Abstract
Background
All‐trans retinoic acid (ATRA) is a natural vitamin A derivative that has a profound effect on the regulation of cell growth, differentiation and death.
Aim
To investigate the tissue dynamic and cellular invasion effects of ATRA in choriocarcinoma (CCA), an aggressive trophoblastic tumour, by using a three‐dimensional organotypic culture model system and cell invasion assay, respectively.
Methods
An organotypic culture model of two CCA cell lines, JAR and JEG, was established. The effects of 1 μM ATRA on proliferation, differentiation and apoptosis on this CCA model were assessed by morphological assessment of the mitotic and apoptotic figures as well as by Ki‐67 and caspase‐related M30 cytoDeath antibody immunohistochemistry and the terminal deoxynucleotidyl transferase‐mediated deoxyuridine triphosphate nick end labelling (TUNEL) assay. The effect of ATRA on p53 and its regulated protein product, WAF1/Cip1, was also evaluated with DO7 and p21WAF1 antibodies, respectively. Moreover, the effect of ATRA on cellular (CCA) invasion was also investigated with Cell Invasion Kit on the JEG cell line.
Results
ATRA was found to induce marked apoptosis in organotypic cultures of both cell lines, as evidenced by increased M30‐positive cells (p<0.0001) and increased TUNEL‐positive cells (p<0.0001) in treated cultures; to decrease proliferation, as evidenced by decreased Ki‐67‐positive cells (p<0.0001); and to decrease p53‐DO7 immunoreactivity (p<0.0001) and increase p21WAF1 (p<0.0001) immunoreactivity. 1.5 μM ATRA was found to effectively inhibit JEG cell invasion in the cell invasion assay.
Conclusion
ATRA treatment was found to inhibit invasion and proliferation and enhance apoptosis, probably by the activation of caspases and induction of differentiation. ATRA and synthetic retinoids may be alternative agents for the treatment of CCA.
Choriocarcinoma (CCA), a highly invasive neoplasm, is a member of gestational trophoblastic disease (GTD) and is characterised by a bilaminar population of cytotrophoblasts and syncytiotrophoblasts, with an absence of chorionic villuses.1,2 In this malignant condition, the neoplastic trophoblasts continue to invade and grow without restraint, resulting in highly vascularised metastasis to organs such as the lungs, brain and liver, and with poor prognosis,3,4,5,6 especially in the pre‐chemotherapy era.
All‐trans retinoic acid (ATRA) is one of the naturally occurring vitamin A derivatives,7 which can be obtained from the diet as preformed retinoids from animal sources and as provitamin carotenoids from plant sources. Vitamin A plays an important part in the maintenance of normal growth, reproduction and cell membrane integrity, mediated through nuclear receptors.7 The activated nuclear receptors control the expression of genes that regulate cell differentiation and growth and the induction of apoptosis. In animals, vitamin A deficiency has been associated with a higher incidence of cancer and increased susceptibility to chemical carcinogens.7
Although the role of retinoids and receptors in GTD is unknown, some early studies have suggested that vitamin A deficiency carries a higher risk of GTD.8 Our previous studies have also shown that the rates of proliferation and apoptosis as well as the patterns of expression of oncogenes, tumour suppressor genes, cathepsin D, tissue inhibitor of metalloproteinases and telomerase have a role in the pathogenesis of GTD.9,10,11,12,13,14,15,16,17,18,19 This study aimed at investigating the effects of ATRA on CCA through effects on cellular response, including proliferation, apoptosis, cell cycle arrest and cell invasion. To conduct experiments in an environment that best reflects the biologically relevant situation, a three‐dimensional organotypic cell culture model was adopted. Organotypic culture allows more vivid in vivo representations of tissue, as intercellular interactions and interactions between the cell and extracellular matrix exist. Such interactions are known to influence gene expression and thus the constitution and behaviour of cells.
Materials and methods
Cell culture
Two human CCA cell lines, JAR and JEG, were obtained from the American Type Culture Collection (Manassas, Virginia, USA). The two cell lines were maintained as monolayer in modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics. All cell lines were kept under sterile condition at 37°C in a humidified atmosphere of 95% air and 5% CO2.
ATRA preparation
ATRA was purchased from Sigma (Sigma Chemical Company, St Louis, Missouri, USA). It was prepared just before use in 100% ethanol and stored as aliquots of 1000× stocks at −20°C. The final concentration of ethanol was 0.001%, which was less than 0.01% and not toxic for cells. As ATRA is sensitive to light, all experiments were carried out in subdued light and the tubes and culture plates containing ATRA were covered with aluminium foil.
Organotypic cultures
Single‐cell suspensions of the CCA cell lines were quantitated with a haematocytometer. Volumes containing 105 cells were centrifuged. The cell pellet was resuspended in a 4°C solution containing modified Eagle's medium and 80% rat‐tail collagen I (Collaborative Biomedical Products, Bedford, Massachusetts, USA). The suspension was poured into Falcon cell culture inserts with transparent membranes containing 0.4 μm pores (Becton Dickinson, Franklin Lakes, New Jersey, USA), which were placed in tissue culture plates. After the collagen was allowed to solidify, a layer of 3×105 cells was placed on top of the collagen gels. The liquid on top of the gel was allowed to evaporate and the medium surrounding the insert was replenished every day. ATRA treatment was started after 1 week of growth and replenished with each change of medium. ATRA was administered at the clinically achievable concentration, 1 μmol/l, by mixing liquid drug stocks into the medium surrounding the insert. Untreated control cultures were treated with the same volume of ethanol used in ATRA treatments. After 1 week of ATRA treatment, the collagen was wrapped in paper before it was put it into a capsule for fixation; it was subsequently embedded in paraffin‐wax and sectioned. Sections from the paraffin‐wax‐embedded blocks were stained with haematoxylin–eosin for histological evaluation. The experiments were repeated in duplicate for each cell line.
Immunohistochemical analysis of M30 cytoDeath, p53 DO‐7, Ki‐67 and p21WAF1
The immunohistochemical analysis for M30, DO7, Ki‐67 and p21WAF1 was carried out, after antigen retrieval by microwave, by using a streptavidin–biotin–peroxidase technique (DAKO, Glostrup, Denmark).11,14,15,19,20 Briefly, formalin‐fixed, paraffin‐wax‐embedded sections (5 μm) were deparaffinised and then immersed in 0.01 M citrate buffer (pH 6) and irradiated in a microwave oven for 9 min at 750 W. The sections were then treated with 3% hydrogen peroxide (H2O2) and 10% rabbit serum to eliminate endogenous peroxidase activity and non‐specific binding, respectively. Four antibodies, including monoclonal mouse anti‐human Ki‐67 antibody (Zymed Laboratories, San Francisco, California, USA), M30 cytoDeath antibody (Boehringer Mannheim, Mannheim, Germany), p21WAF1 antibody (Calbiochem, SanDiego, California, USA) and p53 DO‐7 antibody (DAKO) were applied at neat, 1:50, 1:30 and 1:50 dilutions, respectively, and incubated overnight at 4°C. A biotinylated rabbit anti‐mouse antibody (DAKO) was applied at a 1:100 dilution. Colour development was carried out by applying streptavidin–biotin–peroxidase, 3,3′‐diaminobenzidine tetrahydrochloride and H2O2. Mayer's haematoxylin was used to counterstain the nuclei. The sections were then dehydrated in alcohol, cleared in xylene and mounted. Negative controls were prepared by replacing the first antibody with TRIS‐buffered saline, and a positive control for each antibody was included. A case of hydatidiform mole that was immunoreactive for the five antibodies was used as a positive control in each batch of experiments.11,14,15,20,21
Terminal deoxynucleotidyl transferase‐mediated deoxyuridine triphosphate nick end labelling analysis
Sections were dewaxed in xylene and rehydrated in graded concentrations of ethanol and then in distilled water. Endogenous peroxidase activity was blocked by incubation in 3% H2O2. The slides were then treated with 20 μg/ml proteinase K in 10 mM TRIS (pH 7.2) for 15 min at 37°C and washed in TRIS‐buffered saline. Apoptotic cells were detected by terminal deoxynucleotidyl transferase‐mediated deoxyuridine triphosphate nick end labelling (TUNEL) with the in situ cell detection kit (Boehringer Mannheim), according to the manufacturer's instructions, with minor modifications. Incorporated fluorescein‐deoxyuridine triphosphate was detected by anti‐fluorescein antibody conjugated with horse‐radish peroxidase and subsequent colour development with diaminobenzidine H2O2. Sections were counterstained with Mayer's haematoxylin, dehydrated in ethanol, cleared in xylene and mounted.
Evaluation
Sections were examined at 40× magnification. Cells with clear brown nuclear labelling were defined as positive. All nuclei in the sections were counted. Immunoreactivity for Ki‐67, M30, p53‐DO7, p21WAF1 and TUNEL index in each section was scored and expressed as a percentage of positive nuclei among total number of nuclei counted. In routine haematoxylin–eosin staining, nuclei that were shrunken or fragmented as multiple small pieces of nuclear material in the cell or cell cytoplasm that resides as a small dense segment without nuclear material were identified as apoptotic figures, whereas cells with chromosomes separating in metaphases were identified as mitotic figures. Both mitotic and apoptotic figures were scored and expressed as a percentage of total number of nuclei counted.
Cell invasion assay
Invasion chambers (Chemicon International, Temecula, California, USA) composed of 24‐well culture plates and inserts with an 8‐μm pore polycarbonate filter coated with ECMatrix were used to study the chemoinvasion of the JEG cell line after ATRA treatment. Cells were first trypsinised, centrifuged and resuspended at a density of 106 cells/ml in the culture medium containing different concentrations of ATRA (0, 1, 1.5 and 2 μmol/l). They were then seeded on to the upper chambers of inserts. Lower chambers of the inserts contained the same medium without ATRA. After 24 h of incubation under standard culture conditions, the ECMatrix gel layer and non‐invading cells were removed by wiping with a cotton swab. The invasive cells were then stained on the lower surface of the membrane by dipping inserts on the staining solution provided for 20 min before photographing the cell under a light microscope. In addition, the invasive cells on the lower surface of the membrane were quantified at optical density 560 nm after dissolving the stained cells in 10% acetic acid. All the experiments were duplicated.
Statistical analysis
Data were statistically analysed with SPSS for Windows V.8. Nominal data were analysed by the χ2 test. Numerical data were analysed by the Mann–Whitney U test and analysis of variance, and p<0.05 was considered to be significant.
Results
Effect of ATRA on morphological changes
Table 1 lists the effects of ATRA on the proliferative and apoptotic activities in JEG and JAR CCA cell lines.
Table 1 Effects of ATRA on the proliferative and apoptotic activities in the JEG and JAR choriocarcinoma cell lines.
| JEG | JAR | ||||
|---|---|---|---|---|---|
| ATRA | Positive (%) | p Value | Positive (%) | p Value | |
| Antibody | |||||
| DO7 | − | 63.05 | <0.0001* | 32.10 | 0.202 |
| + | 46.51 | 29.89 | |||
| p21 | − | 31.70 | 0.307 | 23.63 | <0.0001* |
| + | 33.58 | 39.88 | |||
| Ki‐67 | − | 54.78 | <0.0001* | 58.83 | <0.0001* |
| + | 25.55 | 49.19 | |||
| M30 | − | 15.24 | <0.0001* | 11.59 | <0.0001* |
| + | 26.38 | 28.29 | |||
| TUNEL | − | 42.21 | <0.0001* | 49.10 | <0.0001* |
| + | 55.45 | 64.54 | |||
| Morphology | |||||
| Mitotic figure | − | 2.04 | 0.849 | 2.68 | 0.578 |
| + | 1.94 | 1.81 | |||
| Apoptotic figure | − | 6.70 | 0.409 | 7.77 | 0.175 |
| + | 8.73 | 10.90 | |||
ATRA, all‐trans retinoic acid; TUNEL, terminal deoxynucleotidyl transferase‐mediated deoxyuridine triphosphate nick end labelling.
+, presence; −, absence.
*Significant results (p<0.05).
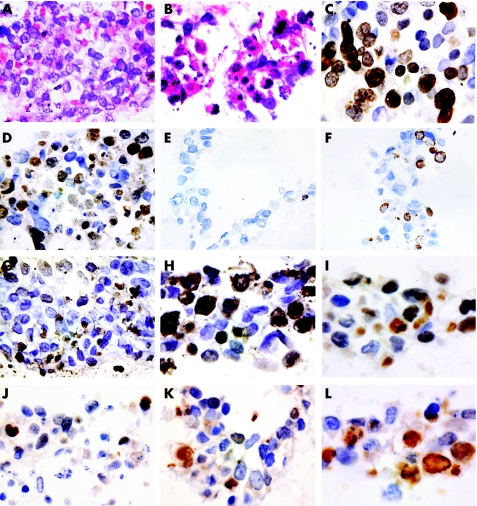
In the absence of ATRA treatment, mitotic figures were found much more often in the sections of both cell lines JAR and JEG. Apoptotic bodies, characterised as dark shrunken nuclei and multiple small fragments of nuclear material (fig 1B), were found much more often in ATRA‐treated organotypic culture from both cell lines. In the absence of ATRA, apoptosis occurred predominantly in cells that were located at the periphery of the colonies, whereas in the presence of ATRA, apoptosis was observed at both the centre and the periphery of the colonies (fig 1A,B). Before and after ATRA treatment, the CCA cells were present as mononuclear cells, with a moderate amount of cytoplasm and hyperchromatic nuclei, compatible with cytotrophoblasts. Multinucleated cells resembling syncytiotrophoblasts were not detected. Thus, no substantial morphological evidence of differentiation was observed in either the JAR or the JEG cell line, regardless of ATRA treatment.
Figure 1 Organotypic cultures of the two choriocarcinoma cell lines, JAR (A–J) and JEG (K,L), were grown in the absence (A,C,E,G,I,K) or in the presence (B,D,F,H,J,L) of 1 μM all‐trans retinoic acid (ATRA) in the medium. The experiments were duplicated for each cell line. Sections (40× magnification) were stained with haematoxylin–eosin (A,B), Ki‐67 (C,D), M30 (E,F), terminal deoxynucleotidyl transferase‐mediated deoxyuridine triphosphate nick end labelling (G,H), p53‐DO7 (I,J) and p21WAF1 (K,L). An increase in apoptotic activity (B,F,H) and reduced proliferation rate (B,D) is seen after ATRA treatment. Reduced p53‐DO7 (J) and increased p21WAF1 (L) expression can also be observed.
Effects of ATRA on proliferative activity
Table 1 summarises the percentage of Ki‐67‐immunoreactive cells (fig 1C,D) in the JAR and JEG cells before and after ATRA application, as an assessment of cell proliferation.21 We found a significant difference in the Ki‐67 immunoreactivity before and after ATRA treatment in both cell lines (p<0.0001). The results suggest that ATRA significantly inhibits the growth of CCA cell lines.
Effects of ATRA on apoptotic activity
The M30 cytoDeath antibody was used to identify early changes in the cytoskeleton related to the action of caspase in apoptosis, whereas the TUNEL technique was used to identify the free 3′‐OH terminal of the fragmented DNA that is formed as a result of DNA endonucleases. M30 and TUNEL positivity was shown as distinct nuclear staining (fig 1E–H). The percentage of M30 cytoDeath‐positive and TUNEL‐positive cells in both the JAR and JEG CCA cell lines induced by ATRA increased significantly (p<0.0001) compared with the sections of culture that did not have ATRA treatment (table 1).
ATRA induces p53 expression in CCA cell lines
p53‐DO7 and p21WAF1 immunoreactivity was also shown as distinct nuclear staining (fig 1I–L) in both the cell lines with and without ATRA treatment. A decrease in p53‐DO7‐positive cells and an increase in p21WAF1‐immunoreactive cells was seen after retinoid treatment in both cell lines (table 1). The reduction in p53‐DO7 expression and the increase in p21WAF1 expression reached significance in the JEG and JAR cell lines, respectively (p<0.0001).
Effect of ATRA on cell invasion assay
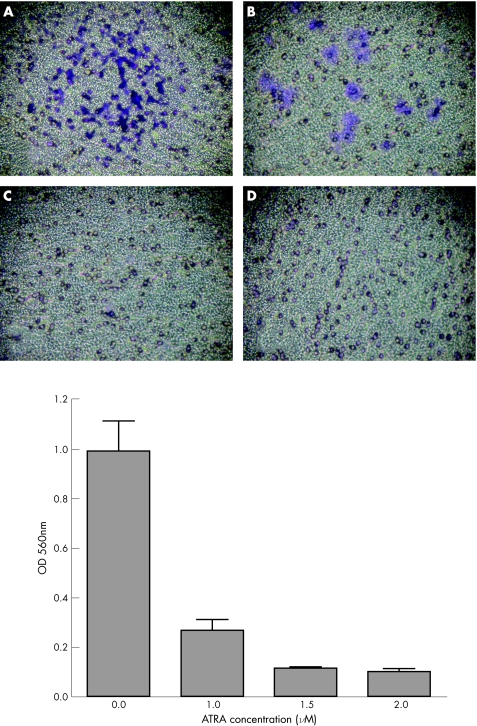
Figure 2 shows the effect of ATRA, at concentrations ranging from 0 to 2 μmol/l, on JEG cell invasion through polycarbonate filter coated with ECMatrix. In the absence of ATRA treatment, JEG cells, which were stained deep blue, invaded the lower surface of the filter (fig 2A). When the JEG cells were treated with up to 1 μM ATRA, we found a marked decrease in the JEG cell invasion (fig 2B). As ATRA concentration reached 1.5 μmol/l, complete inhibition of JEG cell invasion was achieved (fig 2C,D).
Figure 2 Pattern of penetration of ECMatrix basement membrane in JEG cells. A total of 0.4×106 cells were seeded into the chamber and allowed to invade towards 10% fetal bovine serum for 24 h in the absence or presence of all‐trans retinoic acid (ATRA). The cells on the lower surface of the filters were stained blue. ATRA concentrations used were (A) 0; (B) 1 μmol/l; (C) 1.5 μmol/l; and (D) 2 μmol/l. The invasive cells on the lower surface of the membrane were quantified by optical density (OD) at 560 nm after dissolving the stained cells in 10% acetic acid. Values are presented as mean (SEM) on the basis of experiments carried out in duplicate.
Discussion
Induction of apoptosis and differentiation is an important mechanism in cancer treatment. Recently, ATRAs have proved to be effective in the treatment of acute promyelocytic leukaemia.22,23 Retinoic acid analogues have shown promise in chemoprevention of cancers of the breast, ovary, head and neck.
Retinoid acid and its receptors are essential for the growth and development of the mammalian fetus and play a part in trophoblast invasion, implantation and differentiation.24,25,26 Expression of receptor to retinoic acid has been shown in clinical samples of CCA, and JEG‐3, a human CCA cell line, also produces active retinoids.27,28,29,30 In monolayer culture of a CCA cell line, with the use of a 3‐(4.5‐dimethylthiazol‐2‐yl)‐2.5‐diphenyltetrozoliumbromide‐based cell proliferation assay, it was shown that ATRA could inhibit cell proliferation and enhance the effect of standard chemotherapeutic agents, such as methotrexate or actinomycin D.27 Retinoids may thus be useful in the treatment for trophoblastic malignancy, including CCA. The mechanisms of the action of retinoic acid in CCA, however, remain unclear.
This study examined the response of CCA to ATRA treatment in a biologically relevant system. Monolayer cultures of cells are not accurate representations of in vivo tissue, because they lack the interactions among cells and between cells and with the extracellular matrix in vivo. These interactions influence the constitution and behaviour of cells.31 In organotypic models of malignancies,32,33 cancer cells are grown inside and on top of a collagen matrix and such three‐dimensional growth permits a representation of tumours that have invaded stromal tissue. To the best of our knowledge, we are the first to use a three‐dimensional organotypic culture model system to investigate the tissue dynamic effects of ATRA in CCA.
The growth of a tumour is determined by a balance of cell division and cell death.21 Rapidly growing tumours exhibit higher mitotic indices and lower apoptotic indices, which, in combination, usually correlate with aggressive behaviour and poorer prognosis. In this study, immunohistochemical studies clearly showed that ATRA decreased the mitotic activity and increased the apoptotic activity. Morphologically, although reduced mitotic figures and increased apoptotic bodies were observed after ATRA treatment, the difference did not reach statistical significance. One possible explanation is that the morphological manifestations of mitotic or apoptotic figures are the end result of a complicated cascade of events, may only last for a short duration and may not be sensitive enough to reach statistical significance as shown in our immunohistochemical studies with proliferative and apoptotic markers.
ATRA‐induced apoptosis in CCA cultures was shown by morphological changes and also by the M30 cytoDeath antibody and the TUNEL assay. In recent years, proteolytic cleavage of certain cellular proteins is a central biochemical feature of apoptosis. One important group of proteolytic enzymes has been identified as caspases.34 Cytokeratins, in particular cytokeratin 18, which is cleaved by caspase 3 or caspase 7,35 are affected in very early events of apoptosis. M30 cytoDeath can detect a specific formalin‐resistant caspase cleavage site within cytokeratin 18.20 In the present study, the marked increase in M30‐immunoreactive cells after ATRA treatment suggested that activation of caspases may be associated with ATRA‐induced apoptosis in CCA cell lines.
We also investigated whether the ATRA‐induced apoptosis in CCA cell lines related to p53 pathway and cell cycle progression. In our previous study on the issue of p53 mutation in GTD, only wild‐type p53 protein was found to be overexpressed in clinical samples of CCA by c‐DNA‐based sequencing and immunohistochemical method.17 Expression of p53 correlated with proliferative activity. In our present study, p53‐DO7‐positive cells were reduced in both CCA cell lines after ATRA treatment, although statistical significance was shown only in the JEG cell line. A reduction in the Ki‐67 proliferative index was also observed, suggesting that ATRA inhibits proliferation of CCA cells in vitro. These findings suggested that the decrease in p53 expression in CCA cell lines after ATRA treatment may be related to the growth inhibitory effect.
p21WAF1 is a cell cycle regulator and down‐stream effector of p53. Overexpression of p21WAF1 is usually associated with an inhibition of cellular growth, cell differentiation and senescence, as well as with terminal differentiation in various cell lineages.36 Expression of p21WAF1 may be induced by factors related to differentiation and growth arrest.37 We have previously shown that p21WAF1 was expressed predominantly in the differentiated syncytiotrophoblasts, but not in the cytotrophoblasts.14 The JAR and JEG cell lines contain only cytotrophoblastic cells and they retained an undifferentiated morphology after ATRA treatment. Nevertheless, an increase in p21WAF1 immunoreactivity could be shown in ATRA‐treated sections of both cell lines. Thus, although there was no morphological induction of differentiation into syncytiotrophoblasts after ATRA treatment, the overexpression of p21WAF1 suggested the possible presence of subtle biochemical differentiation that may occur before morphological differentiation. This hypothesis was supported by previous reports documenting the induction of giant‐cell formation38 and the secretion of human chorionic gonadotropin, both related to differentiation into syncytiotrophoblasts, after the addition of retinoic acid.27,39,40
Little is known about the effect of ATRA in the invasiveness of CCA. Our results showed that ATRA can effectively inhibit JEG cell invasion in the cell invasion chamber. The mechanisms controlling CCA cell invasiveness are poorly understood so far, but may be closely linked to protease expression.41,42 In CCA cells, we have recently shown the down regulation of E‐cadherin or β‐catenin43 and tissue inhibitor of metalloproteinase‐344 that may be implicated in CCA invasion potential.
Retinoic acid may also affect CCA cells via its inhibitory effect on leptin.45 The expression of leptin and leptin receptor in both complete and partial mole as well as in CCA and placental‐site trophoblastic tumours are up regulated compared with those in normal first trimester.46
In summary, our study shows that ATRA suppresses invasion and proliferation, and induces apoptosis in CCA cells in vitro, implicating the association of p53, p21WAF1 and caspase pathways. Further studies on ATRA and ATRA analogues should be conducted for clinical treatment of CCA. Experiments may be necessary to further validate and delineate the molecular response of CCA cells to ATRA.
Acknowledgements
This study was supported by the HKSAR Research Grant Council Grants (numbers 10203228.13396.21200.324.01 and 10205496.13396.21200.324.01) and the University of Hong Kong Conference and Research Grant. We thank Mr SK Lau for his photographic expertise.
Abbreviations
ATRA - all‐trans retinoic acid
CCA - choriocarcinoma
GTD - gestational trophoblastic disease
TUNEL - terminal deoxynucleotidyl transferase‐mediated deoxyuridine triphosphate nick end labelling
Footnotes
Competing interests: None declared.
References
- 1.Shih I M, Mazur M T, Kurman R J. Gestational trophoblastic disease and related lesion. In: Kurman RJ, ed. Blaustein's pathology of the female genital tract. New York: Springer Verlag, 20021193–1247.
- 2.Paradinas F J, Elston C W. Gestational trophoblastic diseases. In: Fox H, Wells M, eds. Haines & Taylor obstetrical and gynaecological pathology. Edinburgh: Churchill Livingstone, 20031359–1430.
- 3.Cheung A N. Pathology of gestational trophoblastic diseases. Best Pract Res Clin Obstetr Gynaecol 200317849–868. [DOI] [PubMed] [Google Scholar]
- 4.Baergen R N. Gestational choriocarcinoma. Gen Diagn Pathol 1997143127–141. [PubMed] [Google Scholar]
- 5.Ishizuka N, Tomoda Y.Gestational trophoblastic disease. University of Nagoya Press: Nagoya, 199071–96.
- 6.Bagshawe K D. Trophoblastic tumors. In: Coppleson M, ed. Gynecologic oncology. 2nd edn. Edinburgh: Churchill Livingstone, 19921013–1046.
- 7.Miller W H., Jr The emerging role of retinoids and retinoic acid metabolism blocking agents in the treatment of cancer. Cancer 1998831471–1482. [DOI] [PubMed] [Google Scholar]
- 8.Parazzini F, La Vecchia C, Mangili G.et al Dietary factors and risk of trophoblastic disease. Am J Obstet Gynecol 198815893–99. [DOI] [PubMed] [Google Scholar]
- 9.Cheung A N, Srivastava G, Pittaluga S.et al Expression of c‐myc and c‐fms oncogenes in trophoblastic cells in hydatidiform mole and normal human placenta. J Clin Pathol 199346204–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung A N, Ngan H Y, Chen W Z.et al The significance of proliferating cell nuclear antigen in human trophoblastic disease: an immunohistochemical study. Histopathology 199322565–568. [DOI] [PubMed] [Google Scholar]
- 11.Cheung A N, Ngan H Y, Collins R J.et al Assessment of cell proliferation in hydatidiform mole using monoclonal antibody MIB1 to Ki‐67 antigen. J Clin Pathol 199447601–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung A N, Srivastava G, Chung L P.et al Expression of the p53 gene in trophoblastic cells in hydatidiform moles and normal human placentas. J Reprod Med 199439223–227. [PubMed] [Google Scholar]
- 13.Cheung A N, Ngan H Y, Ng W F.et al The expression of cathepsin D, oestrogen receptor and progestogen receptor in hydatidiform mole—an immunohistochemical study. Histopathology 199527341–347. [DOI] [PubMed] [Google Scholar]
- 14.Cheung A N, Shen D H, Khoo U S.et al p21WAF1/CIP1 expression in gestational trophoblastic disease: correlation with clinicopathological parameters, and Ki67 and p53 gene expression. J Clin Pathol 199851159–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung A N, Shen D H, Khoo U S.et al Immunohistochemical and mutational analysis of p53 tumor suppressor gene in gestational trophoblastic disease: correlation with mdm2, proliferation index, and clinicopathologic parameters. Int J Gynecol Cancer 19999123–130. [DOI] [PubMed] [Google Scholar]
- 16.Cheung A N, Zhang D K, Liu Y.et al Telomerase activity in gestational trophoblastic disease. J Clin Pathol 199952588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiu P M, Liu V W S, Ngan H Y S.et al Detection of mitochondrial DNA mutations in gestational trophoblastic disease. Hum Mutat 200322177. [DOI] [PubMed] [Google Scholar]
- 18.Feng H, Cheung A N Y, Xue W C.et al Down‐regulation and promoter methylation of tissue inhibitor of metalloproteinase 3 in choriocarcinoma. Gynecol Oncol 200494375–382. [DOI] [PubMed] [Google Scholar]
- 19.Cheung A N Y. The use of markers on cell proliferation and cell loss in gynaecological cancers. Curr Opin Obstetr Gynaecol 1997932–36. [PubMed] [Google Scholar]
- 20.Wong S Y Y, Ngan H Y S, Chan C C W.et al Apoptosis in gestational trophoblastic disease is correlated with clinical outcome and bcl‐2 expression but not bax expression. Mod Pathol 1999121025–1033. [PubMed] [Google Scholar]
- 21.Chiu P M, Ngan H Y S, Khoo U S.et al Apoptotic activity in gestational trophoblastic disease correlates with clinical outcome—assessment by M30 cytoDeath antibody which recognizes caspase cleavage site within cytokeratin 18. Histopathology 200138243–249. [DOI] [PubMed] [Google Scholar]
- 22.Warrell R P, Jr, Frankel S R, Miller W H., Jret al ifferentiation therapy of acute promyelocytic leukemia with tretinoin (all‐trans‐retinoic acid). N Engl J Med 19913241385–1393. [DOI] [PubMed] [Google Scholar]
- 23.Freemantle S J, Spinella M J, Dmitrovsky E. Retinoids in cancer therapy and chemoprevention: promise meets resistance. Oncogene 2003227305–7315. [DOI] [PubMed] [Google Scholar]
- 24.Tarrade A, Rochette‐Egly C, Guibourdenche J.et al The expression of nuclear retinoid receptors in human implantation. Placenta 200021703–710. [DOI] [PubMed] [Google Scholar]
- 25.Tarrade A, Schoojans K, Guibourdenche J.et al PPAR gamma/RXR alpha heterodimers are involved in human CG beta synthesis and human trophoblast differentiation. Endocrinology 20011424504–4514. [DOI] [PubMed] [Google Scholar]
- 26.Tarrade A, Schoonjans K, Pavan L.et al PPARgamma/RXRalpha heterodimers control human trophoblast invasion. J Clin Endocrinol Metab 2001865017–5024. [DOI] [PubMed] [Google Scholar]
- 27.Yamada S, Okamoto T, Nakanishi T.et al Effects of all‐trans retinoic acid on choriocarcinoma cells in vitro. J Obstet Gynaecol Res 199723125–132. [DOI] [PubMed] [Google Scholar]
- 28.Guibourdenche J, Roulier S, Rochette‐Egly C.et al High retinoid X receptor expression in JEG‐3 choriocarcinoma cells: involvement in cell function modulation by retinoids. J Cell Physiol 1998176595–601. [DOI] [PubMed] [Google Scholar]
- 29.Capparuccia L, Marzioni D, Giordano A.et al PPARgamma expression in normal human placenta, hydatidiform mole and choriocarcinoma. Mol Hum Reprod 20028574–579. [DOI] [PubMed] [Google Scholar]
- 30.Blanchon L, Sauvant P, Bavik C.et al Human choriocarcinoma cell line JEG‐3 produces and secretes active retinoids from retinol. Mol Hum Reprod 20028485–493. [DOI] [PubMed] [Google Scholar]
- 31.Guruswamy S, Lightfoot S, Gold M A.et al Effects of retinoids on cancerous phenotype and apoptosis in organotypic cultures of ovarian carcinoma. J Natl Cancer Inst 200193516–525. [DOI] [PubMed] [Google Scholar]
- 32.Benbrook D M, Rogers R S, Medlin M A.et al Immunohistochemical analysis of proliferation and differentiation in organotypic cultures of cervical tumor cell lines. Tissue Cell 199527269–274. [DOI] [PubMed] [Google Scholar]
- 33.Kamelle S, Sienko A, Benbrook D M. A hormonally responsive model of cycling human endometrium. Fertility Sterility 200278596–602. [DOI] [PubMed] [Google Scholar]
- 34.Thornberry N A, Lazebnik Y. Caspases: enemy within. Science 19982811312–1316. [DOI] [PubMed] [Google Scholar]
- 35.Leers M P, Kolgen W, Bjorklund V.et al Immunocytochemical detection and mapping of a cytokeratin 18 neo‐epitope exposed during early apoptosis. J Pathol. 1999;187567–572. [DOI] [PubMed]
- 36.Parker S B, Eichele G, Zhang P.et al p53 independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science 19952671024–1027. [DOI] [PubMed] [Google Scholar]
- 37.Steinman R A, Hoffman B, Iro A.et al Induction of p21 (WAF‐1/CIP1) during differentiation. Oncogene 199493389–3396. [PubMed] [Google Scholar]
- 38.Yan J, Tanaka S, Oda M.et al Retinoic acid promotes differentiation of trophoblast stem cells to a giant cell fate. Dev Biol 2001235422–432. [DOI] [PubMed] [Google Scholar]
- 39.Kato Y, Braunstein G D. Retinoic acid stimulates placental hormone secretion by choriocarcinoma cell lines in vitro. Endocrinology 1991128401–407. [DOI] [PubMed] [Google Scholar]
- 40.John N J, Linke M, Denker H W. Retinoic acid decreases attachment of JAR choriocarcinoma spheroids to a human endometrial cell monolayer in vitro. Placenta 19931413–24. [DOI] [PubMed] [Google Scholar]
- 41.Fujiwara H, Higuchi T, Sato Y.et al Regulation of human extravillous trophoblastic function by membrane‐bound peptidases. Biochim Biophys Acta 2005175126–32. [DOI] [PubMed] [Google Scholar]
- 42.Okamato T, Niu Rong, Yamada S.et al Reduced expression of tissue inhibitor of metalloproteinase (TIMP)‐2 in gestational trophoblastic diseases. Mol Hum Reprod 20028392–398. [DOI] [PubMed] [Google Scholar]
- 43.Xue W C, Chan K Y K, Feng H C.et al Promoter hypermethylation of multiple genes in hydatidiform mole and choriocarcinoma. J Mol Diagn 20046326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng H, Cheung A N, Xue W C. Down‐regulation and promoter methylation of tissue inhibitor of metalloproteinase 3 in choriocarcinoma. Gynecol Orcol 200494375–382. [DOI] [PubMed] [Google Scholar]
- 45.Hollung K, Rise C P, Drevon C A, Reseland J E. Tissue‐specific regulation of leptin expression and secretion by all‐trans retinoic acid. J Cell Biochem 200492307–315. [DOI] [PubMed] [Google Scholar]
- 46.Li R H, Yu M M, Cheung A N, Wong Y F. Expression of leptin and leptin receptors in gestational trophoblastic diseases. Gynecol Oncol 200495299–306. [DOI] [PubMed] [Google Scholar]