Abstract
Mantle cell lymphoma (MCL) is a B cell neoplasm that most often shows a diffuse growth pattern. Two cases of MCL are reported here, both with a previous diagnosis of lymphoid hyperplasia. Morphologically, germinal centres are hyperplasic with a normal or discretely enlarged mantle zone, where foci of irregularly shaped small lymphocytes are seen. These are positive for CD20, CD5 and cyclin D1, confirming a diagnosis of in situ‐like MCL. This type differs from the mantle zone pattern in that the neoplastic mantle zone is very thin and there is very little or no spread of tumour cells into interfollicular areas. To the best of our knowledge, this is the first report on such a pattern of MCL, which is important to recognise, as it can be confused with lymphoid hyperplasia.
Mantle cell lymphoma (MCL) is a B cell neoplasm, which most often shows a diffuse growth pattern (80%) and presents a more aggressive clinical course than other small B cell non‐Hodgkin's lymphoma.1,2,3 MCL is characterised by the coexpression of B cell markers and CD5 and by the overexpression of cyclin D1, resulting from the t(11;14) (q13;q32) translocation, a hallmark of this type of lymphoma.4,5 More infrequently, MCL presents a nodular (about 15%) or a mantle zone growth pattern.2 MCL with a mantle zone growth pattern can be confused with either marginal zone lymphoma or reactive follicular hyperplasia.6 Our study presents two cases of MCL with a pure mantle zone pattern, representing an in situ‐like MCL.
Case reports
Patient 1 was a 55‐year‐old man presenting with cervical lymphadenopathy and bilateral tonsilar enlargement. A tonsilar biopsy had been performed, with an initial diagnosis of follicular hyperplasia. Patient 2 was a 71‐year‐old woman presenting with multiple cervical lymphadenopathy. A lymph node biopsy had been performed, with the same conclusion as in case 1.
Morphological findings
The tonsilar and node biopsy specimens from both cases showed prominent and heterogeneous follicular hyperplasia with no diffuse or confluent areas. Irregularly shaped follicles were tightly packed, but the interfollicular zones were not infiltrated. Germinal centres were preserved and surrounded by a normal or mildly enlarged, excentric mantle zone. The mantle zone showed a monotonous population of small to medium‐sized cells, with slightly indented and irregular nuclei, inconspicuous nucleoli and scant cytoplasm. The interfollicular zones were filled with a mixed cell population, but no atypical cell was seen. On closer inspection, some follicles without germinal centres (primary follicles) appeared normal, with some irregular cells.
Immunohistochemical findings
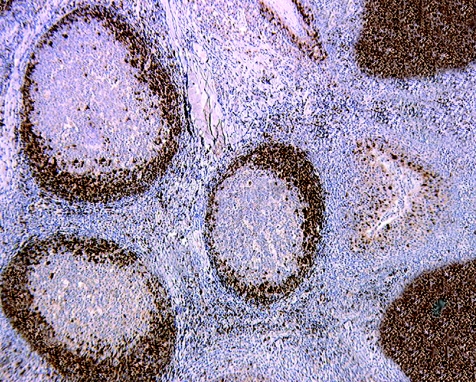
Automated immunohistochemical analysis was carried out on paraffin‐wax sections using a streptavidin–biotin–peroxidase detection system. The follicles were strongly positive for CD20 and CD79a, enhancing the follicular structure of tonsil and lymph node. Few B cells were present in interfollicular zones. Bcl‐2 was detected in the mantle zone, but not in the germinal centres. The mantle zones of secondary and primary follicles were highlighted using antibodies to CD43, CD5 and cyclin D1 (fig 1). We found no cyclin D1 expression in the interfollicular areas, except for rare, scattered elements. Of note, in the tonsil, the epithelial cells served as internal positive control for cyclin D1 staining. Neoplastic cells were negative for CD3, CD10 and CD23. These data and the morphological preservation of the mantle zones prompted us to name this lesion “in situ–like” MCL.
Figure 1 Tonsil: neoplastic cells are positive for cyclin D1 and located in the mantle zone of lymphoid follicles. At the upper and lower coins of the right side, primary follicles show numerous neoplastic cells (immunoperoxidase, anti‐cyclin D1, ×100).
Final staging
Patient 1 was considered to be in Ann Arbor stage IIAa. Fine needle biopsy of a cervical lymph node showed lymphoma cells. The computed tomography scan did not show any other localisation and the bone marrow biopsy specimen remained free of lymphoma cells, even after immunohistochemical analysis. Patient 2 was also classified as stage IIAa. The computed tomography scan showed enlarged thoracic lymph nodes, which were affected. The bone marrow was not infiltrated. In both patients, blood smear examination and systematic gut biopsy specimens were negative. On the basis of these data, the two patients were treated with standard chemotherapy (CHOP—cyclophosphamide, doxorubicin hydrochloride, oncovin and prednisolone), more or less associated with anti‐CD20 antibody (rituximab). Both patients achieved complete remission, but patient 2 relapsed after 1 year with generalised lymphadenopathy and bone marrow infiltration.
Discussion
Lymphoproliferative disorders of small, mature B lymphocytes are represented by distinct entities with different prognosis, sometimes requiring different treatment.3,4,7 The three growth patterns described in MCL did not seem to influence overall survival in one study,1 but others found considerably better clinical outcome for patients with the mantle zone pattern.8 It is also possible that the second case represents the initial stage of the disease in progression.9
The two cases reported in our study represent an extremely rare form of MCL, which may be classified in the group of tumours with pure or true mantle zone growth pattern,10 but with a distinct feature that we designated in situ‐like. Diagnosis may be difficult because the lymph node architecture is preserved. Cytologically, however, monotony in lymphoid cell size and irregularity of nuclear shape suggested the need for immunohistochemical study. As cyclin D1 is not found in appreciable amounts in reactive lymphadenitis (only nuclei of reactive macrophages and stromal cells are consistently positive), this marker was of utmost importance in the diagnosis of MCL in these cases.
In the two cases presented here, the peculiar histological pattern was apparently associated with disease that was more widespread. For patient 1, we were sure that another site (lymph node) was associated, but its histological aspect could not be described. For patient 2, the presence of other putative localisations prompted the clinicians to treat the patient without histological proof. Indeed, in this patient the disease turned out to be more aggressive, as she relapsed 1 year after the onset of the treatment.
The main information obtained from this study is that pathologists should be aware of the existence of an MCL with an in situ‐like growth pattern, and demand an adequate immunohistochemical panel, including a marker for cyclin D1, to differentiate this neoplasm from follicular hyperplasia. In our experience, this presentation, although histologically misleading, was not associated with a strictly localised disease.
Abbreviations
MCL - mantle cell lymphoma
Footnotes
Competing interests: None declared.
References
- 1.Weisenburger D D, Armitage J O. Mantle cell lymphoma—an entity comes of age. Blood 1996874483–4494. [PubMed] [Google Scholar]
- 2.Argatoff L H, Connors J M, Klasa R J.et al Mantle cell lymphoma: a clinicopathologic study of 80 cases. Blood 1997892067–2078. [PubMed] [Google Scholar]
- 3.The Non‐Hodgkin's Lymphoma Classification Project A clinical evaluation of the International Lymphoma Study Group classification of non‐Hodgkin's lymphoma. Blood 1997893909–3918. [PubMed] [Google Scholar]
- 4.Singh N, Wright D H. The value of immunohistochemistry on paraffin wax embedded tissue sections in the differentiation of small lymphocytic and mantle cell lymphomas. J Clin Pathol 19975016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matutes E. New additions to antibody panels in the characterization of chronic lymphoproliferative disorders. J Clin Pathol 200255180–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunt J P, Chan J A, Samoszuk M.et al Hyperplasia of mantle/marginal zone B cells with clear cytoplasm in peripheral lymph nodes. A clinicopathologic study of 35 cases. Am J Clin Pathol 2001116550–559. [DOI] [PubMed] [Google Scholar]
- 7.Zukerberg L R, Medeiros L J, Ferry J A.et al Diffuse low grade B cell lymphomas. Four clinically distinct subtypes defined by a combination of morphologic and immunophenotypic features. Am J Clin Pathol 1993100373–385. [DOI] [PubMed] [Google Scholar]
- 8.Majlis A, Pugh W C, Rodriguez M A.et al Mantle cell lymphoma: correlation of clinical outcome and biologic features with three histologic variants. J Clin Oncol 1997151664–1671. [DOI] [PubMed] [Google Scholar]
- 9.Anagnostopoulos I, Foss H D, Hummel M.et al Extranodal mantle cell lymphoma mimicking marginal zone cell lymphoma. Histopathology 200139561–565. [DOI] [PubMed] [Google Scholar]
- 10.Banks P M, Chan J, Claerly M L.et al Mantle cell lymphoma. A proposal for unification of morphologic, immunologic, and molecular data. Am J Surg Pathol 199216637–640. [DOI] [PubMed] [Google Scholar]