Abstract
Background
Colorectal cancer is associated with a “field change” of increased proliferation throughout the colonic and rectal mucosa. Both proliferation and apoptosis are disrupted during carcinogenesis. Whether altered apoptosis contributes to this field change of microscopic abnormality is, however, unclear. Bcl‐xL is an anti‐apoptotic protein that inhibits apoptosis by preventing release of cytochrome c, a recognised pathway to cell death.
Aim
To determine whether Bcl‐xL inhibition of apoptosis is increased in colorectal mucosa adjacent to colorectal adenocarcinoma over that in normal non‐neoplastic colorectal mucosa.
Patients
Patients undergoing surgical resection for neoplastic (adenocarcinoma) or non‐neoplastic disease of the colorectum (rectal prolapse, diverticular disease or volvulus).
Methods
Formalin‐fixed, paraffin‐wax‐embedded surgical colorectal resection specimens were immunostained for Bcl‐xL protein. Labelling indices were determined by counting the proportion of positively stained cells in mucosal crypts.
Results
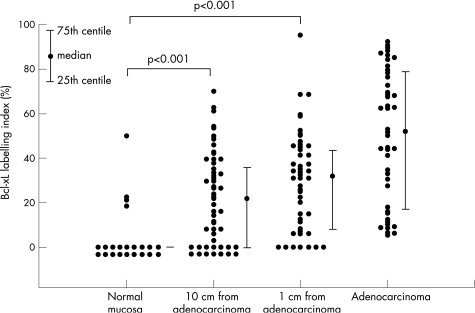
85 patients were studied. Bcl‐xL immunostaining was most marked in the upper third of mucosal crypts. It occurred in a minority of samples from non‐neoplastic colorectal mucosa, but was seen in most mucosal samples adjacent to colorectal adenocarcinoma. Significant increases (p<0.001) were observed in Bcl‐xL labelling indices in the mucosa at 1 cm (n = 46, median labelling index 31.8%, interquartile range 8.3–43.9%) and at 10 cm (n = 52, median labelling index 22.0%, interquartile range 0.0–36.3%) from colorectal carcinoma, compared with normal, non‐neoplastic colorectal mucosa (n = 22, median labelling index 0.0%, interquartile range 0.0–0.0%).
Conclusions
The findings are consistent with a field change of inhibited apoptosis in mucosa adjacent to colorectal carcinoma.
The survival of neoplastic cells depends on increased cell proliferation, reduced cell death or a combination of both.1 Apoptosis is a pre‐determined programme of autonomous cell death and its inhibition results in cellular clonal expansion. The discovery that the Bcl‐2 gene—linked to an immunoglobulin locus by chromosome translocation in follicular lymphoma—inhibits cell death rather than promoting proliferation gave rise to the concept that impaired apoptosis is an important step in tumorigenesis.2 Bcl‐xL is a cytoplasmic anti‐apoptotic protein of 241 amino acids, which shares considerable homology with Bcl‐2.3 After a cytotoxic insult stimulates the apoptosis pathway, Bcl‐xL associates tightly with the mitochondrial outer membrane to prevent cytochrome c release. Unhindered release of cytochrome c leads ultimately to activation of caspases, a family of intracellular cysteine proteases. Activated caspases achieve apoptosis by cleaving DNA and cytoskeletal proteins, causing a loss of cell adhesion. Thus, Bcl‐xL prevents the mobilisation of cell death proteases.
The presence of a colorectal adenoma or carcinoma is associated with a “field change” of increased proliferation throughout the colonic and rectal mucosa.4 Studies on the pattern of this change suggest an increase in the proliferative capacity of cells in the upper portion of colonic crypts—in colonic mucosa from patients with sporadic adenomas and carcinomas compared with mucosa from patients without colonic neoplasia.4,5,6 There may be a dampening of this proliferative effect within 1 cm of the neoplastic lesion; the effect returns at more than 1 cm distance from the lesion.7
Although both proliferation and apoptosis appear to be active during colorectal carcinogenesis,1 a field change in apoptosis has not been reported. We determined whether inhibition of apoptosis, as shown by the presence of Bcl‐xL, was increased in colorectal mucosa adjacent to colorectal adenocarcinoma compared with normal non‐neoplastic colorectal mucosa.
Methods
Recruitment of patients
Patients undergoing elective resection of the colon or rectum at Chelsea & Westminster Hospital, London, UK, between January 2002 and January 2005 were studied. Enrolment in the neoplastic group required a resection of invasive colorectal adenocarcinoma. Non‐neoplastic control samples were obtained from patients undergoing colonic or rectal excision of diverticular disease, rectal prolapse or colonic volvulus. The study was discussed with eligible patients, who were provided with an information sheet and who gave informed consent to participate. The study was approved by the Riverside Ethics Committee, London, UK.
Collection of specimens
Immediately after surgical resection, the colorectal specimen was opened and inspected before formalin fixation. A single tissue sample of 1–2 cm full thickness was taken from all patients in the non‐cancer group. Three tissue samples were taken from patients in the cancer group, each 1–2 cm in size. One sample was from the adenocarcinoma itself, one from 1 cm proximal and another from 10 cm proximal to the carcinoma. Samples were not taken in cases where taking a sample from a small cancer would leave insufficient cancer tissue for subsequent histological analysis as part of an individual patient's clinical management, opening the specimen to facilitate sample collection would interfere with subsequent histological processing, or where a 10‐cm sample was not available because the surgical resection margin was <10 cm.
Immunohistochemistry
Specimens were fixed in 10% formalin for 24 h, dehydrated using an automated Histokinette machine (Reichert‐Jung, Wetzlar, Germany) and embedded in paraffin wax. Tissue sections of 3.5 μm thickness were cut and mounted on slides coated with aminoalkylsilane (Sigma‐Aldrich, Dorset, UK) to promote adherence. Slides for immunohistochemistry were placed in an oven (at 37°C) overnight to further enhance the adherence of specimens to slides. Tissue sections were deparaffinised, antigens were retrieved using microwave heat techniques and endogenous peroxidase activity was blocked (Dako; Dako normal goat serum, Dako, Glostrup, Denmark). Sections were incubated with Bcl‐xL primary antibody (Zymed Laboratories, mouse monoclonal antibodies, Zymed, San Francisco, USA) for 1 h at room temperature. Bound antibody was detected using biotinylated anti‐mouse IgG secondary antibody and streptavidin–peroxidase complex (Dako ABComplex), followed by incubation with diaminobenzidine as the substrate. Sections were counterstained with Mayer's haematoxylin. Reed–Sternberg and multinucleated variant cells in lymph nodes affected by Hodgkin's disease are recognised as positive controls for Bcl‐xL and one positive control slide was included in each batch of immunostaining. The primary antibody was omitted in negative controls. Additionally, one slide for each specimen was stained with haematoxylin and eosin to confirm the diagnosis.
Determination of labelling indices
Immunostained slides were examined using an Olympus U‐CMAD3 microscope at ×400 magnification, without knowledge of the indication for colorectal resection. Two complete mucosal crypts of each slide were photographed at random with an Olympus Camedia digital camera. In disorganised tissue, two photographs of random locations within the tissue section were taken. Photographs were saved on a smart card and analysed on a computer screen, using only a photograph number to avoid creating bias through knowledge of pathology or the patient's name.
Mucosal crypts were divided into three longitudinal compartments4 on the computer screen. Positively stained cells in each compartment and the total number of cells in each compartment were counted by one observer, using a hand‐held numerical counter (VWR International, Leicester, UK). The labelling index of each compartment for each specimen sample was calculated as a mean of the two resulting values. In disorganised mucosa, the number of positively stained cells in three separate areas of 100 cells was counted. The labelling index of each such specimen was calculated as a mean of the six resulting areas of two photographs.
Once labelling indices were determined, the reason for colorectal resection was disclosed and the data were analysed according to the presence or absence of neoplasia. The diagnosis of histological abnormality was confirmed by a specialist gastrointestinal histopathologist.
Statistical analysis
Groups were analysed to determine whether labelling indices followed a normal gaussian distribution (Sigmastat 3.0). Student's t test or Mann–Whitney test was used to identify differences between groups in pairs for parametric and non‐parametric data, respectively. A p value of <0.05 was considered to have a significantly low probability of a type‐1 error occurring.
Results
Patients
Tissue samples were obtained from colorectal resection specimens in a total of 85 patients. Table 1 shows the characteristic details of patients. It was not possible to collect the following samples: one adenocarcinoma sample from a patient with cancer with a small tumour; adenocarcinoma and 1‐cm samples from 17 patients where opening the specimen would have disrupted subsequent histological processing; and 10‐cm samples with resection margins of <10 cm from 11 patients with cancer.
Table 1 Patient characteristics.
| Neoplastic group (n = 63) | Non‐neoplastic group (n = 22) | |
|---|---|---|
| Sex | 32 male, 31 female | 9 male, 13 female |
| Median age (interquartile range) in years | 71 (65–78)* | 64.5 (47–80)* |
*Mann–Whitney test; p = not significant.
Bcl‐xL immunostaining
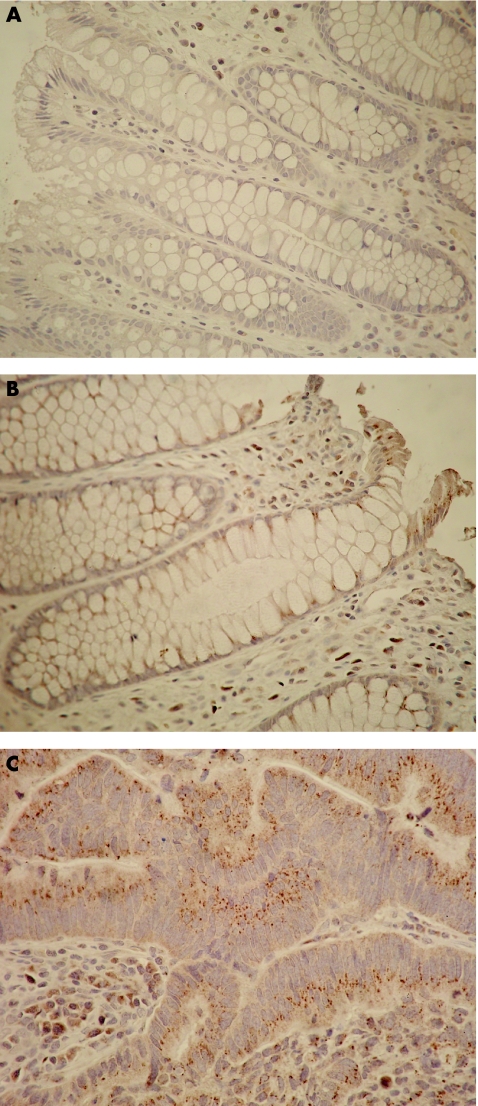
Bcl‐xL immunostaining was observed in the upper thirds, but not in the middle and lower thirds, of colonic crypts. Differences in immunostaining between groups were seen on histological examination of the upper thirds of crypts (fig 1). Labelling indices in crypts from “no‐cancer” control mucosa were not normally distributed (fig 2). Labelling indices for the upper thirds of crypts in mucosa, and collated results for adenocarcinoma, are shown in fig 2. Marked increases in Bcl‐xL labelling indices were detected in mucosa situated at 1 and 10 cm from the adenocarcinoma and in the adenocarcinoma, compared with mucosa from patients without cancer.
Figure 1 Immunostaining of formalin‐fixed paraffin‐wax‐embedded colorectal tissue with anti‐Bcl‐xL antibody. Punctate brown cytoplasmic staining is positive for Bcl‐xL. Blue staining is negative for Bcl‐xL. No immunostaining is observed in normal, non‐neoplastic mucosal crypts (A), but punctate cytoplasmic brown staining occurs in the upper third of a crypt 1 cm from adenocarcinoma (B). Abundant punctate brown immunostaining is observed in adenocarcinoma (C). All figures appear at ×400 magnification.
Figure 2 Labelling indices of colorectal tissue specimens with Bcl‐xL immunohistochemistry. Data points are plotted for each group with adjacent median values. Error bars signify the interquartile range. For normal non‐neoplastic mucosa, the median and interquartile range of labelling indices were 0.0%. In contrast, significant positive Bcl‐xL immunostaining occurs in neoplastic mucosa, as shown by the median values and error bars.
Table 2 shows Bcl‐xL labelling indices grouped according to tumour size and nodal status of the international TNM (tumour–node–metastases) system of cancer staging.8 There was a trend towards an increase in Bcl‐xL labelling index with greater tumour size or node involvement, which was significant for tumour node involvement in the mucosa situated 1 cm from the adenocarcinoma.
Table 2 Median Bcl‐xL labelling indices (%) of all colorectal tissue specimens grouped according to tumour size and nodal status of the international tumour–node–metastases system of cancer staging.
| T1/T2 (n) | T3/T4 (n) | N0 (n) | N1/N2 (n) | |
|---|---|---|---|---|
| Mucosa 10 cm from adenocarcinoma (n = 52, median LI %) | 2.97 (n = 9) | 22.7 (n = 43) | 19.0 (n = 31) | 22.7 (n = 21) |
| IQR (%) | 0.0–35.4 | 6.0–38.5 | 0.0–39.1 | 8.1–32.5 |
| Mucosa 1 cm from adenocarcinoma (n = 46, median LI %) | 31.0 (n = 8) | 32.6 (n = 38) | 22.4* (n = 29) | 36.3* (n = 17) |
| IQR (%) | 2.9–45.7 | 11.3–43.6 | 6.6–34.9 | 32.2–48.3 |
| Adenocarcinoma (n = 45, median LI %) | 35.8 (n = 8) | 53.2 (n = 37) | 50.3 (n = 27) | 63.0 (n = 18) |
| IQR (%) | 6.0–76.9 | 25.1–78.7 | 15.4–75.8 | 34.7–81.5 |
IQR, interquartile range; LI, labelling index; N, nodal status; T, tumour size.
*Mann–Whitney test, p = 0.02.
Five patients in the cancer group had received combined preoperative chemotherapy and radiotherapy, and one patient had received preoperative radiotherapy. All treatments had been completed 1 month before surgery. When results for these six patients were excluded, the significant differences observed in Bcl‐xL immunostaining between mucosa taken from patients with and without colorectal adenocarcinoma persisted (table 3).
Table 3 Labelling indices of colorectal tissue specimens using Bcl‐xL immunohistochemistry, after exclusion of samples from patients treated with chemotherapy and/or radiotherapy.
| Normal mucosa (n = 22) | Mucosa 10 cm from adenocarcinoma (n = 46) | Mucosa 1 cm from adenocarcinoma (n = 44) | Adenocarcinoma (n = 44) | |
|---|---|---|---|---|
| Median LI% (IQR) | 0.0*† (0.0–0.0) | 15.2* (0.0–32.3) | 31.0† (7.8–42.5) | 52.5 (22.7–79.1) |
*Comparison of two groups p<0.001; †Comparison of two groups p<0.001.
IQR, interquartile range; LI, labelling index.
Mann–Whitney test, *†p<0.001.
Discussion
A field change of increased cell proliferation occurs throughout the colonic and rectal mucosa in the presence of colorectal adenocarcinoma.4 It is not clear whether this precedes cancer development or occurs in response to an established neoplasm. Although it is known that both proliferation and apoptosis are disrupted during colorectal carcinogenesis,1 a field change in apoptosis has not been reported.
Previous studies9,10 examining apoptosis inhibition adjacent to colorectal carcinoma relied on the mucosa adjacent to the colorectal cancer for control samples. These studies have suggested that Bcl‐xL has a late role in the development of colorectal cancer. The concept of a field change of histological abnormalities surrounding a tumour implies,4 however, that mucosa adjacent to colorectal cancer is not normal.
We studied patients undergoing resection for non‐neoplastic conditions, and our control group comprised samples from patients without colorectal carcinomas or adenomas. Bcl‐xL expression occurred in a minority of samples from the non‐neoplastic colorectal mucosa, but was seen in most samples from the mucosa adjacent to colorectal carcinoma (fig 2). Changes in apoptosis could not be attributed to chemoradiation, as differences persisted when patients who had received such preoperative treatments were excluded. These findings suggest a field of Bcl‐xL expression in colonic mucosa extending to 10 cm from the site of colorectal adenocarcinoma. This was more marked with larger or lymph node‐positive tumours. It is not clear whether these changes occur adjacent to the colorectal adenocarcinoma, or extend throughout the colon and rectum.
The reproducibility of labelling indices estimated in our study could have been influenced by the number of crypts counted in each slide. Cell proliferation studies suggest little statistical advantage11,12 in estimating the labelling index by counting more than eight crypts per specimen, but similar data are not available for markers of apoptosis or its inhibition. The >15‐fold orders of magnitude labelling index difference identified in the present study, however, are larger than can be explained by reduced reproducibility. Similarly, it is possible that we could have identified labelling in normal mucosa if we had examined eight rather than two crypts. Identification of labelling in normal mucosa may have affected the range of labelling indices for normal mucosa, but is unlikely to have been of sufficient magnitude to affect the median or the significant differences observed.
Our results may also be limited by non‐specific staining. The staining protocol used, however, was optimised to minimise non‐specific staining. In addition, one positive and one negative control slide were included with every set of slides processed, to ensure clean immunostaining. It is unlikely that non‐specific staining can produce differences between groups of the order of magnitude found in our study.
The cause of the increased Bcl‐xL expression identified in the mucosa adjacent to colorectal cancer is unclear. One possible explanation is that upstream regulation of Bcl‐xL protein expression, a poorly understood consequence of cellular damage, is affected by the presence of colorectal cancer. In addition, increased Bcl‐xL expression can result from an early mutational step towards cancer development in a macroscopically normal but altered mucosa. High levels of Bcl‐xL mRNA expression have been reported in colorectal carcinomas and in tissue adjacent to colorectal carcinoma,10 but we are not aware of studies that compare differences in Bcl‐xL mRNA expression in patients with and without colorectal adenocarcinoma.
Acknowledgements
SB was a Stefan Galeski Research Fellow and was supported by the Joint Research Committee of Westminster Medical School Research Trust, Chelsea & Westminster Medical School Research Trust, and Chelsea & Westminster NHS Trust Charity, and by Colon Cancer Concern, London, UK. We thank Professor K Henry, Charing Cross Hospital, for the donation of positive control tissue. This study is part of research performed by SB for a Master of Surgery degree from the University of London.
Footnotes
Competing interests: None.
Ethical approval: This study was approved by the Riverside Ethics Committee, London.
References
- 1.Deschner E E.Kinetics of normal, preneoplastic and neoplastic colon epithelium. San Diego: Academic Press, 199041
- 2.Vaux D L, Cory S, Adams J M. Bcl‐2 gene promotes haemopoietic cell survival and cooperates with c‐Myc to immortalize pre‐B cells. Nature 1988335440–442. [DOI] [PubMed] [Google Scholar]
- 3.Boise L H, Gonzalez‐Garzia M, Postema C E.et al Bcl‐x, a Bcl‐2‐related gene that functions as a dominant regulator of apoptotic cell death. Cell 199374597–608. [DOI] [PubMed] [Google Scholar]
- 4.Terpstra O T, Van Blankenstein M, Dees J.et al Abnormal pattern of cell proliferation in the entire colonic mucosa of patients with colon adenoma or cancer. Gastroenterology 198792704–708. [DOI] [PubMed] [Google Scholar]
- 5.Ponz de Leon M, Roncucci L, DiDonato P.et al Pattern of epithelial cell proliferation in colorectal mucosa of normal subjects and of patients with adenomatous polyps or cancer of the large bowel. Cancer Res 1988484121–4126. [PubMed] [Google Scholar]
- 6.Wilson R G, Smith A N, Bird C C. Immunohistochemical detection of abnormal cell proliferation in colonic mucosa of subjects with polyps. J Clin Pathol 199043744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potten C S, Kellett M, Rew D.et al Proliferation in human gastrointestinal epithelium using bromodeoxyuridine in vivo; data for different sites, proximity to a tumour, and polyposis coli. Gut 199233524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Joint Committee on Cancer Colon and rectum. AJCC cancer staging manual. 6th edn. New York: Springer, 2002113–124.
- 9.Backus H H J, Van Groeningen C J, Vos W.et al Differential expression of cell cycle and apoptosis related proteins in colorectal mucosa, primary colon tumours and liver metastases. J Clin Pathol 200255206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maurer C A, Friess H, Buhler S S.et al Apoptosis inhibiting factor Bcl‐xL might be the crucial member of the Bcl‐2 gene family in colorectal cancer. Dig Dis Sci 1998432641–2648. [DOI] [PubMed] [Google Scholar]
- 11.Lyles C M, Sandler R S, Keku T O.et al Reproducibility and variability of the rectal mucosal proliferation index using proliferating cell nuclear antigen immunohistochemistry. Cancer Epidemiol Biomarkers Prev 19943597–605. [PubMed] [Google Scholar]
- 12.Mills S J, Mathers J C, Chapman P D.et al Colonic crypt cell proliferation state assessed by whole crypt microdissection in sporadic neoplasia and familial adenomatous polyposis. Gut 20014841–46. [DOI] [PMC free article] [PubMed] [Google Scholar]