especially on the surface of endometrial polyps (fig 10).24 These papillary proliferations may be architecturally complex and are often associated with epithelial metaplasias. These are benign proliferations, and in this situation the presence of architectural complexity does not signify a hyperplastic process.
With some epithelial metaplasias—for example, clear‐cell metaplasia, papillary syncytial metaplasia and benign papillary proliferation—the differential diagnosis may be between a metaplastic process and a type‐2 endometrial cancer or an EIC. In such instances, immunohistochemical analysis may be of value, in that EIC and type‐2 cancers usually show diffuse intense nuclear p53 staining, whereas oestrogen receptor is generally negative or weakly positive. By contrast, most metaplastic processes are oestrogen receptor positive and exhibit a weak heterogeneous pattern of p53 staining.25 Many exceptions exist, however, with some clear‐cell carcinomas in particular being p53 negative or weakly positive.
The surface of some endometrial carcinomas, of either the mucinous or endometrioid type, has an appearance that may closely mimic cervical microglandular hyperplasia (fig 11) or various forms of metaplasia such as mucinous metaplasia or papillary syncytial metaplasia.26 These superficial areas are especially likely to be sampled on biopsy and may pose problems in diagnosis.
Figure 8 Florid squamous metaplasia within the endometrium. Assessing the underlying glandular architecture is problematic.
Figure 9 Florid papillary syncytial metaplasia. This may result in consideration of a serous carcinoma or endometrial intraepithelial carcinoma.
Figure 10 Benign papillary proliferation on the surface of an endometrial polyp.
Figure 11 Biopsy from the surface of endometrioid adenocarcinoma, in which the features resemble those of cervical microglandular hyperplasia.
Effects of hormones on the endometrium
Hormones have varying effects on the endometrium and it is essential that the clinician supplies details to the pathologist regarding any hormone treatment. Such information is not always provided. With any unusual, non‐cyclical appearance of the endometrium, the pathologist should consider exogenous hormones. Some hormone preparations, especially those that contain both oestrogen and progestogen (most modern hormone replacement treatment regimens), characteristically result in a weak or poorly developed secretory endometrium,27,28 whereas with other preparations the endometrium is atrophic. Progestogen‐only compounds result in a characteristic morphological appearance with atrophic or weak secretory‐type glands set in an expanded stroma that exhibits varying degrees of pseudodecidualisation. This pseudodecidualisation is often most prominent just beneath the surface glands and is usually accompanied by an inflammatory cell infiltrate, largely comprising natural killer cells. Similar appearances occur with the Mirena coil, an intrauterine device containing progestogen, which is widely used.29 This often produces a polypoid surface and other morphological features, including reactive atypia of the surface glands, epithelial metaplasias, deposition of stromal haemosiderin, calcification, necrosis and stromal myxoid change.29
Effects of tamoxifen on the endometrium
Tamoxifen is widely used as adjuvant therapy in the management of breast cancer. The use of tamoxifen as a prophylactic agent in patients with a family history of breast cancer is now being investigated. The effects of tamoxifen on the endometrium has been the subject of several reviews.30,31,32,33 Although the efficacy of tamoxifen in breast cancer is due to its anti‐oestrogenic properties, tamoxifen may exert a proliferative effect on the endometrium, resulting in the lesions described later in this article. Endometrial samples from women taking tamoxifen tend to be scanty, as tamoxifen may result in fibrosis of the endometrial stroma, making evaluation by biopsy difficult. The fibrosis can result in cystic dilatation of endometrial glands on an obstructive basis and this can be seen on hysteroscopy.
The most common endometrial lesions seen in association with tamoxifen are benign polyps, which may be single or multiple.34,35,36 In some instances, the whole of the endometrium has a polypoid appearance and it may be difficult to discern individual polyps. Tamoxifen‐associated endometrial polyps do not have pathognomonic histological features, although they are often larger than sporadic polyps and are more likely to exhibit epithelial metaplasias, staghorn glands and stromal condensation around glands, reminiscent of a cambium layer.34,35,36 In some cases, this stromal condensation may result in consideration of a low‐grade adenosarcoma. Tamoxifen‐associated polyps should be extensively sampled and examined carefully under the microscope, as small carcinomas, of both the endometrioid and non‐endometrioid type, may occur. Indeed, as discussed previously, there is a peculiar tendency for serous carcinoma and EIC to arise within endometrial polyps, whether sporadic or associated with tamoxifen.18,19
Tamoxifen may result in the full spectrum of endometrial hyperplasias and in the development of endometrial cancers, both endometrioid and non‐endometrioid. As women with breast cancer are more likely to develop endometrial carcinoma due to common risk factors, such as high socioeconomic status, low parity or hyperoestrogenic states, it is difficult to ascertain whether a true association exists between tamoxifen and the development of endometrial cancer. Most epidemiological studies, however, suggest that tamoxifen is associated with an increased risk of developing endometrial cancer, which is two to three times that in patients with breast cancer who are not taking tamoxifen.33,37,38 Clearly, the risk increases with increasing duration and dosage of tamoxifen. As the effects of tamoxifen on the endometrium are believed to be due to oestrogenic activity, it is expected that most endometrial cancers should be endometrioid in type, as these neoplasms are hormone receptor positive. This is, however, controversial, with some studies suggesting that type‐2 cancers, such as serous carcinoma, are proportionally more common in association with tamoxifen.39,40 Indeed, it has been suggested that carcinosarcomas (in reality, metaplastic carcinomas often associated with an epithelial component of type‐2 carcinoma41,42) are particularly likely to be associated with tamoxifen,43,44,45 and adenosarcomas have also been reported.46 Other studies, however, have found most tamoxifen‐associated endometrial neoplasms to be low‐grade endometrioid adenocarcinomas.37,47,48
Endometrial hyperplasia
In my experience, various aspects related to endometrial hyperplasia commonly create problems for pathologists. In the following sections, I discuss some of these problematic areas.
Potential benign mimics of endometrial hyperplasia
Artefacts
Cystic atrophy
Lower uterine segment endotrium
Disordered proliferative endometrium
Secretory endometrium or Arias–Stella effect
Benign papillary proliferations
Endometritis
Endometrial polyps
Exclusion of benign mimics
Before diagnosing an endometrial hyperplasia, it is important to exclude the many benign mimics. The potential benign mimics of endometrial hyperplasia are listed in box 1. Most of these show an increase in the normal gland to stroma ratio, which is a defining feature of complex endometrial hyperplasia and is present in most cases of atypical hyperplasia (complex atypical hyperplasia). One of the most common lesions to be misdiagnosed as a hyperplasia is an endometrial polyp, especially when this is removed piecemeal, and when the gynaecologist is not aware of the presence of a polyp and the suggestion of this is not conveyed to the pathologist. The morphological features of endometrial polyps, as well as several of the other potential benign mimics of endometrial hyperplasia, have been discussed. A secretory endometrium (and Arias–Stella effect endometrium) often shows an increase in the gland to stroma ratio and may be misdiagnosed as an endometrial hyperplasia, especially when subnuclear vacuolation is not obvious. In general, secretory activity is rare in endometrial hyperplasias, although this does occur, especially when hormone treatment has already been instigated. Cystic atrophy is distinguished from simple hyperplasia by the atrophic appearance of the glands, including the lack of proliferative activity. In general, mitotic activity should be identified before diagnosing an endometrial hyperplasia.
Classification of endometrial hyperplasia
Endometrial hyperplasias should be classified according to the 1994 World Health Organization (WHO) scheme.49 Older terms such as cystic hyperplasia and adenomatous hyperplasia should be avoided. The term dysplasia should not be used with regard to the endometrium. The 1994 WHO classification was based on a seminal study of endometrial hyperplasia,50 and divides hyperplasias into simple and complex forms depending on the glandular architecture. In simple hyperplasia, the normal gland to stroma ratio is largely maintained, although there may be a slight increase. In complex hyperplasia, there is an increase in the gland to stroma ratio. Simple and complex hyperplasias are further divided into atypical and non‐atypical categories on the basis of the presence or absence of nuclear atypia. Assessment of nuclear atypia is extremely subjective, resulting in marked interobserver variation, even among specialist gynaecological pathologists, in the classification of endometrial hyperplasia.51,52,53 The 1994 WHO classification results in four categories of endometrial hyperplasia—simple non‐atypical, complex non‐atypical, simple atypical and complex atypical. In practice, atypia usually occurs in endometria with complex architecture and it is uncommon to diagnose simple atypical hyperplasia, although this rarely occurs. As a result, many pathologists use three categories of endometrial hyperplasia—simple, complex and atypical. All these categories have an increased risk of developing an endometrioid‐type endometrial adenocarcinoma, although this is extremely low in simple and complex endometrial hyperplasias (approximately 1% and 3%, respectively),50 which are usually self‐limiting lesions that regress; although once the lesions are diagnosed, hormonal treatment is usually instigated. The risk of developing an endometrioid adenocarcinoma after a diagnosis of atypical hyperplasia is difficult to ascertain, as, once atypical endometrial hyperplasia is diagnosed, it is treated either hormonally or more usually surgically. With atypical hyperplasia, however, there is a considerable risk of transformation into an endometrioid adenocarcinoma, probably of the order of 25–40%.50 Additionally, an endometrioid adenocarcinoma may already be present and not sampled by biopsy. In my experience, many cases diagnosed as atypical endometrial hyperplasias represent well‐differentiated endometrioid adenocarcinoma.
Morphological features of endometrial hyperplasia
In a simple hyperplasia, the normal gland to stroma ratio is maintained or there is slight increase. The endometrium shows proliferative activity, with cystically dilated glands of irregular sizes and shapes. Some glands may exhibit “out‐pouchings”, “infoldings” and “budding”. No nuclear atypia is seen, the nuclei being oval and maintaining their orientation to the underlying basement membrane. A major problem is the distinction between simple endometrial hyperplasia and disordered proliferative endometrium, a term widely used, although the histological features are not well characterised. The morphological features of these overlap and the distinction is poorly reproducible. In my practice, I require to see large numbers of dilated glands to diagnose simple hyperplasia, whereas if only occasional dilated glands are present, I diagnose this as disordered proliferative endometrium, especially if the patient is perimenopausal. Disordered proliferative endometrium is common in the perimenopausal years because of anovulatory cycles. In any case, the management of simple endometrial hyperplasia and disordered proliferative endometrium is usually identical, in the form of progestogenic compounds. Furthermore, a continuum exists between disordered proliferative endometrium and simple hyperplasia. In essence, these are both benign conditions related to prolonged oestrogenic stimulation, with little risk of transformation into endometrioid‐type adenocarcinoma.
In complex hyperplasia, there is an increase in the gland to stroma ratio with glandular crowding. The glands are often closely packed, although some stroma usually remains between individual glands. The glands show proliferative activity and, by definition, there is no nuclear atypia. Simple hyperplasia is usually a generalised condition, whereas most, but not all, cases of complex hyperplasia are focal, often occurring on a background of simple hyperplasia.
In atypical hyperplasia, there is, by definition, nuclear atypia. Assessing nuclear atypia is one of the most subjective and problematic areas in gynaecological pathology, but this assessment is crucial in the management of endometrial hyperplasia, as surgery is generally undertaken for atypical hyperplasia, whereas hormone treatment is common for non‐atypical hyperplasia, although there are many exceptions. Nuclear atypia may be subtle and in its evaluation it is useful to compare the cytology of the atypical glands with that of the residual normal endometrial glands (fig 12). In atypical hyperplasia, the nuclear changes are often accompanied by cytoplasmic changes, such that the cells have more abundant, often eosinophilic, cytoplasm. Cytoplasmic changes, including increased cytoplasmic eosinophilia, may also be seen in various endometrial metaplasias, such as ciliated metaplasia. Nuclear features in atypical hyperplasia, which, as stated earlier, are often subtle, include nuclear stratification, loss of polarity, nuclear rounding and the presence of nucleoli. In fact, rather than overt nuclear atypia, it is often the differing nuclear features between abnormal and normal glands that is characteristic. This is reflected in a well‐publicised suggested alternative terminology, proposed by Mutter and coworkers (discussed later in this review), for the classification of precursor lesions of endometrioid‐type endometrial adenocarcinoma.54 In this classification, the term “endometrial intraepithelial neoplasia” (EIN) is used and rather than true nuclear atypia, cytological demarcation between normal and abnormal areas is evaluated. It should be emphasised that the term EIN refers to the precursor lesion of endometrioid adenocarcinoma and is different from EIC, the precursor of serous adenocarcinoma, which is discussed in detail later in this review.
Figure 12 Atypical endometrial hyperplasia, in which nuclear and cytoplasmic differences are present between atypical glands and residual normal endometrial glands.
Distinction between atypical hyperplasia and grade‐1 endometrioid adenocarcinoma
It may be difficult, or even impossible, in a small biopsy specimen to distinguish between an atypical hyperplasia at the upper end of the spectrum and a grade‐1 endometrioid adenocarcinoma. This is not surprising, as these two lesions are part of a spectrum without clearly defined boundaries. As a reflection of this continuum, it is acceptable in an endometrial biopsy to render a diagnosis of “at least atypical hyperplasia, cannot exclude grade‐1 endometrioid adenocarcinoma”. It has been suggested that in biopsy specimens, these two lesions should be combined under the designation endometrioid neoplasia.53 Usually, the management of atypical hyperplasia and grade‐1 endometrioid adenocarcinoma is identical, comprising hysterectomy. In a young woman with, for example, polycystic ovary syndrome with atypical hyperplasia or grade‐1 endometrioid adenocarcinoma, and who wishes to retain her fertility, conservative management in the form of progestogenic compounds may be undertaken after MRI, to exclude myoinvasive disease.55,56 If such treatment is undertaken, then regular endometrial biopsies should be carried out, for example, at 3‐monthly intervals. In these circumstances, successful pregnancies have ensued, although there is a high risk of subsequent recurrence of hyperplasia or carcinoma.55,56 On the other hand, it is desirable to distinguish between atypical hyperplasia and grade‐1 endometrioid adenocarcinoma, as many clinicians are reluctant to manage grade‐1 adenocarcinoma conservatively. Moreover, if a diagnosis of adenocarcinoma is made on endometrial biopsy, the patient is more likely to undergo full staging surgery, including peritoneal washings, and can be entered into clinical trials.
Several features assist in distinguishing between atypical hyperplasia and grade‐1 endometrioid adenocarcinoma. A desmoplastic stromal response is strong supportive evidence of an adenocarcinoma,57,58 but this is identified only in a minority of biopsies of endometrial carcinoma. A solid back‐to‐back glandular architecture with complete exclusion of stroma is suggestive of adenocarcinoma, as in most atypical hyperplasias a little stroma remains between glands. Bridging between adjacent glands, resulting in a cribriform pattern, as well as the presence of luminal necrosis with polymorph infiltration, suggests an adenocarcinoma. A surface papillary pattern, maze‐like meandering glands and solid non‐squamoid areas also suggest an adenocarcinoma. As stated earlier, however, atypical hyperplasia and grade‐1 endometrioid adenocarcinoma form part of a spectrum without sharp demarcation.
Suggested alternative terminology for classification of precursor lesions of endometrioid adenocarcinoma
EIN terminology has been extensively championed by Mutter and coworkers.54,59,60,61,62 A recent review in this journal has summarised the arguments and I will only briefly discuss them.54 EIN classification is based on the integration of morphological, molecular genetic, morphometric and prognostic data. Briefly, five criteria must be fulfilled for a diagnosis of EIN:
Area of glands exceeding that of stroma (volume percentage stroma <55%)
Cytology differing between architecturally crowded focus and background—that is, cytological demarcation
Maximum linear dimension of the lesion exceeding 1 mm
Exclusion of benign mimics
Exclusion of carcinoma.
It is emphasised that, although the criteria were derived by using morphometry and molecular studies, EIN is diagnosed on standard morphological examination.59
Proponents of the EIN scheme regard most, but not all, cases that would be diagnosed as simple or complex non‐atypical hyperplasia by using the WHO system as a response of the endometrium to oestrogen excess, and they do not use the term hyperplasia.
Opponents of the EIN system point out that implementation of this will require retraining pathologists and clinicians who will be confused by yet another classification of endometrial hyperplasia. As nuclear atypia per se is not a prerequisite for a diagnosis of EIN, some cases that would be classified as complex non‐atypical hyperplasia by using the WHO scheme may be categorised as EIN. My own view is that we should continue to use the 1994 WHO classification until further evidence emerges regarding the reproducibility, practicalities and prognostic implications of the EIN system.
Atypical polypoid adenomyoma
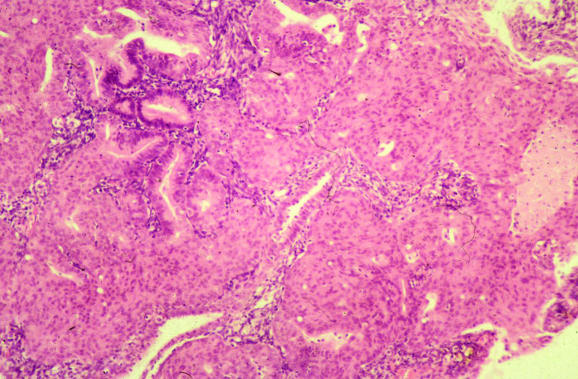
Only a few comments will be made regarding atypical polypoid adenomyoma (APA), concentrating on those issues that are likely to pertain to endometrial biopsy specimens. APA usually occurs in young women and most commonly affects the lower uterine segment.63,64 Although APA is a polypoid lesion, this may be removed piecemeal. Histologically, architecturally complex glands are set in an abundant stroma that usually exhibits smooth‐muscle differentiation (fig 13), although in some cases smooth‐muscle features are not obvious, the stroma having a more fibrous appearance, and the term atypical polypoid adenomyofibroma has been used. Associated cytological atypia is usually mild or moderate. Squamous metaplasia, in the form of morules, sometimes with central necrosis, is often prominent. The main alternative diagnostic consideration on endometrial biopsy is likely to be an endometrioid adenocarcinoma showing myometrial infiltration. In APA, however, the smooth muscle stroma is generally more cellular and disorganised than normal myometrium. Furthermore, in a biopsy specimen from an endometrioid adenocarcinoma, it would be extremely unusual to see only myoinvasive disease in the absence of free tumour fragments.
Figure 13 Atypical polypoid adenomyoma composed of architecturally complex glands, showing squamous metaplasia, with a surrounding myomatous stroma.
Adenofibroma and adenosarcoma
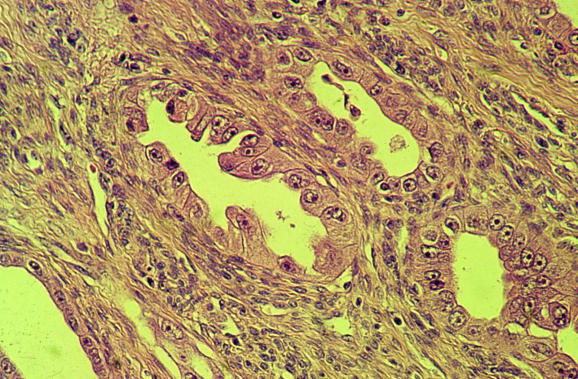
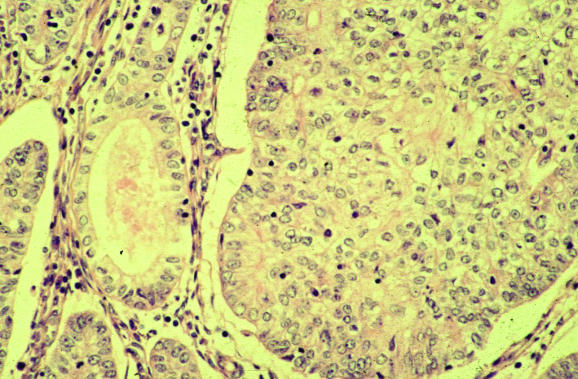
Adenofibroma, with a benign stromal component, and adenosarcoma, with a malignant one, are two of the mixed epithelial and mesenchymal (mixed Mullerian) tumours of the uterus.65 In both, the epithelial element is benign. Carcinosarcomas (malignant epithelial and mesenchymal components) will not be discussed, as these have been recently reviewed.41,42 Both adenofibroma and adenosarcoma are rare neoplasms that are rarely seen in endometrial biopsy specimens, but adenosarcoma is much more common. Some authorities do not recognise adenofibroma, but prefer to regard all the lesions in the adenofibroma–adenosarcoma spectrum as adenosarcomas, albeit some with a low‐grade malignant stromal component. The management of adenofibroma and adenosarcoma is identical, comprising hysterectomy. In most cases, this is curative, unless the sarcomatous component is high grade or there is sarcomatous overgrowth or myometrial infiltration. In a small percentage of cases without these features, there is vaginal recurrence after hysterectomy. Adenofibroma and adenosarcoma are polypoid lesions, the adenosarcoma sometimes forming multiple polyps, and may rarely be identified on endometrial biopsy. As the malignant stromal component in adenosarcoma is sometimes subtle and low grade, adenosarcoma may present as recurrent endometrial polyps, with the correct diagnosis being made only after examination of multiple biopsies. Depending on what areas have been sampled on biopsy, adenosarcoma may also be initially diagnosed as a pure mesenchymal lesion. Morphologically, adenosarcoma is usually composed of leaf‐like papillary projections, with an architecture reminiscent of phyllodes tumour of the breast. The epithelial element, which lines the papillary projections, is benign and may be simple cuboidal or columnar, mucinous, ciliated or even squamous in type. One of the most useful clues suggesting a diagnosis of adenosarcoma is the increased cellularity of the stroma around the epithelial elements, resulting in a cambium layer (fig 14). In these areas, the stromal cells are often mildly atypical and it is here that mitotic figures are usually identified, although these may be sparse and may vary in number from area to area. According to the recent WHO classification of tumours of the breast and female genital organs,66 a mitotic count of ⩾1 HPF is necessary for a diagnosis of adenosarcoma. In practice, however, with a mitotic count of less than this and if the characteristic leaf‐like architecture and cambium layer are present, many would diagnose adenosarcoma rather than adenofibroma. I agree with this view and reiterate that hysterectomy is the management of choice for an adenofibroma, as the lesion should be seen in its entirety to exclude the presence of areas diagnostic of adenosarcoma. Rarely, sex cord‐like foci may be present in the stromal component of an adenosarcoma, similar to those seen in endometrial stromal sarcomas.67 Heterologous elements may also be present.
Figure 14 Adenosarcoma with increased stromal cellularity and mitotic activity beneath the epithelium, resulting in a cambium layer.
Endometrial carcinoma
Issues relevant only to the diagnosis of endometrial carcinoma on biopsy specimens will be considered. In 1983, Bokhman68 first proposed that there were two major variants of endometrial cancer—namely type 1 (the prototype of which is endometrioid carcinoma) and type 2 (the prototype of which is serous carcinoma). It is imperative that an endometrial cancer is both typed and graded, if appropriate, on an endometrial biopsy (the term endometrial adenocarcinoma does not suffice, as endometrial adenocarcinomas may be of several different morphological subtypes). This is especially so in countries with a well‐developed gynaecological oncology service, such as the UK, where different referral patterns exist for cancers of type 1 and type 2. For example, many endometrioid adenocarcinomas (grade 1 and grade 2) are dealt with in a cancer unit, whereas type‐2 cancers, including serous and clear‐cell carcinoma, are usually managed at a cancer centre. More extensive surgical staging may be undertaken with a type‐2 cancer—for example, omentectomy and lymphadenectomy may be carried out. Similarly, in many regions, grade‐3 endometrioid carcinomas are managed in a gynaecological oncology cancer centre. It is controversial whether serous carcinoma (and other type‐2 carcinomas such as clear cell) should be graded, and most take the view that these are by definition grade‐3 tumours. I believe that it is useful to state this on the pathology report, especially if dealing with general gynaecologists who are not routinely occupied with the management of gynaecological neoplasms. All endometrioid carcinomas (and other type‐1 carcinomas such as mucinous carcinoma) should be graded by using the revised 1988 International Federation of Gynecology and Obstetrics grading system.69 In this system, for an architectural grade‐1 or grade‐2 tumour to be upgraded to a grade‐2 or grade‐3 neoplasm, respectively, high‐grade (grade 3) nuclei should be present.69,70 Grade‐3 nuclei are defined as enlarged and pleomorphic, with abnormal coarsely clumped chromatin and large irregular nucleoli.69 In assessing the proportion of solid and non‐solid elements, squamous foci should be ignored. Assessing whether solid areas are squamous or non‐squamous is often difficult, as squamous foci may exhibit a variety of patterns, including a clear cell and spindle cell morphology.71
Take‐home messages
In the evaluation of an endometrial biopsy specimen, an adequate clinical history is important, including details regarding the use of exogenous hormones.
In a postmenopausal woman with an atrophic endometrium and no focal lesion on ultrasound scan, the presence of scant endometrial tissue in an outpatient biopsy is the norm.
When an endometrial biopsy is carried out for abnormal uterine bleeding, the pathologist should always consider the possibility of a polyp.
Serous proliferations, either serous carcinoma or endometrial intraepithelial carcinoma, have a propensity to arise in endometrial polyps.
All endometrial cancers should be typed and, if appropriate, graded, even for small biopsy specimens.
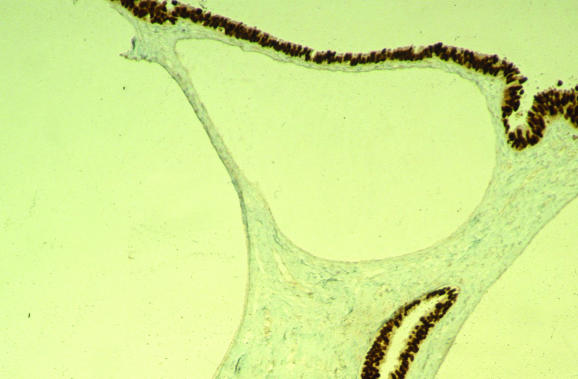
Differentiating between an endometrioid and a serous carcinoma may be problematic on occasion. An endometrioid carcinoma with a papillary growth pattern may be misdiagnosed as a serous carcinoma and, conversely, serous carcinoma with a glandular growth pattern and little or no papillary formation may be mistaken for an endometrioid carcinoma. A diagnosis of a papillary adenocarcinoma should not be made without specification of the morphological type. Architecturally well‐differentiated endometrioid carcinomas usually have low‐grade nuclei; and in an architecturally well‐differentiated neoplasm with grade‐3 nuclei and without papillary formation, a glandular variant of serous carcinoma should be considered (fig 15). In the distinction between an endometrioid and a serous carcinoma, immunohistochemical analysis may be of value.72 Endometrioid adenocarcinomas, especially when grade 1 or grade 2, are usually oestrogen receptor positive and p53 negative. Conversely, serous carcinomas usually show diffuse nuclear p53 reactivity and are oestrogen receptor negative.73,74,75 Many exceptions exist, however, with occasional serous carcinomas being p53 negative and a considerable proportion exhibiting oestrogen receptor positivity, albeit often focal. Conversely, some endometrioid adenocarcinomas are p53 positive, although grade‐1 and grade‐2 neoplasms only rarely exhibit the diffuse strong nuclear reactivity that is characteristic of serous carcinoma. Mixed endometrioid and serous carcinomas are not uncommon. Staining with oestrogen receptor and p53 may also be of value in identifying small foci of EIC in a polypoid or non‐polypoid endometrium (fig 16) or in suggesting a diagnosis of serous neoplasia when only small fragments of tissue are present in an endometrial biopsy specimen.72
Figure 15 Glandular variant of serous carcinoma without papillary formation. The glands are lined by markedly atypical nuclei, characteristic of serous neoplasia.
Figure 16 Endometrial intraepithelial carcinoma exhibiting diffuse intense p53 positivity. The residual atrophic glands are negative.
As referred to previously, serous carcinoma and its presumed precursor lesion EIC (also variously termed endometrial carcinoma in situ or surface serous carcinoma) have a marked propensity to arise in or to be associated with otherwise benign endometrial polyps.18,19,76,77 I use the term “presumed precursor lesion”, as it is possible that EIC, defined as the replacement of residual atrophic glands by cells with markedly atypical nuclei that are characteristic of serous neoplasia without endometrial stromal, myometrial or vascular invasion,76,78,79 actually represents a growth pattern of serous carcinoma and not a precursor lesion. Endometrial polyps, especially when large and occurring in elderly patients, should be carefully scrutinised for small serous proliferations.
A commonly encountered problem in endometrial carcinoma is in determining the nature of clear‐cell areas. This may indicate a pure clear‐cell carcinoma or a component of clear‐cell carcinoma, a variant of type‐2 endometrial cancer usually with markedly atypical nuclei, sometimes with a hobnail pattern. Occasional clear‐cell carcinomas, however, have relatively bland nuclei. Negative staining for oestrogen receptor and diffuse p53 reactivity favour a clear‐cell carcinoma, although a significant proportion of clear‐cell carcinomas do not show this immunophenotype.80 Clear cells are not uncommon in endometrioid adenocarcinomas. This may represent a non‐specific change in clear cells, a secretory variant of endometrioid carcinoma or clear‐cell squamous areas (fig 17).71 Clearing of the cytoplasm in an endometrioid adenocarcinoma may also be a result of prior progestogen treatment.
Figure 17 Endometrioid adenocarcinoma of the endometrium with clear‐cell squamous areas.
Abbreviations
APA - atypical polypoid adenomyoma
EIC - endometrial intraepithelial carcinoma
EIN - endometrial intraepithelial neoplasia
WHO - World Health Organization
Footnotes
Competing interests: None declared.
References
- 1.Crum C P, Hornstein M D, Nucci M R. Hertig and beyond: a systematic and practical approach to the endometrial biopsy. Adv Anat Pathol 200310301–318. [DOI] [PubMed] [Google Scholar]
- 2.Phillips V, McCluggage W G. Results of a questionnaire regarding criteria for adequacy of endometrial biopsies. J Clin Pathol 200558417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakour S H, Khan K S, Gupta J K. Controlled analysis of factors associated with insufficient sample on outpatient endometrial biopsy. BJOG 20001071312–1314. [DOI] [PubMed] [Google Scholar]
- 4.Buckley C H, Fox H. Inflammation of the endometrium. In Buckley CH, Fox H, eds. Biopsy pathology of the endometrium. London: Chapman and Hall, 200291–111.
- 5.Mazur M T, Kurman R J. Endometritis. In: Mazur MT, Kurman RJ, eds. Diagnosis of endometrial biopsies and curettings. Berlin: Springer, 2005147–162.
- 6.Zaino R J. Endometritis. In: Zaino RJ, ed. Interpretation of endometrial biopsies and curettings. Philadelphia: Lippencott‐Raven, 1996174–180.
- 7.Bennett A E, Rathore S, Rhatigan R M. Focal necrotizing endometritis: a clinicopathologic study of 15 cases. Int J Gynecol Pathol 199918220–225. [PubMed] [Google Scholar]
- 8.Euscher E, Nuovo G J. Detection of kappa‐ and lamda‐expressing cells in the endometrium by in‐situ hybridisation. Int J Gynecol Pathol 200221383–390. [DOI] [PubMed] [Google Scholar]
- 9.Leung A S Y, Vinyuvat S, Leong F J W M.et al Anti‐CD38 and VS38 antibodies for detection of plasma cells in the diagnosis of chronic endometritis. Appl Immunohistochem 19975189–193. [Google Scholar]
- 10.Bayer‐Garner I B, Korourian S. Plasma cells in chronic endometritis are easily identified when stained with syndecan‐1. Mod Pathol 200114877–879. [DOI] [PubMed] [Google Scholar]
- 11.Bayer‐Garner I B, Nickell J A. Routine syndecan‐1 immunhistochemistry aids in the diagnosis of chronic endometritis. Arch Pathol Lab Med 20041281000–1003. [DOI] [PubMed] [Google Scholar]
- 12.Disep B, Innes B A, Cochrane H R.et al Immunohistochemical characterisation of endometrial leucocytes in endometritis. Histopathology 200445625–632. [DOI] [PubMed] [Google Scholar]
- 13.Morris H, Edward J, Tiltman A.et al Endometrioid lymphoid tissue: an immunohistochemical study. J Clin Pathol 198538644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleatzeris L D, Bulmer J N, Warren A.et al Endometrial lymphoid tissue in the timed endometrial biopsy: morphometric and immunohistochemical aspects. Am J Obstet Gynaecol 19921676667–6674. [DOI] [PubMed] [Google Scholar]
- 15.Marshall R J, Jones D B. An immunohistochemical study of lymphoid tissue in human endometrium. Int J Gynecol Pathol 19887225–235. [DOI] [PubMed] [Google Scholar]
- 16.Kamat B R, Isacson P. The immunohistochemical distribution of leucocyte subpopulations in human endometrium. Am J Pathol 198612766–73. [PMC free article] [PubMed] [Google Scholar]
- 17.Kim K R, Peng R, Ro J Y.et al A diagnostically useful histopathologic feature of endometrial polyp: the long axis of endometrial glands arranged paralled to surface epithelium. Am J Surg Pathol 2004281057–1062. [DOI] [PubMed] [Google Scholar]
- 18.McCluggage W G, Sumathi V P, McManus D T. Uterine serous carcinoma and endometrial intraepithelial carcinoma arising in endometrial polyps: report of 5 cases, including 2 associated with tamoxifen therapy. Hum Pathol 200334939–943. [DOI] [PubMed] [Google Scholar]
- 19.Silva E G, Jenkins R. Serous carcinoma in endometrial polyps. Mod Pathol 19903120–128. [PubMed] [Google Scholar]
- 20.McCluggage W G. Metaplasias in the female genital tract. In: Lowe D, Underwood J, eds. Recent advances in histopathology. Vol. 20. London, UK: Royal Society of Medicine Press Ltd, 2003
- 21.Hendrickson M R, Kempson R L. Endometrial epithelial metaplasias: proliferations frequently misdiagnosed as adenocarcinoma. Report of 89 cases and proposed classification. Am J Surg Pathol 19804525–542. [PubMed] [Google Scholar]
- 22.Zaman S S, Mazur M T. Endometrial papillary syncytial change: a non‐specific alteration associated with active breakdown. Am J Clin Pathol 199399741–745. [DOI] [PubMed] [Google Scholar]
- 23.Nucci M R, Prasad C J, Crum C P.et al Mucinous endometrial epithelial proliferations: a morphologic spectrum of changes with diverse clinical significance. Mod Pathol 1999121137–1142. [PubMed] [Google Scholar]
- 24.Lehman M B, Hart W R. Simple and complex hyperplastic papillary proliferations of the endometrium: a clinicopathologic study of nine cases of apparently localised papillary lesions with fibrovascular stromal cores and epithelial metaplasia. Am J Surg Pathol 2001251347–1354. [DOI] [PubMed] [Google Scholar]
- 25.Quddus M R, Sung C J, Zheng W.et al p53 immunoreactivity in endometrial metaplasia with dysfunctional uterine bleeding. Histopathology 19993544–49. [DOI] [PubMed] [Google Scholar]
- 26.Jacques S M, Qureshi F, Lawrence W D. Surface epithelial changes in endometrial adenocarcinoma: diagnostic pitfalls in curettage specimens. Int J Gynecol Pathol 199514191–197. [DOI] [PubMed] [Google Scholar]
- 27.Feeley K M, Wells M. Hormone replacement therapy and the endometrium. J Clin Pathol 200154435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piegsa K, Calder A, Davis J A.et al Endometrial status in post‐menopausal women on long‐term continuous combined hormone replacement therapy (Kliofem). A comparative study of endometrial biopsy, outpatient hysteroscopy and transvaginal ultrasound. Eur J Obstet Gynecol Reprod Biol 199772175–180. [DOI] [PubMed] [Google Scholar]
- 29.Phillips V, Graham C T, Manek S.et al The effects of the levonorgestrel intrauterine system (Mirena coil) on endometrial morphology. J Clin Pathol 200356305–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Houghton J P, Ioffe O B, Silverberg S G.et al Metastatic breast lobular carcinoma involving tamoxifen‐associated endometrial polyps: report of two cases and review of tamoxifen‐associated polypoid uterine lesions. Mod Pathol 200316395–398. [DOI] [PubMed] [Google Scholar]
- 31.Ismail S M. Pathology of endometrium treated with tamoxifen. J Clin Pathol 199447827–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neven P, De Muylder X, Van Belle Y.et al Tamoxifen and the uterus and endometrium. Lancet 19891375. [PubMed] [Google Scholar]
- 33.Seidman J D, Kurman R J. Tamoxifen and the endometrium. Int J Gynecol Pathol 199918293–296. [PubMed] [Google Scholar]
- 34.Schlesinger C, Kamoi S, Ascher S M.et al Endometrial polyps: a comparison study of patients receiving tamoxifen with two control groups. Int J Gynecol Pathol 199817302–311. [PubMed] [Google Scholar]
- 35.Kennedy M M, Baigrie C F, Manek S. Tamoxifen and the endometrium: review of 102 cases and comparison with HRT‐related and non‐HRT‐related endometrial pathology. Int J Gynecol Pathol 199918130–137. [PubMed] [Google Scholar]
- 36.Nuovo M A, Nuovo G J, McCaffrey R M.et al Endometrial polyps in postmenopausal patients receiving tamoxifen. Int J Gynecol Pathol 19898125–131. [DOI] [PubMed] [Google Scholar]
- 37.Fisher B, Constantino J P, Redmond C K.et al Endometrial cancer in tamoxifen‐treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B‐14. J Natl Cancer Inst 199486527–537. [DOI] [PubMed] [Google Scholar]
- 38.Rutqvist L E, Johansson H, Signomklao T.et al Adjuvant tamoxifen therapy for early stage breast cancer and second primary malignancies. J Natl Cancer Inst 199587645–651. [DOI] [PubMed] [Google Scholar]
- 39.Magriples U, Naftulia F, Schwartz P E.et al High‐grade endometrial carcinoma in tamoxifen‐treated breast cancer patients. J Clin Oncol 199311485–490. [DOI] [PubMed] [Google Scholar]
- 40.Silva E G, Tornos C S, Follen‐Mitchell M. Malignant neoplasms of the uterine corpus in patients treated for breast carcinoma: the effects of tamoxifen. Int J Gynecol Pathol 199413248–258. [DOI] [PubMed] [Google Scholar]
- 41.McCluggage W G. Malignant biphasic uterine tumours: carcinosarcomas or metaplastic carcinomas? J Clin Pathol 200255321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCluggage W G. Uterine carcinosarcomas (malignant mixed Mullerian tumors) are metaplastic carcinomas. Int J Gynecol Cancer 200212687–690. [DOI] [PubMed] [Google Scholar]
- 43.McCluggage W G, Abdulkader M, Price J H.et al Uterine carcinosarcoma in patients receiving tamoxifen. A report of 19 cases. Int J Gynecol Cancer 200010280–284. [DOI] [PubMed] [Google Scholar]
- 44.McCluggage W G, McManus D T, Lioe T F.et al Uterine carcinosarcoma in association with tamoxifen therapy. BJOG 1997104748–750. [DOI] [PubMed] [Google Scholar]
- 45.Evans M J, Langlois N E I, Kitchener H L.et al Is there an association between long term tamoxifen treatment and the development of carcinosarcoma (malignant mixed Mullerian tumour) of the uterus? Int J Gynecol Cancer 19955310–313. [DOI] [PubMed] [Google Scholar]
- 46.Clement P B, Oliva E, Young R H. Mullerian adenosarcoma of the uterine corpus associated with tamoxifen therapy: a report of six cases and a review of tamoxifen‐associated endometrial lesions. Int J Gynecol Pathol 199615222–229. [DOI] [PubMed] [Google Scholar]
- 47.Cohen L, Azaria R, Fishman A.et al Endometrial cancers in postmenopausal breast cancer patients with tamoxifen treatment. Int J Gynecol Pathol 199918304–310. [DOI] [PubMed] [Google Scholar]
- 48.Barakat R R, Wong G, Curtin J P.et al Tamoxifen use in breast cancer patients who subsequently develop corpus cancer is not associated with a higher incidence of adverse histologic features. Gynecol Oncol 199455164–168. [DOI] [PubMed] [Google Scholar]
- 49.Scully R E, Poulson H, Sobin L H.International histological classification and typing of female genital tract tumours. Berlin: Springer, 1994
- 50.Kurman R J, Kaminski P F, Norris H J. The behaviour of endometrial hyperplasia. A long‐term study of “untreated” hyperplasia in 170 patients. Cancer 198556403–412. [DOI] [PubMed] [Google Scholar]
- 51.Skov B G, Broholm H, Engel U.et al Comparison of the reproducibility of the WHO classifications of 1975 and 1994 of endometrial hyperplasia. Int J Gynecol Pathol 19971633–37. [DOI] [PubMed] [Google Scholar]
- 52.Kendall B S, Ronnett B M, Isacson C.et al Reproducibility of the diagnosis of endometrial hyperplasia, atypical hyperplasia, and well differentiated carcinoma. Am J Surg Pathol 1998221012–1019. [DOI] [PubMed] [Google Scholar]
- 53.Bergeron C, Nogales F F, Masseroli M.et al Multicentric European study testing the reproducibility of the WHO classification of endometrial hyperplasia with a proposal of a simplified working classification for biopsy and curettage specimens. Am J Surg Pathol 1999231102–1108. [DOI] [PubMed] [Google Scholar]
- 54.Baak J P A, Mutter G L. EIN and WHO 94. Considering the classification of endometrial hyperplasia. J Clin Pathol 2005581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Montz F J, Bristow R E, Bovicelli A.et al Intrauterine progesterone treatment of early endometrial cancer. Am J Obstet Gynecol 2002186651–657. [DOI] [PubMed] [Google Scholar]
- 56.Randall T C, Kurman R J. Progestin treatment of atypical hyperplasia and well differentiated carcinoma of the endometrium in women under age 40. Obstet Gynecol 199790434–440. [DOI] [PubMed] [Google Scholar]
- 57.Kurman R J, Norris H J. Evaluation of criteria for distinguishing atypical endometrial hyperplasia from well‐differentiated carcinoma. Cancer 1982152547–2559. [DOI] [PubMed] [Google Scholar]
- 58.Kurman R J, Norris H J. Endometrial stromal invasion in the diagnosis of well‐differentiated carcinoma. Am J Surg Pathol 19848719–720. [PubMed] [Google Scholar]
- 59.Hecht J L, Ince T A, Baak J P.et al Prediction of endometrial carcinoma by subjective endometrial intraepithelial neoplasia diagnosis. Mod Pathol 200518324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mutter G L, The Endometrial Collaborative Group Endometrial intraepithelial neoplasia (EIN): will it bring order to chaos? Gynecol Oncol 200076287–290. [DOI] [PubMed] [Google Scholar]
- 61.Mutter G L, Baak J P A, Crum P C.et al Endometrial precancer diagnosis by histopathology, clonal analysis and computerised morphometry. J Pathol 2000190462–469. [DOI] [PubMed] [Google Scholar]
- 62.Mutter G L. Diagnosis of premalignant endometrial disease. J Clin Pathol 200255326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mazur M T. Atypical polypoid adenomyomas of the endometrium. Am J Surg Pathol 19815473–482. [DOI] [PubMed] [Google Scholar]
- 64.Young R H, Treger T, Scully R E. Atypical polypoid adenomyoma of the uterus. A report of 27 cases. Am J Clin Pathol 198686139–145. [DOI] [PubMed] [Google Scholar]
- 65.Zaloudek C J, Norris H J. Adenofibroma and adenosarcoma of the uterus: a clinicopathologic study of 35 cases. Cancer 198148354–366. [DOI] [PubMed] [Google Scholar]
- 66.McCluggage W G, Haller U, Kurman R J.et al Tumours of the uterine corpus. Mixed epithelial and mesenchymal tumours. In: Tavassoli FA, Devilee P, eds. World Health Organization classification of tumours. Pathology and genetics of tumours of the breast and female genital organs. Lyon: IARC Press, 2003245–249.
- 67.Clement P B, Scully R E. Mullerian adenosarcomas of the uterus with sex cord‐like elements: a clinicopathological analysis of eight cases. Am J Clin Pathol 198991664–672. [DOI] [PubMed] [Google Scholar]
- 68.Bokhman J V. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 19831510–17. [DOI] [PubMed] [Google Scholar]
- 69.Zaino R J, Kurman R J, Diana K L.et al The utility of the revised International Federation and Gynecology and Obstetrics histologic grading of endometrial adenocarcinoma using a defined nuclear grading system: a Gynecologic Oncology Group study. Cancer 19957581–86. [DOI] [PubMed] [Google Scholar]
- 70.Alkushi A, Abdul‐Rahman Z H, Lim P.et al Description of a novel system for grading of endometrial carcinoma and comparison with existing grading systems. Am J Surg Pathol 200529295–304. [DOI] [PubMed] [Google Scholar]
- 71.Clement P B, Young R H. Endometrioid carcinoma of the uterine corpus: a review of its pathology with emphasis on recent advances and problematic aspects. Adv Anat Pathol 20029145–184. [DOI] [PubMed] [Google Scholar]
- 72.McCluggage W G. A critical appraisal of the value of immunohistochemistry in the diagnosis of ovarian neoplasms. Adv Anat Pathol 200411162–171. [DOI] [PubMed] [Google Scholar]
- 73.Alkushi A, Lim P, Coldman A.et al Interpretation of p53 immunoreactivity in endometrial carcinoma: establishing a biologically relevant cut‐off level. Int J Gynecol Pathol 200423129–137. [DOI] [PubMed] [Google Scholar]
- 74.Lax S F, Kendall B, Tashiro H.et al The frequency of p53, K‐ras mutations and microsatellite instability in uterine endometrioid and serous carcinoma: evidence of distinct molecular genetic pathways. Cancer 200088814–824. [PubMed] [Google Scholar]
- 75.Zheng W, Cuo P, Zheng M.et al p53 overexpression and bcl‐2 persistence in endometrial carcinoma: comparison of papillary serous and endometrioid subtypes. Gynecol Oncol 199661167–174. [DOI] [PubMed] [Google Scholar]
- 76.Wheeler D T, Bell K A, Kurman R J.et al Minimal uterine serous carcinoma: diagnosis and clinicopathologic correlation. Am J Surg Pathol 200024797–806. [DOI] [PubMed] [Google Scholar]
- 77.Hui P, Kelly M, O'Malley D M.et al Minimal uterine serous carcinoma: a clinicopathological study of 40 cases. Mod Pathol 20051875–82. [DOI] [PubMed] [Google Scholar]
- 78.Ambros R A, Sherman M E, Zahn C M.et al Endometrial intraepithelial carcinoma: distinctive lesion specifically associated with tumors displaying serous differentiation. Hum Pathol 1995261260–1267. [DOI] [PubMed] [Google Scholar]
- 79.Spiegl G W. Endometrial carcinoma in situ in postmenopausal women. Am J Surg Pathol 199519417–432. [DOI] [PubMed] [Google Scholar]
- 80.Lax S F, Pizer E S, Ronnett B M.et al Clear cell carcinoma of the endometrium is characterised by a distinctive profile of p53, Ki‐67, estrogen and progesterone receptor expression. Hum Pathol 199829551–558. [DOI] [PubMed] [Google Scholar]