Abstract
Background
Minimal change disease (MCD) and diffuse mesangial proliferation (DMP) are the most common pathomorphological forms of nephrotic syndrome glomerulopathies in children. The clinical course of DMP can be characterised by either DMP‐sensitivity (DMP‐S) or DMP‐resistance (DMP‐R) to steroids, resulting in an unfavourable course of the glomerulopathy. Although the clinical processes of DMP‐S and DMP‐R are initially identical, resistance to steroids may be foreseen by the immunohistochemical expression of cytoskeleton‐associated proteins in podocytes.
Aims
To estimate the immunohistochemical expression of ezrin in children with MCD, DMP and focal segmental glomerulosclerosis (FSGS) and to evaluate its usefulness in predicting resistance to steroids.
Materials and methods
Renal biopsy specimens of patients with MCD (n = 15), DMP (n = 16) and FSGS (n = 6) were taken. The control tissue consisted of normal‐appearing cortex taken from kidneys resected for localised neoplasms (n = 6). The indirect immunohistochemical protocol for the use of a monoclonal antibody directed against ezrin was used.
Results
The immunohistochemical expression of ezrin in cases progressively reduced from MCD to DMP‐S to DMP‐R to FSGS. Except for DMP‐R and FSGS (p>0.05), the difference in ezrin expression in podocytes was significant.
Conclusion
Ezrin can be a potent marker of podocyte injury (podocytopathy) and may help in the histological qualification of MCD, DMP and FSGS. The increased permeability of the filtration barrier in steroid‐resistant and proteinuric glomerulopathies may be a consequence of subcellular changes in podocyte‐associated proteins following decreased expression of ezrin.
Proteinuria is the main clinical symptom of idiopathic nephrotic syndrome (INS) glomerulopathies in children,1,2 and is caused by impaired glomerular permeability after immune or non‐immune inflammatory processes affecting the glomeruli.
The most frequent pathomorphological forms of INS are minimal change disease (MCD), mesangial proliferative glomerulonephritis and focal segmental glomerulosclerosis (FSGS).3 However, not every case of mesangial cell proliferation can be regarded as a mesangial proliferative glomerulonephritis. The condition in which an increase in the number of mesangial cells is not followed by evidence of glomerulonephritis is called diffuse mesangial proliferation (DMP) and its prognostic relevance in patients with INS is not well established.4 DMP constitutes up to 10% of glomerulopathies, but our clinical observation indicates that the course of DMP in about 30% of those patients reflects the FSGS process.
The benignity of INS, which is usually observed in children, may be complicated in some patients by a resistance to steroids, with constant proteinuria (this can occur in patients with DMP, but mainly in those with FSGS). Clinical severity in INS increases progressively from MCD to DMP and FSGS.5,6 Moreover, constant and extensive proteinuria is due to the progression of impaired renal filtration and follows both qualitative and quantitative changes of the podocytes.
Podocytes have essential roles in the formation and maintenance of the glomerular filtration barrier of the kidney.7 They contribute considerably to the filtering properties of hydraulic and molecular permeability. Podocytes on the glomerular basement membrane are the terminal elements in the filtration barrier and exhibit an elaborate morphology with large arborisation of cell processes, which are based on a well‐developed cytoskeleton.8
One of the most specific and distinguishing features of podocytes is the presence of cytoskeletal proteins (intermediate filaments),9 which regulate organelle distribution or cell motion and contraction.10 The structural integrity of podocytes depends on cytoskeleton‐associated proteins, particularly podocalyxin, synaptopodin and ezrin.11,12,13 Podocalyxin is believed to be the most sensitive marker of podocytes. It forms and maintains the structure and function of podocyte foot processes, partly by virtue of its negative charge.11 At these sites, podocalyxin may be responsible for maintaining the distance between the foot processes of neighbouring podocytes and the endothelium. In addition to podocytes, podocalyxin is also expressed in extra‐glomerular endothelial cells as a coexpression with CD34.14 Because of its connection to ezrin, through sodium or hydrogen‐exchanger regulator factor 2, podocalyxin may serve as an excellent reference of localisation of these peptides in podocytes.15
Ezrin belongs to a family of plasma membrane cytoskeleton‐linking proteins. It interacts with the actin cytoskeleton and the plasma membrane at specific cellular localisations and is associated with signal transduction16 and growth control.17 Moreover, ezrin is known as a marker for activated or damaged podocytes in experimental models.18
In line with the above known facts, our aim was to analyse the immunohistochemical expression of ezrin in children with glomerulopathies and to evaluate the potent usefulness of this marker in the prediction of an unfavourable course of nephrotic syndrome.
Patients and methods
Biopsy specimens
In all, 37 renal biopsies, performed at the time and in the manner recommended by the Report of the International Study of Kidney Disease in Children,19 were obtained from the Department of Pediatric Nephrology, Poznan University of Medical Sciences, Poznan, Poland. The research protocol was approved by the local ethics committee of the university and fulfils the standards recommended by the Helsinki Convention. The diagnosis of MCD, DMP and FSGS was established according to the World Health Organization definitions.20 The study group consisted of 15 patients with MCD and 16 patients with DMP, including 8 steroid‐sensitive patients, 8 steroid‐resistant patients and 6 patients with FSGS. The control tissue (n = 6) consisted of macroscopically normal‐appearing cortex taken from kidneys resected for localised neoplasms. Table 1 summarises the clinical parameters of all the patients in the study group, at the time of the renal biopsy.
Table 1 Clinical parameters of patients with glomerulopathies at the time of renal biopsy.
| Histological diagnosis | Median age (years) | No of patients (n) with | |||||
|---|---|---|---|---|---|---|---|
| Male sex | Proteinuria >50 mg/kg/day | Proteinuria 0–50 mg/kg/day | Erythrocyturia* | Increased serum creatinine concentration | Steroid resistance | ||
| MCD (n = 15) | 15 | 9 | 7 | 8 | 2 | 2 | 0 |
| DMP (n = 16) | 14 | 9 | 8 | 8 | 10 | 2 | 8 |
| FSGS (n = 6) | 13.5 | 3 | 1 | 5 | 6 | 6 | 6 |
DMP, diffuse mesangial proliferation; FSGS, focal segmental glomerulosclerosis; MCD, minimal change disease.
*More than five erythrocytes per high‐power field.
Immunohistochemistry
The biopsy material for immunohistochemical analysis was fixed in Bouin's solution for 24 h, washed, dehydrated and subsequently embedded in paraffin wax. For bright field microscopy, sections of 6 µm thickness were dewaxed and processed according to the indirect avidin–biotin or peroxidase technique21 using a polyclonal rabbit anti‐podocalyxin, C‐terminus, antibody 0601, dilution 1:2000, a kind gift from Dr MG Farquhar (San Diego, California, USA), and ezrin (1:200, clone 18, Transduction Labs, Lexington, Kentucky, USA), respectively. The reaction with endogenous peroxidases was abolished by pretreatment of the sections with 0.03% hydrogen peroxide in phosphate‐buffered saline before incubation with the antibodies. Binding of the antibodies required microwave pretreatment of the slides (800 W, citrate buffer, 10 min). After incubation with the primary antibodies for 24 h at 4°C, the sections were incubated for 1 h at room temperature with biotin‐conjugated goat anti‐mouse immunoglobulin (Ig)G or goat anti‐rabbit IgG (diluted 1:300, Sigma, Munich, Germany). Subsequently, the sections were incubated with an avidin–biotin or peroxidase reagent (Vectastain Elite; Vector, California, USA) for 45 min at room temperature. The activity of peroxidase was visualised using 0.5% 3,3′‐diaminobenzidine in tris (hydroxymethyl) amino‐methane or hydrochloric acid (pH 7.6) containing 0.3% hydrogen peroxide.
Controls
For all the samples, the primary antibodies were replaced by a non‐immune serum (normal mouse or rabbit serum or phosphate‐buffered saline). No reaction products were observed in the normal serum control incubations.
For double immunofluorescence with ezrin and podocalyxin, the sections were dewaxed and incubated with anti‐podocalyxin (1:400) at 4°C overnight, followed by incubation with Alexa Flour 488 goat anti‐rabbit secondary antibody (1:200) at 37°C for 30 min. Ezrin antibody (1:20) was applied at 4°C overnight, followed by incubation with Texas Red goat anti‐rabbit secondary antibody at 37°C for 30 min. The slides were then washed, mounted in buffered glycerin‐gelatine and observed with an Olympus BX60 microscope (Olympus, Tokyo, Japan). Pictures were taken with a CCD camera connected to Analysis soft‐imaging system (Münster, Germany).
Quantitative analysis
Owing to the scarce material of the clinical biopsy specimens, we were unable to assess complete serial sections of individual glomeruli. Instead, we measured the area of immunoreactive cells and determined their value as a percentage of the whole glomerular area, of at least five glomeruli in four subsequent sections. Changes of antigen distribution in renal glomeruli were evaluated using Micro Image V.4.0 software (Olympus, MS Windows 98) and the area showing marker expression on each colour image was measured using an “eyedropper” tool for each group of patients. The estimates of ezrin expression were mathematically analysed to examine changes of this marker in the various glomerulopathies and the differences between groups were evaluated by one‐way analysis of variance test. To determine the significance between group means in the analysis of variance, the nearest significant difference test (Tukey test) was used as the multiple comparison test. Significance was set at p<0.05.22
Results
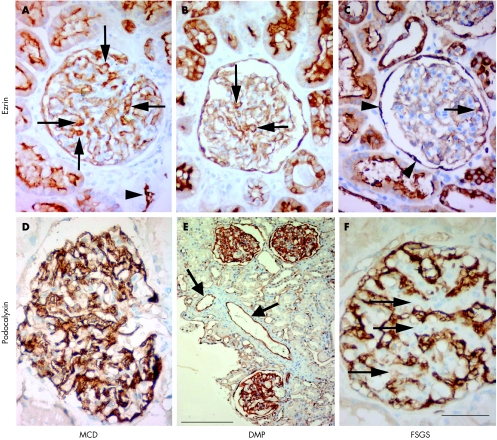
Immunohistochemical expression of ezrin was observed in the cytoplasm of the renal podocytes and in the apical areas of epithelial cells of the proximal tubuli (fig 1A–C). The immunohistochemical expression of podocalyxin in podocytes decreased progressively from MCD to FSGS (fig 1D–F).
Figure 1 Light microscopy of renal glomeruli in children with immunohistochemical reactions for ezrin (A–C) and podocalyxin (D–F). Scale bar: 50 μm (A–D,F), 200 µm (E). (A) Immunohistochemical expression of ezrin in podocytes (arrows), parietal glomerular epithelial cells (Bowman's capsule) and on the apical surface of tubular epithelial cells (arrowhead) in a10‐year‐old child with minimal change disease (MCD). (B) Renal glomerulus of a 9‐year‐old child with diffuse mesangial proliferation (DMP). Ezrin immunoreactivity in podocytes (arrows) and tubular epithelial cells. Note the decreased number of ezrin‐positive podocytes and the lower staining intensity compared to A. (C) Glomerulus of an 11‐year‐old child with focal segmental glomerulosclerosis (FSGS) with only individual ezrin‐positive podocytes (arrow). Parietal glomerular epithelial cells (arrowheads) and tubular epithelial cells show positive reactions for ezrin. (D) Intensive immunohistochemical reaction for podocalyxin in the glomeruli (10‐year‐old child with MCD). (E) Renal cortex of an 11‐year‐old child with DMP and steroid‐resistant idiopathic nephrotic syndrome. Some podocytes with a positive reaction for podocalyxin. The arrows show extra‐glomerular endothelial cells with a positive reaction for podocalyxin. (F) Renal glomerulus of a 9‐year‐old child with FSGS. There is no reaction for podocalyxin in areas of sclerosis (arrows).
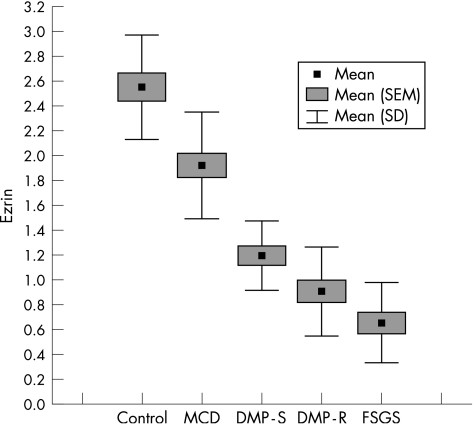
We also observed that the expression of ezrin decreased progressively from MCD to DMP to FSGS (fig 2). The distribution of ezrin in the extra‐glomeruli structures (tubular epithelial cells) did not change in the specimens and served as a specific positive control for the incubations that were carried out (fig 1B). In the cases with glomerulosclerosis, we observed only individual ezrin‐positive podocytes, which were mainly localised at the periphery of the glomeruli.
Figure 2 Box plot distribution of data for ezrin expression by the percentage of glomerular area in the control and study groups. DMP‐R, diffuse mesengial proliferation‐resistant; DMP‐S, diffuse mesengial proliferation‐sensitivity; FSGS, focal segmental glomerulosclerosis; MCD, minimal change disease.
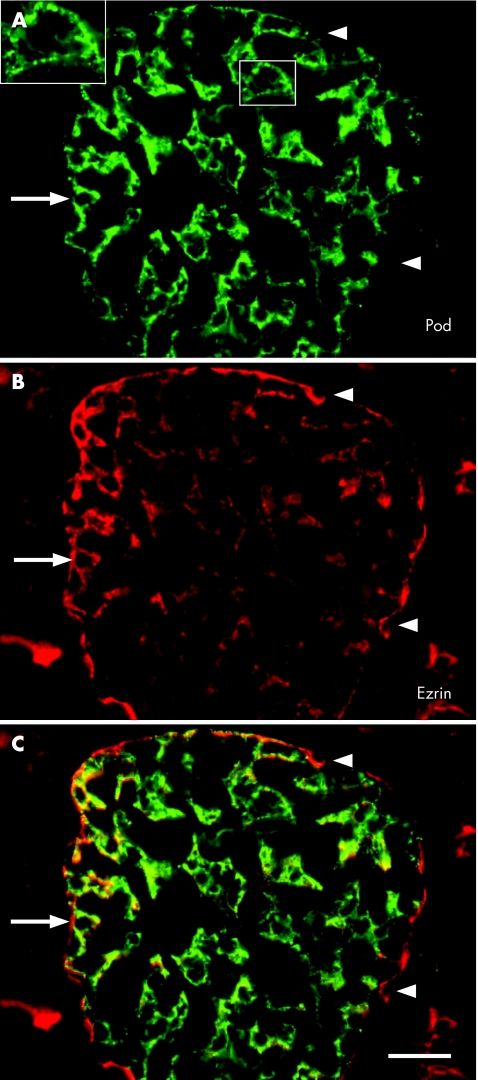
In the double immunofluorescence assays, we observed the coexpression of ezrin and podocalyxin in renal podocytes (fig 3A–C). Significant differences were observed among all groups in the expression of ezrin in podocytes (p<0.04; fig 2; table 2), except for that between DMP‐R and FSGS (p>0.05).
Figure 3 (A–C) Podocalyxin (pod) and ezrin in a renal glomerulus of a 10‐year‐old child presenting with diffuse mesengial proliferation. The large inset (magnified from the small inset) shows the granular pattern of podocalyxin distribution. Note the largely overlapping immunofluorescence of both antibodies in podocytes (arrow; yellow in C). In addition, ezrin is located on the apical cell membranes (probably microvilli) of proximal tubuli and in parietal glomerular cells (Bowman's capsule; arrowhead in (B)). (C) Scale bar 20 µm.
Table 2 Significance of the differences between the control and study groups in the percentage of glomerular area with immunohistochemical expression of ezrin and podocalyxin, estimated by one‐way analysis of variance and Tukey tests.
| Ezrin expression | Control | MCD | DMP‐S | DMP‐R | FSGS |
|---|---|---|---|---|---|
| Control | — | 0.001 | 0.001 | 0.001 | 0.001 |
| MCD | 0.001 | — | 0.001 | 0.001 | 0.001 |
| DMP‐S | 0.001 | 0.001 | — | 0.037 | 0.001 |
| DMP‐R | 0.001 | 0.001 | 0.037 | — | 0.068 |
| FSGS | 0.001 | 0.001 | 0.001 | 0.068 | — |
DMP‐R, diffuse mesengial proliferation‐resistant; DMP‐S, diffuse mesengial proliferation‐sensitivity; FSGS, focal segmental glomerulosclerosis; MCD, minimal change disease; —, absence of the trait in the given group.
Discussion
The discrepancies in the clinical course of DMP in children who were either steroid‐sensitive or steroid‐resistant and had constant proteinuria prompted us to investigate the podocyte cytoskeleton‐associated proteins responsible for the shape and function of the renal filtration barrier.
Pathological podocytes in proteinuric glomerular disease are ultrastructurally characterised by vacuolisation, loss of slit diaphragms, effacement of foot processes and, occasionally, detachment from the glomerular basement membrane.23 Moreover, podocyte injury is believed to trigger the formation of extracapillary lesions as crescents, and precedes sclerosis in virtually all models of diseases of the glomerular filtering barrier.24 Therefore, estimation of podocyte injury is of clinical importance, especially as there are only a few reports investigating podocyte injury in glomerulopathies using immunohistochemical methods.25,26,27
The major finding in our study was the decreased immunohistochemical expression of ezrin in glomeruli in patients with steroid‐resistant INS. The immunohistochemical distribution of this marker, parallel to the increasing clinical severity in INS from MCD to DMP to FSGS, signals a worse response to steroid treatment. This finding suggests, firstly, that severe injury to podocytes leads to their impaired support and function of the filter barrier and matches our earlier observation of the re‐expression of cytokeratin 18 in podocytes in steroid‐resistant nephrotic syndrome with glomerular immaturity (children up to the age of 2 years).28 Secondly, reduced distribution of synaptopodin and podocalyxin in primary glomerulopathies is associated with an unfavourable clinical course.29 The reduced expression of ezrin in podocytes in patients with steroid‐resistant DMP and FSGS showed undoubted podocytopathy and may reflect the subcellular rearrangement as well as the loss of podocytes seen during the progression of nephrotic syndrome glomerulopathies in children.30 Moreover, the absence of a statistical difference between DMP‐R and FSGS suggests advanced pathological changes in the podocytes in DMP that is running a steroid‐resistant clinical course.
Additionally, in the immunofluorescence part of the study, we carried out double incubations of ezrin and podocalyxin (a well‐known podocyte marker) in renal glomeruli, which showed the colocalisation of these two proteins in podocytes. The changed immunohistochemical expression of podocalyxin in nephrotic syndrome glomerulopathies can partly show the subcellular re‐arrangement of podocytes, as described in our previous study.29
It must be emphasised that the expression of ezrin was also observed in tubular epithelial cells. However, we observed the same pattern of ezrin stained in the epithelial cells of tubuli in all the glomerulopathies studied and in the control samples, which indicates normal subcellular structures of this part of the nephron. It suggests that, despite the clear contribution that ezrin makes to the stability or regulation of several tubular membrane transporters, it does not immunohistochemically respond to heavy proteinuria in this part of the nephron. Kuusniemi et al31 indicated that tubular epithelial cells were resistant to heavy proteinuria (in congenital nephrotic syndrome) and did not show epithelial–mesenchymal transition. It also confirms other observations from animal studies where the usefulness of ezrin as an indicator of injury progression was proved.18 It was, however, difficult to explain the different intensity of immunohistochemical expression of ezrin in various experimental glomerular diseases.18 Moreover, the possibility of ezrin application in monitoring podocytopathy is still controversial in clinical practice because of an inadequate number of renal re‐biopsies (International Study of Kidney Disease in Children criteria).19,20 Hence, these results need further investigation in a greater number of INS cases.
Conclusions
Ezrin seems to be a potent marker of podocyte injury (podocytopathy) and may help in the histological differentiation of minimal change disease, diffuse mesangial proliferation and glomerulosclerosis.
The increased permeability of the filtration barrier in steroid‐resistant and proteinuric glomerulopathies may be a consequence of subcellular changes in podocyte‐associated proteins after decreased expression of ezrin.
Acknowledgements
We thank Dr MG Farquhar, San Diego, California, USA, for the generous donation of the podocalyxin antibody, Mrs S Bramke and Mrs A Neisser, University of Technology Dresden, Germany, for their skilful technical work and Professor Geoffrey Show for his language editing. The research was supported by the Polish State Committee for Scientific Research–grant 2 PO5E 07130.
Abbreviations
DMP - diffuse mesangial proliferation
DMP‐R - diffuse mesangial proliferation‐resistance
DMP‐S - diffuse mesangial proliferation‐sensitivity
FSGS - focal segmental glomerulosclerosis
INS - idiopathic nephrotic syndrome
MCD - minimal change disease
Footnotes
Competing interests: None.
References
- 1.Churg J, Habib R, White R H R. Pathology of the nephrotic syndrome in children. Lancet 19707601299–1302. [DOI] [PubMed] [Google Scholar]
- 2.Glassock R. The nephrotic syndrome. Hosp Pract 197914105–9, 11518, 1239. [DOI] [PubMed] [Google Scholar]
- 3.White R H R, Glasgow E F, Mills R J. Clinicopathological study of nephrotic syndrome in childhood. Lancet 197011353–1358. [DOI] [PubMed] [Google Scholar]
- 4.Waldherr R, Gubler M C, Levy M.et al The significance of pure diffuse mesangial proliferation in idiopathic nephrotic syndrome. Clin Nephrol 197810171–179. [PubMed] [Google Scholar]
- 5.Brown E. The nephrotic syndrome. Postgrad Med J 1985611057–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abeyagunawardena A, Brogan P A, Trompeter R S. Immunosuppressive therapy of childhood idiopathic nephrotic syndrome. Expert Opin Pharmacother 20023513–519. [DOI] [PubMed] [Google Scholar]
- 7.Daniels B S. The role of glomerular epithelial cells in the maintenance of the glomerular filtration barrier. Am J Nephrol 199313318–323. [DOI] [PubMed] [Google Scholar]
- 8.Vasmant D, Maurice M, Feldmann G. Cytoskeleton ultrastructure of podocytes and glomerular endothelial cells in man and in the rat. Anat Rec 198421017–24. [DOI] [PubMed] [Google Scholar]
- 9.Drenckhahn D, Franke R P. Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Invest 198859673–682. [PubMed] [Google Scholar]
- 10.Moll R, Franke W, Shiller D.et al The catalog of human cytokeratin polypeptides: patterns of expression of specific cytokeratins in normal epithelia, tumors and cultured cells. Cell 19823111–24. [DOI] [PubMed] [Google Scholar]
- 11.Kerjaschki D, Sharkey D J, Farquhar M G. Identification and characterization of podocalyxin‐the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol 1984981591–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mundel P, Heid H W, Mundel T M.et al Synaptopodin: An actin associated protein in telencephalic dendrites and renal podocytes. J Cell Biol 1997139193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tekada T. Podocyte cytoskeleton is connected to the integral membrane protein podocalyxin through Na+/H+‐exchanger regulatory factor 2 and ezrin. Clin Exp Nephrol 20037260–269. [DOI] [PubMed] [Google Scholar]
- 14.Horvat R, Hovorka A, Dekan G.et al Endothelial cell membranes contain podocalyxin: the major sialoprotein of visceral glomerular epithelial cells. J Cell Biol 1986102486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orlando R, Takeda T, Zak B.et al The glomerular epithelial cell anti‐adhesin, podocalyxin, associates with the actin cytoskeleton through interactions with ezrin. J Am Soc Nephrol 2001121589–1598. [DOI] [PubMed] [Google Scholar]
- 16.Turunen O, Wahlstrom T, Vaheri A. Ezrin has a COOH‐terminal actin‐binding site that is conserved in the ezrin protein family. J Cell Biol 19941261445–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arpin M, Algrain M, Louvard D. Membrane‐actin microfilament connections: an increasing diversity of players related to band 4.1. Curr Opin Cell Biol 1994l6136–141. [DOI] [PubMed] [Google Scholar]
- 18.Hugo C, Nangaku M, Shankland S J.et al The plasma membrane‐actin linking protein, ezrin, is a glomerular epithelial cell marker in glomerulogenesis, in the adult kidney and in glomerular injury. Kidney Int 1998541934–1944. [DOI] [PubMed] [Google Scholar]
- 19.Report of the International Study of Kidney Disease in Children The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. J Pediatr 198198561–564. [DOI] [PubMed] [Google Scholar]
- 20.Gulati S, Sharma A, Sharma R.et al Do current recommendations for kidney biopsy in nephrotic syndrome need modifications? Pediatr Nephrol 200217404–408. [DOI] [PubMed] [Google Scholar]
- 21.Hsu S, Raine L, Fanger H. Use of avidin‐biotin peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 198129577–580. [DOI] [PubMed] [Google Scholar]
- 22.Armitage P.Statistical methods in medical investigations [in Polish]. 1st edn. Warszawa: PZWL, 197897–103.
- 23.Andrews P. Morphological alterations of the glomelular (visceral) epithelium in response to pathological and experimental situations. J Electron Microsc Tech 19889115–144. [DOI] [PubMed] [Google Scholar]
- 24.Kriz W, Lemley K V. The role of the podocyte in glomerulosclerosis. Curr Opin Nephrol Hypertens 19998489–497. [DOI] [PubMed] [Google Scholar]
- 25.Koop K, Eikmans M, Baelde H.et al Expression of podocyte‐associated molecules in acquired human kidney diseases. J Am Soc Nephrol 2003142063–2071. [DOI] [PubMed] [Google Scholar]
- 26.Horinouchi I, Nakazato H, Kawano T.et al In situ evaluation of podocin in normal and glomerular diseases. Kidney Int 2003642092–2099. [DOI] [PubMed] [Google Scholar]
- 27.Srivastava T, Garola R E, Whiting J M.et al Synaptopodin expression in idiopathic nephrotic syndrome of childhood. Kidney Int 200159118–125. [DOI] [PubMed] [Google Scholar]
- 28.Ostalska‐Nowicka D, Zachwieja J, Nowicki M.et al Expression of intermediate filaments of podocytes within nephrotic syndrome glomerulopathies in children. Histochem Cell Biol 2004121109–113. [DOI] [PubMed] [Google Scholar]
- 29.Ostalska‐Nowicka D, Zachwieja J, Nowicki M.et al Vascular endothelial growth factor (VEGF‐C1)‐dependent inflammatory response of podocytes in nephrotic syndrome glomerulopathies in children: an imunohistochemical approach. Histopathology 200546176–183. [DOI] [PubMed] [Google Scholar]
- 30.Mundel P, Shankland S. Podocyte biology and response to injury. J Am Soc Nephrol 2002133005–3015. [DOI] [PubMed] [Google Scholar]
- 31.Kuusniemi A M, Lapatto R, Holmberg C. Kidneys with heavy proteinuria show fibrosis, inflammation, and oxidative stress, but no tubular phenotyping change. Kidney Int 200568121–132. [DOI] [PubMed] [Google Scholar]