Abstract
Background
Deleted in liver cancer‐1 (DLC‐1) is a tumour suppressor gene that is inactive in liver carcinogenesis. It encodes a ρ‐guanosine triphosphatase‐activating protein (ρ‐GAP) and maps to one of the deleted regions (8p21.3–22). Little is known, however, about the methylation status of the DLC‐1 promoter in myeloma cells.
Aim
To identify whether methylation of DLC‐1 was associated in pathogenesis of multiple myeloma.
Methods
Reverse transcription‐polymerase chain reaction (RT‐PCR) was used to detect DLC‐1 transcripts in RPMI 8226, U266, OPM‐2 and XG‐2 cell lines. The methylation status was determined by methylation‐specific PCR followed by bisulphite DNA sequencing in these four cell lines and in the bone marrow of 14 patients with multiple myeloma and 4 normal patients. DLC‐1 mRNA expression in cells with or without treatment with 5‐aza‐deoxycytidine (5‐aza‐CdR) or trichostatin A (TSA) was investigated by real‐time RT‐PCR.
Results
RPMI 8226 and U266 showed complete methylation and XG‐2 showed partial methylation. DLC‐1 was expressed only in OPM‐2 cell lines that showed no methylation. DLC‐1 methylation was shown in 11 of 14 (78%) patients with multiple myeloma and none of the normal controls. The exposure of cell lines to 5‐aza‐CdR or TSA resulted in the up regulation of DLC‐1 gene expression.
Conclusions
DLC‐1 methylation is often present in multiple myeloma and has a key role in DLC‐1 silencing.
Multiple myeloma is a plasma cell neoplasm. These malignant plasma cells arise from a postgerminal centre B cell, which adheres to the marrow stroma and triggers subsequent bone resorption and the paracrine cytokine loop.1 Cytogenetic studies show a wide variety of translocations in specific subtypes of the disease, and structurally altered genes have important roles in cell proliferation, differentiation and gene transcription.2,3
Evidence increasingly shows that, in addition to genetic aberrations, epigenetic processes have a major role in carcinogenesis. For patients with multiple myeloma, only a limited number of studies to date have dealt with the issue of gene methylation, including methylation of BNIP3, p15, p16, death‐associated protein kinase, E‐cadherin, methylguanine DNA methyl transferase, Ras Association Family 1A (RASSF1A) and suppressor of cytosine signalling 1.4,5,6
The deleted in liver cancer‐1 gene (DLC‐1), which has been mapped to 8p21.3–p22,7 is deleted in primary hepatocellular carcinoma (HCC) as well as in HCC cell lines, and exerts inhibitory effects on HCC cell lines.8DLC‐1 is the human homologue of rat p122, a member of ρ‐guanosine triphosphatase (GTPase)‐activating proteins (GAPs). The ρ family proteins function as important regulators in the organisation of the actin cytoskeleton. Dysregulation of the ρ‐GTPase‐associated signalling pathways has been reported in tumours.9DLC‐1 is therefore a negative regulator of the ρ family of small GTPases. Hypermethylation of the gene without DLC‐1 expression was found in several cell lines of HCC and of tumours of the breast, colon and prostate, showing that aberrant promoter methylation is responsible for DLC‐1 silencing in some cancer cells.10 The mechanisms underlying DLC‐1 gene expression in myeloma cells were unknown. In this study, we assessed the status of the DLC‐1 promoter hypermethylation in multiple myeloma cell lines and bone marrow of patients.
Methods
Cell culture
Human multiple myeloma cell lines, RPMI 8226, U266, OPM‐2 and XG‐2, were used in this study. Cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 1% (v/v) penicillin and 100 µg/ml streptomycin, and maintained at 37°C in a 5% CO2 atmosphere.
Bone marrow samples
Fourteen untreated patients with multiple myeloma (9 men, 5 women; median age 62 years; stage I, n = 2; stage II, n = 3; and stage III, n = 9) seen in the Department of Hematology, Hua Shan Hospital, Shanghai, China, between July 2003 and January 2005 were studied. The patients were diagnosed according to the criteria given by Durie and Salmon.11 The bone marrow samples of patients with non‐haematological diseases served as controls (n = 4).
Reverse transcriptase‐polymerase chain reaction and quantitative real‐time polymerase chain reaction
Total RNA was extracted from the cells with RNeasy Mini kits (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Total RNA was reverse transcribed and amplified using Superscript First‐Strand Synthesis System for reverse transcriptase‐polymerase chain reaction (RT‐PCR; Invitrogen, Life Technologies, Carlsbad, Germany).
Routine RT‐PCR for detection of DLC‐1 expression in the cell lines was carried out by HotStar Taq DNA Polymerase (Qiagen). The PCR consisted of 35 cycles of DNA denaturing at 95°C, followed by 30 s of primer pair annealing to the DNA target sequence at 57°C, followed by 40 s of primer pair extension at 72°C. Samples were defined as DLC‐1 positive if a band was detectable after 35 cycles of amplification.
Gene expression of cells after exposure to 5‐aza‐deoxycytidine (5‐aza‐CdR) or trichostatin A (TSA) was assessed by real‐time RT‐PCR with the ABI PRISM 7700 Sequence Detection System instrument and software (Applied Biosystems, Foster City, California, USA). Glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) was used as an endogenous reference gene for normalising variance in the quality of RNA and the amount of input cDNA. Primers for DLC‐1 and GAPDH were synthesised according to the Primer 3 program (http://frodo.wi.mit.edu/cgi‐bin/primer3/primer3_www.cgi). Primers for DLC‐1 mRNA were as follows: 5′‐GGACACCATGATCCTAACAC‐3′(sense); 5′‐CTCATCCTCGTCTGAATCGT‐3′(antisense). Primers for GAPDH were as follows: 5′‐GAAGGTGAAGGTCGGAGTCA‐3′(sense); 5′‐GAAGATGGTGATGGGATTTC‐3′ (antisense). After an initial denaturation step at 95°C for 10 min, 40 cycles of amplification were carried out. Each cycle comprised denaturation at 94°C for 15 s, primer annealing at 55°C for 30 s and elongation at 72°C for 40 s. Amplification was immediately followed by a melting cycle of 2 min at 95°C, 2 min at 68°C and a stepwise temperature increase of 0.2°C/s until 90°C, with fluorescence acquisition at each temperature transition. Thus, determination of the individual melting temperature for a DNA fragment can be used to characterise the amplicon and to differentiate a specific PCR product from unspecific products or primer dimer. PCR was carried out using SYBR green. Relative quantification of the mRNA levels of the DLC‐1 was determined using computed tomography. Briefly, the amount of DLC‐1 was normalised to the endogeneous reference gene (GAPDH) and its expression in treated samples was calculated relative to the non‐treated sample (control).
Methylation‐specific PCR
Genomic DNA was isolated from bone marrow aspirates using proteinase K (Gibco, Lofer, Austria) digestion, followed by phenol–chloroform extraction and ethanol precipitation. The methylation status of CpG (cytosine‐deoxyribose phosphates followed immediately by a guanine‐deoxyribose phosphate) islands of the DLC‐1 gene was determined using methylation‐specific PCR (MSP). The DNA was chemically modified using bisulphite with the CpGenome DNA Modification Kit (Intergen, Oxford, UK). Bisulphite‐modified DNA was amplified with primers described by Kim et al:12 methylated (M) DLC‐1 gene 5′‐CCCAACGAAAAAACCCGACTAACG‐3′ (sense) and 5′‐TTTAAAGATCGAAACGAGGGAGCG‐3′ (antisense); unmethylated (U) DLC‐1 gene 5′‐AAACCCAACAAAAAAACCCAACTAACA‐3′ (sense) and 5′‐TTTTTTAAAGATTGAAATGAGGGAGTG‐3′ (antisense). Methylated DNA (CpGenome Universal Methylated DNA; Intergen) was used as a positive control. MSP was carried out in a thermal cycler (9700; PE Biosystems, Foster City, California, USA) with the following cycling conditions: 95°C for 12 min, 35 cycles of 95°C for 45 s, 55°C for 30 s, 72°C for 30 s and a final extension of 10 min at 72°C. The PCR mixture contained 50 ng bisulphite‐treated DNA, 0.2 mM deoxynucleotide triphosphates, 2 mM magnesium chloride (MgCl2), 10 pmol of each primer, 1×PCR buffer II and 2.5 units of AmpliTaq Gold (PE Biosystems) in a final volume of 50 μl. PCR products were analysed on 2% agarose gels and were visualised under ultraviolet illumination. Methylated and unmethylated status was determined by the presence of fragments in samples amplified with the M and U primers, respectively.
Sodium bisulphite‐treated genomic DNA sequencing
Sodium bisulphite‐treated genomic DNA samples were amplified using a pair of universal primers as follows: 5′‐GTTTTTAGTTAGGATATGGT‐3′ (sense) and 5′‐CTTCTTTCTACACATCAAACA‐3′ (antisense). The PCR products were cloned into the terminal transferase activity cloning system. Eight recombinant clones were randomly picked from each transfection for nucleotide sequence analysis to determine the proportion of hypomethylated promoter sequence.
Treatment with 5‐aza‐CdR or TSA
Three study treatment groups were established: treatment with 0.3 μM TSA, 0.5 μM 5‐aza‐CdR or culture medium alone. Cells treated with TSA were assessed after 24 h and those treated with 5‐aza‐CdR were assessed after 96 h.
Results
Expression of DLC‐1 in multiple myeloma cell lines
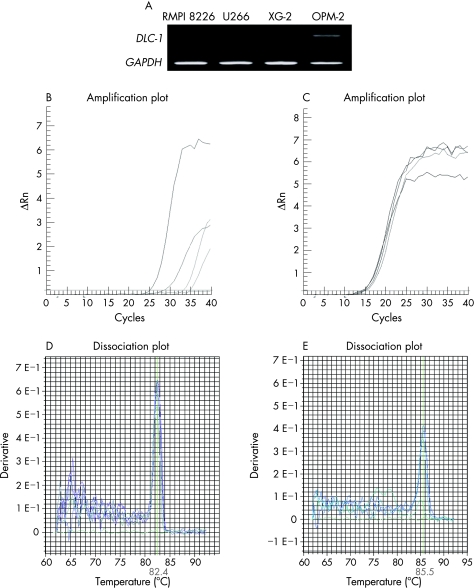
We first determined the expression of the DLC‐1 gene in four multiple myeloma cell lines, using a pair of sequence‐specific primers in PCR. GAPDH mRNA was used as a control for cDNA integrity. DLC‐1 mRNA was detected only in the OPM‐2 cell line but not in RPMI 8226, U266 or XG‐2 cell lines (fig 1A). Expression of DLC‐1 and internal control was also determined by real‐time PCR (fig 1B,C). Amplification of the two amplicons was observed by the appearance of their expected fluorescence melting temperature (Tm) peaks. The Tm peak for GAPDH was at 82.4°C (fig 1D), but that for the human GAPDH was at 85.5°C (fig 1E).
Figure 1 Expression of the deleted in liver cancer‐1 gene (DLC‐1) in multiple myeloma cell lines. DLC‐1 expression was detected by conventional reverse transcription‐polymerase chain reaction (RT‐PCR) in four cell lines; glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) served as a control for equal loading. (A) DLC‐1 mRNA was detected only in the OPM‐2 cell line but not in RPMI 8226, U266 or XG‐2 multiple myeloma cell lines. (B) DLC‐1 expression was detected by real‐time amplification with SYBR green fluorescence in four cell lines. (C) GAPDH was used as an internal control. Melt derivative analysis of the multiplex fluorescence PCR showing the amplification of specific products of (D) DLC‐1 at melting temperature (Tm) 85.5°C and (E) GAPDH at Tm 82.4°C.
Methylation status in multiple myeloma cell lines and bone marrow of patients
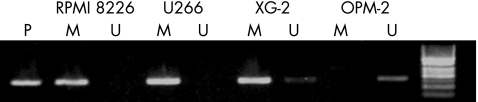
MSP was used to determine whether the loss of DLC‐1 gene expression among the multiple myeloma cell lines was related to differences in the methylation status of their promoters. Of the four cell lines examined, three of them showed methylation of DLC‐1 (fig 2), including two cell lines (RPMI 8226 and U266) showing complete methylation, XG‐2 showed partial methylation and DCL‐1 was expressed only in OPM‐2 cell lines that showed no methylation. These results suggest that methylation of the CpG sites strongly correlates to the loss of DLC‐1 expression.
Figure 2 Analysis of promoter methylation of the deleted in liver cancer‐1 gene in multiple myeloma cell lines. M, amplified products with primers recognising the methylated gene sequence; P, positive control; U, amplified products with primers recognising the unmethylated gene sequence.
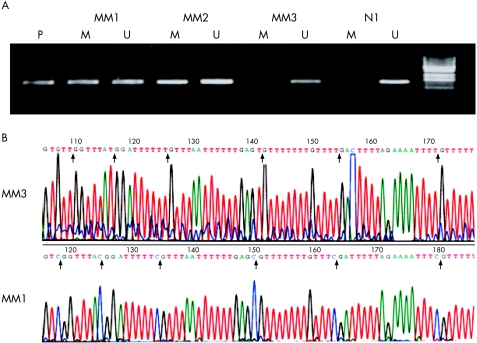
To examine the prevalence of DLC‐1 methylation in non‐cultured cells, we analysed the DLC‐1 methylation status in samples from patients with primary multiple myeloma by MSP (fig 3A). By using MSP, we found DLC‐1 methylation in 11 of 14 (78%) primary multiple myeloma samples compared with none in the four bone marrow samples from normal controls (fig 3). In all of the patients with primary multiple myeloma and DLC‐1 methylation, unmethylated DNA bands were observed. The presence of unmethylated DNA may be caused by contamination by other cells in the bone marrow; hemizygotic methylation, however, cannot be excluded.
Figure 3 (A) Representative methylation‐specific polymerase chain reaction analysis of samples from patients with multiple myeloma. (B) Partial sequence data obtained from bisulphite genomic sequencing; M, amplified products with primers recognising the methylated gene sequence; MM1, MM2, MM3, myeloma samples; N, normal bone marrow sample; P, positive control; U, amplified products with primers recognising the unmethylated gene sequence. Vertical arrows indicate the positions of cytosine on cytosine‐deoxyribose phosphates followed immediately by a guanine‐deoxyribose phosphate (CpG). Upper column, all cytosines are converted to thymidine by bisulphite treatment in MM3, indicating that it was unmethylated. Lower column, all cytosines on seven CpG sites remained unchanged in MM1, which was methylated.
Bisulphite genomic sequencing
To further verify the methylation status of DLC‐1 in the cell lines and in bone marrow of patients, we subjected cell lines U266 and RPMI 8226 and a randomly selected methylated sample to bisulphite sequencing and examined a total of 35 CpG sites located −7 to −257 bp upstream of the ATG translation codon for hypermethylation. In agreement with the results obtained from the MSP study, the analysis of eight clones from the U266 cell line showed hypermethylation of all CpG sites, and RPMI 8226 showed 94% (32/35) hypermethylation of all CpG sites.
We next examined additional multiple myeloma samples (fig 3B). Sample MM1 showed extensive hypermethylation of these CpG dinucleotides, whereas sample MM3 remained unmethylated.
Up regulation of DLC‐1 gene expression in multiple myeloma cell lines by 5‐aza‐CdR or TSA
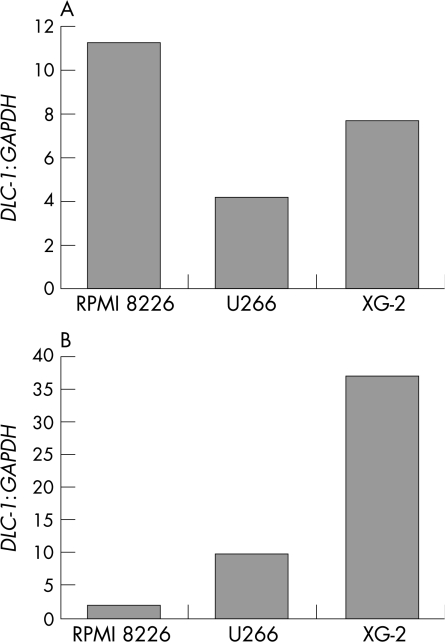
To determine whether the inhibition of cytosine methylation or histone deacetylation could induce DLC‐1 mRNA expression in these four cell lines with methylation of the CpG island, we treated the four cell lines with either the cytosine methylation inhibitor 5‐aza‐CdR or the histone deacetylase inhibitor (HDAC) TSA (fig 4). The 5‐aza‐CdR treatment alone with a concentration of 0.5 μmol/l was able to up regulate DLC‐1 expression levels 11.2‐fold (RPMI 8226), 4.1‐fold (U266) and 7.7‐fold (XG‐2) when compared with the untreated cells. The greater activation of DLC‐1 mRNA was also seen by TSA treatment alone (9.6‐fold in U266 and 37.3‐fold in XG‐2), although this was not observed in RPMI cells. These results suggest that both DNA methylation and histone deacetylation are associated with a gene silencing mechanism in multiple myeloma cell lines.
Figure 4 The role of 5‐aza‐deoxycytidine (5‐aza‐CdR) or trichostatin A (TSA) in restoration of deleted in liver cancer‐1 gene (DLC‐1) expression. RPMI 8226, U266 and XG‐2, which showed DLC‐1 methylation, were treated with (A) 0.5 μM 5‐aza‐CdR or (B) 0.3 μM TSA. The bars show the level of DLC‐1 expression determined by real‐time polymerase chain reaction normalised to glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) compared with untreated cell.
Discussion
The DLC‐1 gene is located on chromosome 8p21.3–22, a region of LOH in a variety of cancers.13,14 Somatic mutation of the DLC‐1 gene, however, seems to be rare in some tumours.15,16,17,18,19 In this study, we tested the methylation status in multiple myeloma cell lines and bone marrow samples of patients. By MSP assay, we found frequent methylation of the DLC‐1 gene in 75% of the multiple myeloma cell lines and 78% of the bone marrow samples from patients with multiple myeloma. These results show a high frequency of aberrant promoters of DLC‐1, which contrasted with previous investigations on the infrequent methylation rate in breast cancer,20 gastric cancer,12 liver cancer21 and brain tumour.19
We showed that the expression of DLC‐1 was highly suppressed in three methylated cell lines compared with that in one non‐methylated cell line. These results suggest that DNA methylation of the regulatory promoter region is critical for transcriptional regulation of the DLC‐1 gene and has an important role in DLC‐1 inactivation. The results are consistent with the finding of restoration of DLC‐1 expression in methylated cell lines after 5‐aza‐CdR treatment.22
Recent data suggest that hyperacetylated histones have an important role in regulating gene expression. We therefore examined the effect of the HDAC, TSA, on the expression of DLC‐1. TSA treatment influences the expression level of U266 and XG‐2, but not of RPMI 8226, suggesting that chromatin modification in the DLC‐1 promoter region may vary between cell types. TSA by itself was more efficient than 5‐aza‐CdR in inducing DLC‐1 transcripts, supporting that HDAC is a promising new treatment strategy for malignant multiple myeloma.23
Take‐home messages
By methylation‐specific polymerase chain reaction assay, we found frequent methylation of the deleted in liver cancer‐1 gene (DLC‐1) in 75% multiple myeloma cell lines and in 78% bone marrow samples of patients with multiple myeloma. DLC‐1 may affect the pathogenesis of multiple myeloma.
The inhibition of cytosine methylation or histone deacetylation may induce DLC‐1 mRNA expression in methylated cell lines, suggesting a potential new treatment strategy for multiple myeloma.
DNA methylation of the regulatory promoter region may be critical for transcriptional regulation of the DLC‐1 gene and have an important role in DLC‐1 inactivation, which is a promising marker for early detection, prediction of cancer risk and prognosis of disease in myeloma.
Migration is one of the important biological processes for myeloma cell invasion and dissemination, but little is known about the mechanisms regulating this phenomenon. Previous studies show that Wnt was implicated in the morphological change in myeloma cells through ρ‐A,24 an important ρ‐GTPase family. The ρ‐A pathway is known to regulate motility through reorganisation of actin cytoskeletin and stress fibre and focal adhesion formation.25 Raised levels of expression of ρ (ρ‐A, ρ‐B and ρ‐C) have been corrected with tumour stage or enhanced metastasis in tumours, including breast cancer,26 melanomas27 and pancreatic ductal adenocarcinoma.28 Thus, the ρ‐A pathway is likely to have a critical role in malignant cell invasion in multiple myeloma. It is not surprising that ρ‐GTPases are potential candidates for cancer treatment.
Inactivation of DLC‐1, a ρ‐GAP with methylation, may potentially result in up regulation of the GTPase activity of ρ family proteins. The ρ‐GAP domain of the DLC‐1 genes is very likely to be associated with the inhibitory effect on tumour cell proliferation. Given the altered DNA, methylation is one of the most promising markers for early detection, prediction of cancer risk and prognosis of disease; the high frequency of methylation in the promoter of DLC‐1 suggests a potential clinical application in the detection of multiple myeloma.
This is the first study to show that DLC‐1 in multiple myeloma is epigenetically regulated and can be targeted by HDAC and demethylating agents. Additional studies on a larger spectrum of patients with multiple myeloma are needed to clarify the clinical implications of the DLC‐1 methylation and also the correspondence between the genetic changes in DLC‐1 and protein expression and the role of DNA methylation, particularly when the restoration of proliferation‐associated genes silenced by aberrant DNA hypermethylation emerges as an anticancer target in haematological malignancies.
Acknowledgements
We thank our colleagues in the Department of Hematology for helpful discussion and valuable contributions.
Abbreviations
5‐aza‐CdR - 5‐aza‐deoxycytidine
CpG - cytosine‐deoxyribose phosphates followed immediately by a guanine‐deoxyribose phosphate
DLC‐1 - deleted in liver cancer‐1 gene
GAPDH - glyceraldehyde‐3‐phosphate dehydrogenase
GTPase - guanosine triphosphatase
HCC - hepatocellular carcinoma
HDAC - histone deacetylase inhibitor
MSP - methylation‐specific polymerase chain reaction
ρ‐GAP - ρ‐guanosine triphosphatase‐activating protein
RT‐PCR - reverse transcription‐polymerase chain reaction
Tm - melting temperature
TSA - trichostatin A
Footnotes
Competing interests: None declared.
References
- 1.Kuehl W M, Bergsagel P L. Multiple myeloma: evolving genetic events and host interactions. Nat Rev Cancer 20022175–187. [DOI] [PubMed] [Google Scholar]
- 2.Zandecki M, Lai J L, Facon T. Multiple myeloma: almost all patients are cytogenetically abnormal. Br J Haematol 199694217–227. [DOI] [PubMed] [Google Scholar]
- 3.Seidl S, Kaufmann H, Drach J. New insights into the pathophysiology of multiple myeloma. Lancet Oncol 20034557–564. [DOI] [PubMed] [Google Scholar]
- 4.Murai M, Toyota M, Satoh A.et al Aberrant DNA methylation associated with silencing BNIP3 gene expression in haematopoietic tumours. Br J Cancer 2005921165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galm O, Wilop S, Reichelt J.et al DNA methylation changes in multiple myeloma. Leukemia 2004181687–1692. [DOI] [PubMed] [Google Scholar]
- 6.Seidl S, Ackermann J, Kaufmann H.et al DNA‐methylation analysis identifies the E‐cadherin gene as a potential marker of disease progression in patients with monoclonal gammopathies. Cancer 20041002598–2606. [DOI] [PubMed] [Google Scholar]
- 7.Yuan B Z, Miller M J, Keck C L.et al Cloning, characterization, and chromosomal localization of a gene frequently deleted in human liver cancer (DLC‐1) homologous to rat RhoGAP. Cancer Res 1998582196–2199. [PubMed] [Google Scholar]
- 8.Ng I O, Liang Z D, Cao L.et al DLC–1 is deleted in primary hepatocellular carcinoma and exerts inhibitory effects on the proliferation of hepatoma cell lines with deleted DLC‐1. Cancer Res 2000606581–6584. [PubMed] [Google Scholar]
- 9.Schmitz A A, Govek E E, Bottner B.et al Rho GTPases: signaling, migration, and invasion. Exp Cell Res 20002611–12. [DOI] [PubMed] [Google Scholar]
- 10.Yuan B Z, Durkin M E, Popescu N C. Promoter hypermethylation of DLC‐1, a candidate tumor suppressor gene, in several common human cancers. Cancer Genet Cytogenet 2003140113–117. [DOI] [PubMed] [Google Scholar]
- 11.Durie B G, Salmon S E. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer 197536842–854. [DOI] [PubMed] [Google Scholar]
- 12.Kim T Y, Jong H S, Song S H.et al Transcriptional silencing of the DLC‐1 tumor suppressor gene by epigenetic mechanism in gastric cancer cells. Oncogene 2003223943–3951. [DOI] [PubMed] [Google Scholar]
- 13.Yuan B Z, Zhou X, Durkin M E.et al DLC‐1 gene inhibits human breast cancer cell growth and in vivo tumorigenicity. Oncogene 200322445–450. [DOI] [PubMed] [Google Scholar]
- 14.Yaremko M L, Kutza C, Lyzak J.et al Loss of heterozygosity from the short arm of chromosome 8 is associated with invasive behavior in breast cancer. Genes Chromosomes Cancer 199616189–195. [DOI] [PubMed] [Google Scholar]
- 15.Park S W, Durkin M E, Thorgeirsson S S.et al DNA variants of DLC‐1, a candidate tumor suppressor gene in human hepatocellular carcinoma. Int J Oncol 200323133–137. [PubMed] [Google Scholar]
- 16.Hewitt C, Wilson P, McGlinn E.et al DLC1 is unlikely to be a primary target for deletions on chromosome arm 8p22 in head and neck squamous cell carcinoma. Cancer Lett 2004209207–213. [DOI] [PubMed] [Google Scholar]
- 17.Yin X L, Pang J C, Ng H K. Identification of a region of homozygous deletion on 8p22–23.1 in medulloblastoma. Oncogene 2002211461–1468. [DOI] [PubMed] [Google Scholar]
- 18.Wilson P J, McGlinn E, Marsh A.et al Sequence variants of DLC1 in colorectal and ovarian tumours. Hum Mutat 200015156–165. [DOI] [PubMed] [Google Scholar]
- 19.Pang J C, Chang Q, Chung Y F.et al Epigenetic inactivation of DLC‐1 in supratentorial primitive neuroectodermal tumor. Hum Pathol 20053636–43. [DOI] [PubMed] [Google Scholar]
- 20.Teramoto A, Tsukuda K, Yano M.et al Less frequent promoter hypermethylation of DLC‐1 gene in primary breast cancers. Oncol Rep 200412141–144. [PubMed] [Google Scholar]
- 21.Wong C M, Lee J M, Ching Y P.et al Genetic and epigenetic alterations of DLC‐1 gene in hepatocellular carcinoma. Cancer Res 2003637646–7651. [PubMed] [Google Scholar]
- 22.Yuan B Z, Jefferson A M, Baldwin K T.et al DLC‐1 operates as a tumor suppressor gene in human non‐small cell lung carcinomas. Oncogene 2004231405–1411. [DOI] [PubMed] [Google Scholar]
- 23.Podar K, Hideshima T, Chauhan D.et al Targeting signalling pathways for the treatment of multiple myeloma. Expert Opin Ther Targets 20059359–381. [DOI] [PubMed] [Google Scholar]
- 24.Qiang Y W, Walsh K, Yao L.et al Wnts induce migration/invasion of myeloma plasma cells. Blood 20051061786–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall A. Rho GTPases and the actin cytoskeleton. Science 1998279509–514. [DOI] [PubMed] [Google Scholar]
- 26.Lin M, van Golen K L. Rho‐regulatory proteins in breast cancer cell motility and invasion. Breast Cancer Res Treat 20048449–60. [DOI] [PubMed] [Google Scholar]
- 27.Collisson E A, Kleer C, Wu M.et al Atorvastatin prevents RhoC isoprenylation, invasion, and metastasis in human melanoma cells. Mol Cancer Ther 20032941–948. [PMC free article] [PubMed] [Google Scholar]
- 28.Suwa H, Ohshio G, Imamura T.et al Overexpression of the rhoC gene correlates with progression of ductal adenocarcinoma of the pancreas. Br J Cancer 199877147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]