X‐linked sideroblastic anaemia (XLSA; OMIM 301 300) is characterised by accumulation of inorganic iron in erythroblast mitochondria, visualised on staining as distinctive perinuclear rings. It arises from a deficiency of the erythroid specific isoenzyme of δ‐aminolaevulinate synthase (ALAS2; E.C. 2.3.1.3.7), caused mainly by mutations affecting the catalytic or substrate‐binding domains.1,2 ALAS2 uses pyridoxal‐5‐phosphate as a cofactor to catalyse the first, rate‐limiting step of erythroid haem synthesis and pyridoxine treatment can alleviate anaemia in many cases, although the response is variable and affected by factors such as mutation, age and iron load.3,4
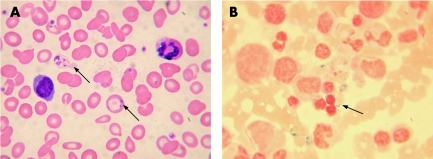
A 40‐year‐old man presented with microcytic anaemia (haemoglobin 7.9 g/dl, mean corpuscular volume 58 fl, and mean corpuscular haemoglobin 15 pg), no evidence of thalassaemia, raised serum ferritin and 88% saturated transferrin. The blood film was dimorphic, with basophilic stippling and Pappenheimer bodies (fig 1A). Bone marrow cytology showed dysplastic erythropoiesis and ringed sideroblasts (fig 1B). After a 10‐week course of 200 mg pyridoxine twice daily, the haemoglobin concentration rose to 12.4 g/dl. A liver biopsy, undertaken because of increased liver transaminases, showed heavy parenchymal iron deposition, but no fibrosis or cirrhosis. Screening for HFE gene mutations was negative. During fortnightly venesections over 8 months, haemoglobin remained stable, serum ferritin fell to 125 µg/l and liver transaminases returned to normal. The patient remains on pyridoxine and on a three‐monthly maintenance venesection. Family history included a maternal grandfather and a brother (haemoglobin 13.1 g/dl, mean corpuscular volume 82 fl, mean corpuscular haemoglobin 26 pg) with microcytic anaemia.
Figure 1 Blood film and bone marrow smear from the index case. (A) Wright–Giemsa stain of the peripheral blood showing dimorphic red cells and the presence of Pappenheimer bodies (indicated by arrows). (B) Perls's stain of the bone marrow aspirate showing the presence of ringed sideroblasts (indicated by the arrow).
Sequencing the coding region and intron or exon boundaries of ALAS2 detected a T918C change on exon 7, predicting substitution of the highly conserved isoleucine by threonine at codon 289. The Ile289Thr mutation was present in the affected brother but not in an unaffected brother. Screening 150 alleles from 75 healthy women, mostly of northern European ancestry, and 45 alleles from 34 (11 women, 23 men) unrelated patients with congenital or inherited sideroblastic anaemia excluded ALAS2T918C as a common (⩾5%), but not rare, north European polymorphism.
The novel mutation Ile289Thr is therefore probably responsible for the development of sideroblastic anaemia in this family, despite a variable associated phenotype not uncommon for this disorder.3 Ile289 is located in a highly conserved region close to previously reported mutations, Gly291Ser and Lys299Gln, also associated with marked responses to pyridoxine,4 and to His285, predicted by homology to have an important steric relationship with bound pyridoxal‐5‐phosphate.4,5
Acknowledgements
We thank Mrs Angela McVeigh for technical assistance and Dr P J Wilson for clinical data on the patient's family history.
References
- 1.Anderson K E, Sassa S, Bishop D F.et al Disorders of heme biosynthesis: X‐linked sideroblastic anemia and the porphyrias. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, eds. The metabolic and molecular bases of inherited disease. Vol 2, 8th edn. New York, NY: McGraw‐Hill, 20012991–3062.
- 2.Bottomley S S. Sideroblastic anaemias. In: Greer JP, Foerster J, Lukens JN, Rodgers GM, Paraskevas F, Glader B, eds. Wintrobe's clinical hematology. Vol 1, 11th edn. Philadelphia: Lippincott Williams & Wilkins, 20041011–1033.
- 3.May A, Bishop D. The molecular biology and pyridoxine responsiveness of X‐linked sideroblastic anaemia. Haematologica 19988356–70. [PubMed] [Google Scholar]
- 4.Shoolingin‐Jordan P M, Al‐Daihan S, Alexeev D.et al 5‐Aminolevulinic acid synthase: mechanism, mutations and medicine. Biochim Biophys Acta 20031647361–366. [DOI] [PubMed] [Google Scholar]
- 5.Alexeev D, Alexeeva M, Baxter R L.et al The crystal structure of 8‐amino‐7‐oxononanoate synthase: a bacterial PLP‐dependent, acyl‐coA‐condensing enzyme. J Mol Biol 1998284401–419. [DOI] [PubMed] [Google Scholar]