Abstract
Background
Malaria is currently diagnosed almost exclusively by microscopy in clinical laboratories. The introduction of rapid diagnostic tests (RDTs) may be useful in achieving rapid detection of malaria parasites, especially in situations where malaria is not often seen or where staff are inexperienced.
Aim
To explore the use of RDT in UK laboratories.
Methods
The current use of RDTs was surveyed in UK laboratories subscribing to the United Kingdom National External Quality Assessment Scheme blood parasitology and haematology schemes.
Results
An overall survey response rate of 60.3% was seen. RDTs were found to be the preferred choice, either alone or in conjunction with microscopy in 31.2% of the samples examined during normal working hours and in 44.3% of the specimens examined on call.
Conclusions
During on‐call hours, the use of RDTs was observed to increase and RDTs changed the diagnosis in 12% of laboratories. No established protocol for RDT use was, however, observed in the UK. A protocol that needs to be validated in the laboratory setting is suggested.
The diagnosis of malaria in clinical laboratories in the UK has, until recently, depended almost exclusively on microscopy and this technique remains the most widely used. Although the polymerase chain reaction (PCR) has a sensitivity and specificity superior to blood film microscopy, using PCR for the primary diagnosis of malaria is an unrealistic goal for most UK laboratories.
In the UK, roughly 2000 imported cases of malaria are reported each year, a major proportion of the 12 000 cases reported annually in Europe.
Furthermore, the case mortality from Plasmodium falciparum species is reported to be as high as 3.6%, even up to 20% in some non‐endemic countries.1,2 Prompt and accurate diagnosis and treatment of malaria should be a priority in any region; but in a non‐endemic area, many of the malaria cases are seen in non‐immune travellers in whom even very low malaria parasitaemias can result in serious illness. The situation is compounded if the diagnosis is delayed by lack of familiarity with the clinical presentations of malaria or by difficulty in detecting and speciating malaria parasites in blood films.
Rapid diagnostic tests (RDTs) to aid the diagnosis of malaria can be important in the future for the detection of malaria parasites both in UK practice and in countries that are endemic to malaria, under certain circumstances.
RDTs use immunochromatographic methods to detect malaria antigens from parasites in lysed blood. They usually use a test strip bearing monoclonal antibodies that are directed against the target parasite antigens and are commercially available in kits provided with all the reagents.3 The depth and sophistication of training required to carry out the tests and to interpret the results are substantially less than that required to achieve proficiency in malaria microscopy.
As yet, there is no established protocol for the use of RDTs in UK laboratory practice. To rationalise this, we surveyed the current use of RDTs in UK laboratories.
Aims
This study aimed at:
ascertaining the methods of diagnosing malaria deployed in UK laboratories and comparing the pattern of use in normal and on‐call hours;
identifying the circumstances in which RDTs are used;
identifying methods of diagnosing malaria in different laboratory settings;
ascertaining the level of knowledge about RDTs in end users;
suggesting a protocol for RDT use in the detection of malaria in UK laboratories.
Method
An anonymous questionnaire was devised and sent to all laboratories participating in the United Kingdom National External Quality Assessment Schemes for blood parasitology and for general haematology. Although this scheme is open to non‐UK participants, this paper considers only UK laboratories, as the policies for RDT use may vary in different healthcare systems across Europe. In all, 542 (450 haematology and 92 microbiology) laboratories were asked to complete the questionnaire.
Information from completed questionnaires was entered onto an Access database. Following entry of all the data, analysis was carried out on Excel. On the basis of the information obtained on the current deployment of RDTs, a protocol for their use in the UK laboratories was devised.
Results
The response rate was 55.4% (51/92) for parasitology laboratories, 61.3% (276/450) for haematology laboratories and 60.3% (327/542) overall.
The laboratories were situated in different locations, such as district general hospitals (59.6%), teaching hospitals (20.5%), private hospitals (12.5%) and other categories (5.8%). The location was not recorded in 1.6%. The catchment area served was described as urban (57.5%), rural (24.5%) and urban and rural (15.6%). An answer was not recorded in 2.4% of questionnaires.
Most laboratories in this study are asked to carry out relatively few blood films for malaria over the course of a year. Of the 327 laboratories, 167 are asked to carry out between 0 and 100 blood films per year and 36 of them examine less than 10 films per year. In all, 123 laboratories view between 100 and 1000 malaria films per year and 15 laboratories examine more than this, the maximum stated being 4000. An answer was not recorded in 22 questionnaires.
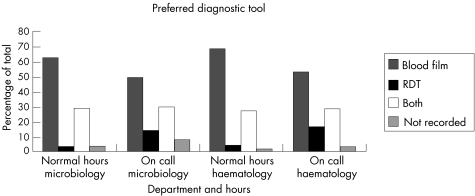
Overall, RDTs are being used as the preferred choice, either alone or in conjunction with microscopy in 31.2% of samples examined in normal working hours and in 44.3% of specimens examined on call. The pattern of use was very similar in haematology and microbiology laboratories (fig 1).
Figure 1 Preferred diagnostic tool for diagnosis of malaria in haematology and microbiology laboratories. RDT, rapid diagnostic test.
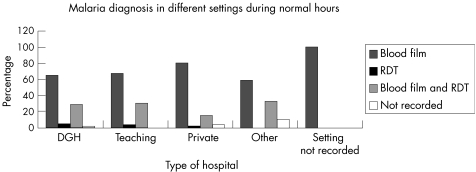
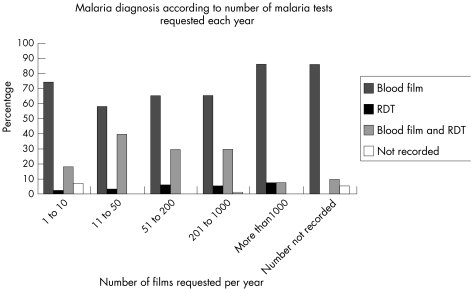
The pattern of use was similar in different laboratory settings (fig 2) and among laboratories with differing exposure to malaria (fig 3).
Figure 2 Diagnosis of malaria in different settings during normal hours. DGH, district general hospital; RDT, rapid diagnostic test.
Figure 3 Diagnosis of malaria according to number of malaria diagnoses requested per year. RDT, rapid diagnostic test
Laboratory choice of RDT was a histidine‐rich protein 2 and aldolase‐based test in 43.1% and a Plasmodium lactate dehydrogenase‐based test in 29.4%; 4.8% of responders used both types of RDT, whereas 11% used none and 11.7% did not answer this question.
Of the responders, 12.2% stated that RDTs revised the malaria diagnosis in their laboratories; 60.2% said it did not; and 27.6% did not answer this question. Of the 40 (12.2%) responders who said that RDTs did revise the diagnosis, 36 carried out analysis of 1–10 films per year; 9 in district general hospitals, 1 in a teaching hospital and 26 in private hospitals.
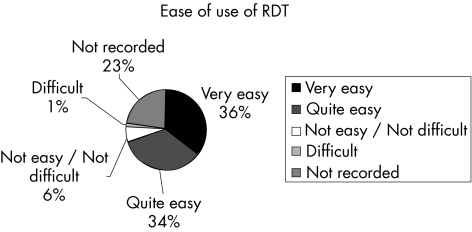
Ease of use is an important factor in a test gaining widespread use in laboratory practice. Figure 4 summarises the responses to the question of how easy it is to use RDTs.
Figure 4 Responses to the question of ease of use of rapid diagnostic tests.
In all, 72.2% of responders commented that RDTs are very easy or quite easy to use. When the 22% non‐responders are removed from analysis, 92.5% of those who answered this question regarded RDTs for diagnosis of malaria as very easy or quite easy to use.
Awareness of the sensitivity of a given test is essential for its correct deployment in laboratory practice; yet, 221 responders (67.6% of questionnaires returned) did not answer this particular question. Furthermore, the range of answers given (60–100%) was relatively wide. The most frequent sensitivity quoted was 93.4% (n = 50) and this may reflect the product information in the kit instructions for one of the commercial tests.
External quality assessment is fundamental to the practice of good clinical governance,4 so it is surprising that 8.9% of responders did not believe they would find it helpful to have an external quality assessment scheme for RDTs for malaria. Although 68.2% of those completing the questionnaires would like an external quality assessment for these tests, it was disappointing that 22.9% of returned questionnaires did not contain a response to this straightforward question.
Take‐home messages
In UK laboratories rapid diagnostic tests (RDTs) for malaria are the preferred choice, either alone or in conjunction with microscopy, in 31.2% of samples examined in normal working hours and in 44.3% of specimens examined on call.
In all 40 (12.2%) laboratories, which examined between 1 and 10 blood films for malaria per year, stated that RDTs revised their diagnosis.
Light microscopy remains the preferred method of choice for diagnosing malaria in a clinical laboratory. RDTs are most useful in aiding inexperienced microscopists, but should not be seen as a replacement for microscopy where it is currently available.
We have suggested a protocol for the use of RDTs in diagnosis of malaria in the UK.
Discussion
Although PCR is more sensitive and specific than microscopy for the diagnosis of malarial infection, it is available in relatively few UK centres, and even then is not usually deployed as the preferred diagnostic method. Thus, light microscopy remains the method of choice for diagnosing malaria in a clinical laboratory.2 It is, however, operator dependent, time consuming5,6 and requires regular examination of sufficient specimens to maintain competency. In non‐endemic areas staff time is expensive, and in some instances malaria parasites are rarely seen in a given laboratory. According to our survey, most laboratories in the UK are being asked to examine relatively few malaria films over the course of a year; in fact most laboratories are asked to examine <100 films per year. The introduction of RDTs has added a new dimension to the diagnosis of malaria and RDTs are now part of routine practice in a large proportion of laboratories. Table 1 compares RDTs with light microscopy for diagnosis of malaria.
Table 1 Comparison of microscopy with rapid diagnostic tests.
| Microscopy | Rapid diagnostic test | |
|---|---|---|
| Time required to carry out | Approximately 1 h | Less than 30 minutes |
| Training required | Extensive | Relatively little |
| Sensitivity | 50 parasites/μl if skilled microscopist | >90% v blood film if >100 Pfalciparum parasites per μl |
| Specificity | Operator dependent | Some false positives |
| Specificity ranges from 92% to 95% for Pfalciparum | ||
| Detects Pfalciparum | Yes | Yes |
| Differentiates sexual stages (gametocytes) from asexual stages | Yes | No |
| Differentiates P falciparum from non‐falciparum parasites | Yes | Partially, cannot speciate non‐falciparum parasites |
As with any laboratory technique, there are advantages and disadvantages for RDTs and for blood films. The most important problems to discuss regarding RDT, as echoed in the free text comments made by the responders, are as follows:
Sensitivity decreases markedly if <100 parasites/μl are present.1,2 Thus, an RDT can be less sensitive than a highly skilled microscopist who may detect as few as 50 parasites/μl, but may be more sensitive than a microscopist who has little and infrequent experience of observing malaria parasites. Highly skilled malaria microscopists are relatively scarce, so RDTs have a clear role in supplementing microscopy on call or in cases where the staff is not expert in the diagnosis of malaria.
Histidine‐rich protein 2‐based RDTs detect only P falciparum. Although aldolase‐based or Plasmodium lactate dehydrogenase‐based RDTs can detect non‐falciparum parasites, they are not yet able to differentiate between P vivax, P ovale or P malariae. Furthermore, if P falciparum is present, these RDTs may give a positive reaction in the falciparum antigen line and in the non‐falciparum malaria antigen line, making a diagnosis of mixed infection impossible. This has therapeutic implications, as primaquine treatment may be required in those patients who do have P vivax or P ovale in addition to P falciparum, but also acts as a source of confusion when laboratories correctly see only P falciparum in a blood film but the RDT result on the same specimen shows a pattern which could indicate a mixed infection. This is a common source of inquiries to the HPA Malaria Reference Laboratory (JE Williams, personal communication, 2005). It would be helpful if kit instructions were clearer on this point. This confirms the need to undertake microscopy in parallel to RDT, and to consult a reference laboratory in case of doubt.
RDTs cannot distinguish between sexual and asexual stages of malaria. Thus, patients adequately treated for P falciparum malaria and with only gametocytes in their peripheral blood may still give a positive RDT result. Caution is required in using RDTs alone as an indication of treatment success, although Plasmodium lactate dehydrogenase‐based RDTs may have a role in cases where microscopy is not available.
These comments reinforce the needs expressed by the World Health Organization, for example, improvement in current test performance characteristics and adequate shelf life.7
Several clinical trials on RDT use have been conducted in a variety of clinical settings, reporting 88–100% sensitivity and 92–95% specificity for the detection of P falciparum.3,8 As an example of practice in non‐endemic areas, Rubio et al1 looked at RDT use in Spanish hospitals. They too reported both false positives and false negatives, but their data suggest that RDTs do have a place in the investigation of returning travellers thought to have malaria.
Most responders believed that RDTs are easy to use, which is not unexpected in a survey of laboratory‐trained personnel. This contrasts with the situation in travellers, where marked failure in performance compared with that in health professionals was noted.9
Enrolment in external quality assessment schemes for the tests carried out in a given laboratory is one criterion used during laboratory inspection visits, so the lack of response to our question on this in 22.9% of returned questionnaires is disappointing.
RDTs cannot and must not be seen as replacement for microscopy where it is currently available. They do, however, have a place in laboratory practice, and our results show that they are now used relatively often in the UK (fig 1). Indeed, an RDT is a preferred diagnostic tool either alone or in conjunction with microscopy in 31.2% of laboratories during normal hours and 44.3% during on‐call hours. They are already influencing management directly, as our data show that RDTs change the diagnosis made at microscopy in 12.2% of laboratories.
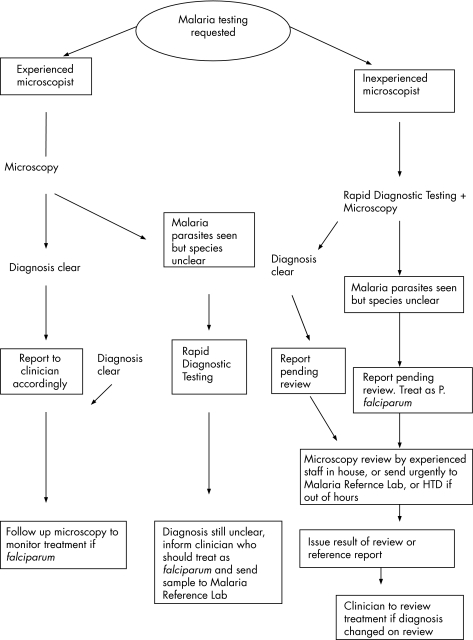
In accordance with best‐practice guidelines, there should be local protocols drawn up for the examination of specimens for the diagnosis of parasitic infections in routine diagnostic laboratories.10 Currently, there are no agreed guidelines for the use of RDTs in the diagnosis of malaria in the UK so, in the light of this study, we have compiled a suggested protocol for their use (fig 5).
Figure 5 Suggested protocol for the use of rapid diagnostic tests with light microscopy for diagnosis of malaria in the UK. HTD; Hospital for Tropical Diseases.
This algorithm does not deal with the issue of near‐patient testing. RDTs may have a role in regions where patients commonly present to primary care practitioners, rather than to accident and emergency departments. In general practice, for example, patients with positive dipstick results could be referred directly to hospital, therefore reducing time to treatment. This would require training of primary care staff to use RDTs in the community setting and is worth studying. Greater confidence in the robustness of RDTs outside a controlled laboratory environment and clear care pathways for their use are, however, required before such studies can be undertaken.
Conclusions
Although microscopy is the mainstay of routine malaria diagnosis in clinical laboratories, RDTs have become increasingly popular in UK practice. They are potentially most useful in aiding inexperienced microscopists. They certainly have a place in the diagnostic laboratories in the UK, where malaria is rarely seen. As there is no uniform protocol yet for the use of RDTs, we have suggested one that needs to be validated in routine practice.
Abbreviations
HTD - Hospital for Tropical Disease
PCR - polymerase chain reaction
RDT - rapid diagnostic test
Footnotes
Competing interests: None.
References
- 1.Rubio J M, Buhigas M, Subirats M.et al Limited level of accuracy provided by available rapid diagnosis tests for malaria enhances the need for PCR‐based reference laboratories. J Clin Microbiol 2001392736–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Malaria diagnosis new perspectives. Report of the Joint WHO/USAID Informal Consultation. Geneva: World Health Organization, October, 1999
- 3.Moody A H. Rapid diagnostic tests for malarial parasites. Clin Microbiol Rev 20021566–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halligan A, Donaldson L. Implementing clinical governance: turning vision into reality. BMJ 200181413–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moody A H, Chiodini P L. Methods for the detection of blood parasites. Clin Lab Haematol 200022189–201. [DOI] [PubMed] [Google Scholar]
- 6.Kettelhut M M, Chiodini P L, Edwards H.et al External quality assessment schemes raise standards: evidence from the UKNEQAS parasitology subschemes. J Clin Pathol 200356927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization Malaria rapid diagnosis: making it work. In: Proceedings of Informal Consultation on Field Trials and Quality Assurances on Malaria Rapid Diagnostic Tests Manila, Philippines, 20–23 January 2003
- 8.Palmer C J, Lindo J F, Winslow I.et al Evaluation of the optimal test for rapid diagnosis of Plasmodium vivax and Plasmodium falciparum malaria. J Clin Microbiol 199836203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jelinek T, Amsler L, Grobusch M P.et al Self‐use of rapid tests for malaria diagnosis by tourists. Lancet 19993541609. [DOI] [PubMed] [Google Scholar]
- 10.Francis J, Barrett S P, Chiodini P L. Best practice guidelines for the examination of specimens for the diagnosis of parasitic infections in routine diagnostic laboratories. J Clin Pathol 200356888–891. [DOI] [PMC free article] [PubMed] [Google Scholar]