Abstract
Background
Fascin, an actin‐binding protein, is usually expressed at a low level in normal epithelium, but is markedly up regulated in several types of carcinomas. Reports on fascin expression in oesophageal squamous cell carcinoma (ESCC) and precancerous lesions remain rare.
Aim
To show the roles of fascin in the progression from normal epithelium to invasive ESCC.
Methods
Fascin expression in 102 sections embedded in paraffin wax, including samples of normal mucosa (n = 20), dysplasia (n = 10), ESCC (n = 62) and special sections (n = 10) of a full‐length mucosa layer from the distant margin to the cancer focus of the excised oesophagus, and 49 fresh specimens of ESCC was analysed by immunohistochemistry, western blot and real‐time reverse transcription‐polymerase chain reaction. Fascin expression in ESCC cell lines was also investigated.
Results
In the immunohistochemical study, the positive rate of fascin was significantly higher in the tumour tissue than in the normal epithelium (p = 0.020), but no significant difference was shown between ESCC and dysplasia (p = 1.000). Immunostaining for fascin was only apparent in the basal layer of the normal epithelium. However, in the dysplasia, positive staining was observed in most of the heterogeneous cells from the basal layer to the granular layer of the epithelium. Fascin expression was seen to increase progressively from the normal epithelium to invasive ESCC. Up regulation of fascin was observed in 87.76% (43/49) and 77.55% (38/49) of the specimens, respectively, using western blot and real‐time reverse transcription‐polymerase chain reaction assays; 80% (4/5) of ESCC cell lines also expressed fascin at a high level. Furthermore, overexpression of fascin was markedly correlated with cell proliferation and lymph node metastasis.
Conclusions
These findings suggested that fascin was associated with the transformation and development of ESCC and implicated the potential of fascin as a novel biomarker that would allow the tumour to be identified at an early stage in high‐risk individuals.
Oesophageal squamous cell carcinoma (ESCC) is the fourth most common malignancy in China, with relatively high mortality. It is hard to diagnose ESCC at an early stage of disease development and advanced ESCC frequently exhibits local invasion and lymph node metastasis,1 which is one of the important reasons for its poor prognosis. So the identification of people at high risk of developing ESCC, combined with the appropriate screening tests, offers the best chance of reducing the morbidity and mortality of this disease.2 Our previous work has identified that fascin, an actin‐binding protein associated with cell motility, is markedly up regulated in the progression of immortalised human oesophageal epithelial cell line (SHEE) to malignantly transformed oesophageal cell line (SHEEmt).3,4 This suggested that fascin may be associated with the carcinogenesis of ESCC.
Fascin was originally found in the extracts of unfertilised sea urchin eggs as an actin‐binding protein.5 The expression of fascin was greatly increased in many transformed cells, as well as in specialised normal cells including neuronal cells and antigen‐presenting dendritic cells.6 Recently, overexpression of fascin was reported as being markedly associated with lymph‐node metastasis or poor prognosis in several human epithelial cancers such as colonic, gastric, breast, skin and urothelial carcinomas.7,8,9,10,11 Hashimoto et al12 also reported that the immunostaining intensity of fascin was usually increased in ESCC compared with that in normal epithelium and overexpression of fascin was markedly associated with a poor prognosis. Although this was an important finding, the role of fascin expression in the progression of normal epithelium to invasive ESCC was still unknown.
On the basis of our previous study of fascin in oesophageal epithelial cell lines, we continued our work in an attempt to show the role of fascin in the transformation and development of ESCC. Besides investigating the normal epithelium and invasive cancer of the oesophagus, we also investigated fascin expression in a sequence of histopathological lesions that appeared during the progression from normal epithelium to invasive ESCC, including chronic oesophagitis, mild to severe dysplasia and carcinoma in situ. In addition, fascin expression and its correlation with cell proliferation and clinicopathological features in ESCC were also examined.
Materials and methods
Patients and surgical specimens
A total of 102 sections were embedded in paraffin wax: normal mucosa (n = 20), dysplasia (n = 10), ESCC (n = 62) and special sections (n = 10). They were taken from a full‐length mucosa layer from the distant margin to the cancer focus of the excised oesophagus, collected from 2001 to 2003, and were acquired from the Clinicopathologic Department of the First Affiliated Hospital of Shantou University, Shantou, China, and the Pathology Department of the Medical College of Shantou University. The sections of normal mucosa and dysplasia, which were histologically evaluated by haematoxylin and eosin staining, were taken from the matched distal resected margin of ESCC samples. Frozen tumour tissues and corresponding normal tissues were obtained for western blot and real‐time reverse transcription‐polymerase chain reaction (RT‐PCR) from 49 patients with primary ESCC who underwent surgery in the same hospital from 2003 to 2004. All the patients had not received radiotherapy or chemotherapy before surgery. Information on sex, age, stage of disease and histopathological factors were retrieved from the medical record (table 1). All the tumours were confirmed as ESCCs by the Clinicopathologic Department of the hospital. All cases were classified according to the fifth edition of the tumour‐node‐metastasis classification of the International Union against Cancer. Evaluation of tumour differentiation was based on histological criteria of the guidelines of the World Health Organization Pathological Classification of Tumours.
Table 1 Clinicopathological features of patients with oesophageal squamous cell carcinoma.
| Parameters | IHC (n = 62) | WB and PCR (n = 49) |
|---|---|---|
| Mean age in years (range) | 57 (40–75) | 58 (37–74) |
| <57 (32) | <58 (23) | |
| >57 (30) | >58 (26) | |
| Sex | ||
| Male | 50 | 40 |
| Female | 12 | 9 |
| Primary tumour | ||
| T1/T2 | 5 | 9 |
| T3/T4 | 57 | 40 |
| Regional lymph node | ||
| N0 | 37 | 22 |
| N1 | 25 | 27 |
| Distant metastasis | ||
| M0 | 60 | 47 |
| M1 | 2 | 2 |
| Stage | ||
| I/IIA/IIB | 38 | 19 |
| III/IV | 24 | 30 |
| Histopathology | ||
| Well differentiated (G1) | 21 | 13 |
| Moderately differentiated (G2) | 21 | 31 |
| Poorly differentiated (G3) | 20 | 5 |
IHC, immunohistochemistry; PCR, polymerase chain reaction; WB, western blot.
Specimens were fixed in 10% formaldehyde solution and embedded in blocks of paraffin wax, then cut into 4‐μm‐thick sections. A small portion from each resected tissue sample was immediately frozen in liquid nitrogen and stored at −70°C. The study was approved by the ethics committee of the First Affiliated Hospital of Shantou University and written informed consent to use resected samples for research was obtained from all patients undergoing surgery.
Immunohistochemical staining
Immunohistochemical staining was carried out using the streptavidin–peroxidase‐conjugated method. Briefly, each tissue section was deparaffinised in dimethylbenzene, rehydrated through a graded ethanol series, and then incubated with fresh 3% hydrogen peroxide for 10 min. After rinsing with phosphate‐buffered saline (PBS), antigen retrieval from the tissue was carried out by autoclaving in 0.01 M citrate buffer (pH 6.0) at 120°C for 3 min. Next, sections were incubated with 10% normal goat serum in PBS for 15 min at room temperature to block non‐specific binding. After rinsing with PBS, slides were incubated overnight at 4°C with mouse antihuman fascin monoclonal antibody, clone 55k‐2 (1:800 dilution in PBS containing 0.01% Triton X‐100, Dako Biot Co, Glostrup, Denmark). After rinsing with PBS, tissue sections were incubated for 15 min at room temperature with a polymer helper solution (Polymer Detection System Kit, GBI Biot Co, USA), then rinsed again with PBS. Slides were then incubated for 20 min at room temperature with streptavidin–peroxidase‐conjugated antimouse or antirabbit immunoglobulin G (IgG; Polymer Detection System Kit, GBI Biot Co). Subsequently, they were stained with 0.003% 3,3‐diaminobenzide tetrahydrochloride and 0.005% hydrogen peroxide in 0.05 M TRIS–hydrochloric acid (HCl; pH 7.2), counterstained with Mayer's haematoxylin, dehydrated and mounted.
Negative controls were prepared by substituting PBS for primary antibody. A metastatic breast carcinoma, shown previously to have immunoreactivity, was used as a positive control to confirm the immunoreactivity in each series of experiments.
Fascin‐positive samples were defined as those showing brown signals in the cytoplasm. When >5% of the cells in a given specimen were positively stained, we defined it as positive. All sections were evaluated independently by two investigators without any prior knowledge of the patients' clinical information. When the opinions of the two evaluators were different, agreement was reached by careful discussion.
Cell cultures
Human ESCC cell lines EC109, EC18, EC171, EC8712 and SHEEC were cultured in 199 medium (GIBCO, Grand Island, New York, USA) supplemented with 10% heat‐inactivated bovine serum. Cells were cultured in flasks in a humidified atmosphere of 5% CO2 at 37°C.
Western blot analysis
Tissues or cells were lysed in a sample buffer (50 mM TRIS–HCl, pH 8.0, 150 mM sodium chloride, 1% Triton X‐100, 100 μg/ml phenylmethylsulphonyl fluoride) on ice for 30 min. The lysates were then centrifuged for 5 min (12 000 rpm, 4°C). The protein concentration was estimated by the Bradford method. Equal amounts of tissue or cell lysates (50 μg) were electrophoresed on 10% polyacrylamide gel and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, Massachusetts, USA). The membranes were then blocked with 5% skim milk—phosphate‐buffered saline Tween (0.01 M PBS (pH 7.4), 0.05% Tween 20) for 1 h and incubated at room temperature for 1 h with the primary antibody as described above. The membrane was subsequently incubated at room temperature for 1 h with horseradish peroxidase‐linked goat antimouse IgG and analysed using Western Blotting Luminol Reagent (Santa Cruz Biotechnology, San Diego, California, USA). Image acquisition and quantitative analysis were carried out using the FluorChem 8900 image analysis system (Alpha Innotech, St San Leandro, California, USA). To evaluate the approximative proliferation level of the cells in every sample, the level of proliferating cell nuclear antigen (PCNA) was measured with anti‐PCNA monoclonal antibody (Dako Biot Co). To verify the relative amounts of protein in each lane, the level of β‐actin as an internal control was measured with anti‐β‐actin monoclonal antibody (Sigma, St Louis, Missouri, USA). The level of protein expression in tumour and in the corresponding normal tissue was calculated from the signal intensity. The level of fascin and PCNA expression were finally evaluated by the ratio of their presence in the tumour to the corresponding normal epithelial tissue (T:N ratio).
Preparation of total RNA and quantitative real‐time RT‐PCR
Total RNA was extracted from frozen stored tissues using the TRIzol reagent (Invitrogen, Grand Island, New York, USA) method.13 Reverse transcription was carried out in a total volume of 20 µl, using 1 µg total RNA, oligo‐dT primer, random primers and avian myeloblastosis virus reverse transcriptase (Promega, Madison, Wisconsin, USA). All PCR reactions were carried out using an ABI Prism 7000 Sequence Detection System (Perkin‐Elmer Applied Biosystems, USA). For the normalisation of our results, β‐actin was used as the internal control. Primers and probes for fascin and β‐actin were chosen with the assistance of Primer Express 2.0 software (Perkin‐Elmer Applied Biosystems). To avoid amplification of contaminating genomic DNA, one of the two primers of each gene was intron spanning. The PCR primers used for fascin were as follows: forward primer 5′‐AGCAAGAATGCCAGCTGCTACT‐3′ and reverse primer 5′‐GGTCACAAACTTGCCATTGGA‐3′. According to the fascin gene structure, a PCR product of 84 bp was obtained. For β‐actin, the forward primer was 5′‐GCGCGGCTACAGCTTCA‐3′ and the reverse primer was 5′‐TCTCCTTAATGTCACGCACGAT‐3′, and the sequences of the labelled probes were as follows: 5′‐TCGAGTGGCGTGACCGGC‐3′ for fascin and 5′‐CACCACGGCCGAGCGGGA‐3′ for β‐actin. The 50‐µl‐volume PCR reaction mixture was preheated at 93°C for 3 min, followed by 40 cycles at 93°C for 45 s and 55°C for 1 min. A non‐template control and the standard curve group (with 10‐fold serial dilutions of pGEM‐T Easy Vector containing truncated fascin cDNA as templates4) were included in each PCR run. The relative copy number of fascin mRNA was evaluated by the absolute mRNA copy number ratio of fascin to β‐actin.
Immunofluorescent staining
Cells were seeded on coverslips and incubated for 24 h. After washing with PBS, cells were fixed with 100% methanol at −10°C for 15 min and then treated with 0.2% Triton X‐100 in PBS for 10 min. Cells were subsequently incubated with a blocking solution (10% normal goat serum in PBS) for 20 min and incubated with primary antibody overnight at 4°C. The cells were washed and incubated with fluorescein‐conjugated anti‐mouse IgG (Zymed Biot Co, San Francisco, California, USA) as a secondary antibody for 30 min at 37°C. The cells were washed and nuclei were counterstained using propidium iodide (Sigma), mounted in glycerol and examined with a fluorescence microscope.
Statistical analysis
The relationship between fascin‐positive rate and clinicopathological features was immunohistochemically analysed using the χ2 test. Using western blot analysis, the relationship between fascin and PCNA expression levels and clinicopathological features was analysed using the independent sample t test. In real‐time RT‐PCR, the difference between the levels of fascin mRNA in tumour and in normal tissues was analysed using the paired sample t test. p Value <0.05 was considered to be significant.
Results
Fascin expression in ESCC
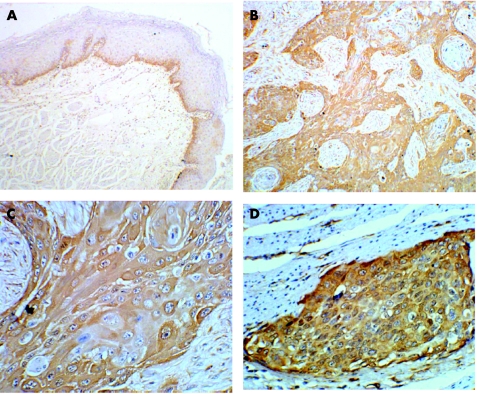
Positive immunostaining for fascin could be observed in a cytoplasmic pattern both in cancerous tissue and normal mucosa of the oesophagus. In ESCC, most of the cancer cells showed intense immunostaining, whereas weak to moderately positive signals were seen in the basal layer of some normal epithelium of the oesophagus (fig 1). Overexpression of fascin was found more often in ESCC than in the normal mucosa (p = 0.020). However, no difference was observed between the frequency of overexpression of fascin in ESCC and dysplasia of the oesophagus (p = 1.000; table 2), nor between normal mucosa and dysplasia of the oesophagus (p = 0.255; table 2). Nevertheless, a more intensive staining for fascin always presented in more of the epithelium with dysplasia than in the normal epithelium. By western blot, the level of fascin expression was increased in 87.76% (43/49) of ESCC cases, and fascin expression in ESCC was 2.43 (2.09) times that in the corresponding normal mucosa of the oesophagus (table 3).
Figure 1 Immunohistochemical staining of fascin expression in normal human oesophageal epithelium and tissue from oesophageal squamous cell carcinoma (ESCC). Paraffin‐wax‐embedded tissue sections were stained using an immunoperoxidase method, as described in Materials and methods. (A) In normal oesophageal epithelium, immunostaining for fascin (brown signal) was only located in the basal layer (×100). In ESCC, intense immunoreactivity for fascin was presented at the cytoplasm of most of the cancer cells ((B) ×100, (C) ×400). (D) Intense immunostaining for fascin was present in the cancer nest that invaded the muscle tissue of the oesophagus, and was enhanced at the periphery of the invasive cancer nest (×400).
Table 2 Relationship between clinicopathological features and fascin positivity in immunohistochemistry.
| Parameters | Positive rate (%) | p Value |
|---|---|---|
| Mean age in years (range) | ||
| <57 | 62.50 (20/32) | 0.732 |
| >57 | 66.67 (20/30) | |
| Sex | ||
| Male | 66.00 (33/50) | 0.618 |
| Female | 58.33 (7/12) | |
| Primary tumour | ||
| T1/T2 | 80.00 (4/5) | 0.789 |
| T3/T4 | 63.16 (36/57) | |
| Regional lymph node | ||
| N0 | 56.76 (20/37) | 0.036 |
| N1 | 80.00 (20/25) | |
| Distant metastasis | ||
| M0 | 65.00 (39/60) | 1.000 |
| M1 | 50.00 (1/2) | |
| Stage | ||
| I/IIA/IIB | 55.26 (21/38) | 0.055 |
| III/IV | 79.17 (19/24) | |
| Histopathology | ||
| Well differentiated (G1) | 57.14 (12/21) | 0.467 |
| Moderately differentiated (G2) | 61.90 (13/21) | |
| Poorly differentiated (G3) | 75.00 (15/20) | |
| ESCC | 64.52 (40/62) | 1.000 |
| Dysplasia | 60.00 (6/10) | |
| ESCC | 64.52 (40/62) | 0.020 |
| Normal mucosa | 35.00 (7/20) | |
| Dysplasia | 60.00 (6/10) | 0.255 |
| Normal mucosa | 35.00 (7/20) |
ESCC, oesophageal squamous cell carcinoma.
Table 3 Relationship between clinicopathological features and level of fascin expression in western blot.
| Parameters | Level of fascin expression, T:N ratio, mean (SD) | n | p Value |
|---|---|---|---|
| Mean age in years (range) | |||
| <58 | 2.75 (2.57) | 23 | 0.312 |
| >58 | 2.14 (1.54) | 26 | |
| Sex | |||
| Male | 2.22 (1.32) | 40 | 0.432 |
| Female | 3.35 (4.06) | 9 | |
| Primary tumour | |||
| T1/T2 | 3.19 (4.09) | 9 | 0.520 |
| T3/T4 | 2.26 (1.33) | 40 | |
| Regional lymph node | |||
| N0 | 1.76 (0.87) | 22 | 0.040 |
| N1 | 2.98 (2.60) | 27 | |
| Distant metastasis | |||
| M0 | 2.45 (2.13) | 47 | 0.780 |
| M1 | 2.02 (0.64) | 2 | |
| Stage | |||
| I/IIA/IIB | 2.35 (2.91) | 19 | 0.839 |
| III/IV | 2.48 (1.40) | 30 | |
| Histopathology | |||
| Well differentiated (G1) | 1.83 (0.97) | 13 | p1/p2 = 0.323 |
| Moderately differentiated (G2) | 2.53 (2.34) | 31 | p2/p3 = 0.389 |
| Poorly differentiated (G3) | 3.48 (2.41) | 5 | p1/p3 = 0.049 |
T:N ratio, ratio of tumour:corresponding normal tissue.
To verify whether there is an increase of fascin mRNA in ESCC, we carried out real‐time RT‐PCR. The result showed that the level of fascin mRNA in ESCC was 5.53 (7.29) times that in the corresponding normal mucosa of the oesophagus (p<0.001).
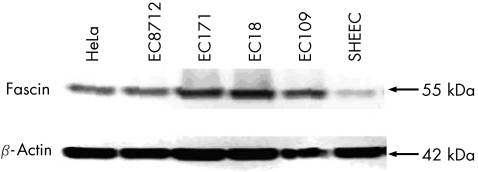
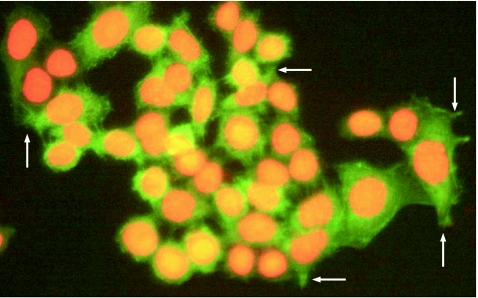
In western blot, the level of fascin expression was higher in 80% (4/5) of oesophageal cancer cell lines (fig 2) than in the positive controls (HeLa cell line).6 The localisation of fascin expression in the ESCC cell line was defined by immunofluorescent staining. Besides, in the cytoplasm, immunofluorescence for fascin was also seen in the membrane protrusions (fig 3).
Figure 2 Expression of fascin protein in five oesophageal squamous cell carcinoma (ESCC) cell lines. The HeLa cell line served as a positive control for fascin expression. Expression of β‐actin served as an internal control for equal loading. In all, 80% (4/5) of ESCC cell lines (EC109, EC18, EC171, EC8712) showed higher fascin expression compared with the HeLa cell line.
Figure 3 Representative fascin expression in oesophageal squamous cell carcinoma cell line EC109. Cells seeded and fixed in the coverslips were stained by immunofluorescence, as described in Materials and methods. The green fluorescence corresponding to fascin was seen in the cytoplasm of the cancer cells. In addition, fascin was also located in membrane protrusions (shown by arrows). Nuclei were counterstained with propidium iodide (red fluorescence). Original magnification ×400.
Fascin expression in the progression from normal epithelium to invasive carcinoma of the oesophagus
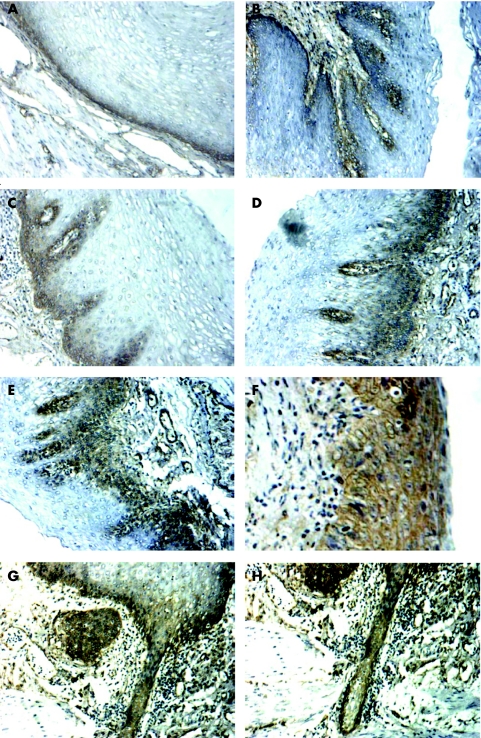
Using immunohistochemistry, we investigated the immunoreactivity of fascin in 10 special sections of a full‐length mucosa layer from the distant margin to the cancer focus of the excised oesophagus. Besides the normal epithelium and invasive cancer of the oesophagus, these 10 special sections contained various pathological oesophageal lesions. In normal epithelium (3/10), chronic oesophagitis (3/6) and simple hyperplasia (3/5), positive immunostaining was apparent only in the basal layer and the lower spinous layer of the oesophageal epithelium (fig 4A–C; the numerator represents the quantity of fascin‐positive cases and the denominator represents the quantity of each lesion of oesophagus in 10 sections), whereas in mild and moderate dysplasia (6/9) positive staining was observed in most of the heterogeneous cells from the basal layer to the granular layer of the epithelium (fig 4D,E). In the case of carcinoma in situ (4/5), the full‐thickness epithelium showed intensive immunostaining for fascin (fig 4F). We also observed that the infiltrative tumour margins (2/2) breaking through the basement membrane during transformation to invasive cancer showed intensive immunostaining for fascin (fig 4G,H).
Figure 4 Fascin expression in the progression from normal epithelium to invasive carcinoma of the oesophagus. Ten special sections of full‐length mucosa layer from the distant margin to the cancer focus of the excised oesophagus were immunohistochemically investigated as described in Materials and methods. In normal epithelium (A) and in mild chronic oesophagitis (B), positive immunostaining was only apparent in the basal layer of the epithelium. In simple hyperplasia (C), positive immunostaining was apparent in the basal layer and the lower spinous layer of the oesophageal epithelium, whereas in mild and moderate dysplasia (D, E) positive staining was observed in most of the heterogeneous cells from the basal layer to the granular layer of epithelium. Lastly, the full‐thickness epithelium showed intensive immunostaining for fascin in the case of carcinoma in situ (F). The infiltrative tumour margins (G, H) breaking through the basement membrane of epithelium during the transformation to invasive ESCC showed intensive immunostaining for fascin. Original magnification ×200.
Correlation between the level of fascin expression and cell proliferation
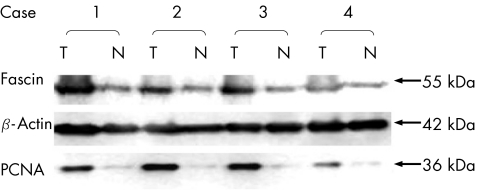
We used western blot to examine the level of PCNA expression. Overexpression of PCNA was observed in 87.76% (43/49) of tumour tissue (T) compared with the corresponding normal epithelium (N). The level of PCNA expression was much higher in the group overexpressing fascin (10.43 (9.37)) than in the group expressing low levels of fascin (0.88 (0.84); p<0.001; table 4; fig 5). The cases in which fascin was up regulated (T/N>1; n = 43) showed high levels of PCNA expression (T/N>1; n = 43).
Table 4 Relationship between levels of expression of fascin and proliferating cell nuclear antigen.
| Group | Level of PCNA expression, T:N ratio, mean (SD) | n | p Value |
|---|---|---|---|
| Fascin T/N<1 | 0.88 (0.84) | 6 | <0.001 |
| Fascin T/N>1 | 10.43 (9.37) | 43 |
PCNA, proliferating cell nuclear antigen; T:N ratio, ratio of tumour:corresponding normal tissue.
Figure 5 Western blot analysis of fascin and proliferating cell nuclear antigen (PCNA) in four oesophageal carcinoma tissues (T) and in the corresponding normal oesophageal tissues (N). The molecular weights (kDa) of the immunoreactivity are shown on the right. Expression of β‐actin was simultaneously tested as an internal control.
Correlation between clinicopathological features and the level of fascin expression in ESCC
In ESCC, a significant correlation was observed between fascin expression and lymph node metastasis both in the analysis of immunohistochemistry (p = 0.036; table 2) and western blot (p = 0.040; table 3). Up regulation of fascin in the matched distal resected margin of ESCC specimens (including normal mucosa and dysplasia, n = 30) also significantly correlated with lymph‐node metastasis. The fascin‐positive margin (n = 13) presented more lymph‐node metastasis potential than the fascin‐negative margin (n = 17; 11/13 v 4/17; p = 0.003). No significant correlation was found between frequency of fascin overexpression and the degree of differentiation of cancer cells in the immunohistochemical study, whereas a significant difference in the level of fascin expression was found between well‐differentiated and poorly differentiated groups using western blot (p = 0.049). The poorer the ESCC differentiation, the higher the fascin expression.
The correlation between fascin expression and other clinicopathological factors, such as pathological tumour‐node‐metastasis classification and stage grouping, was simultaneously investigated (tables 2 and 3). However, there was no significant association between fascin expression and factors such as age and sex.
Discussion
Cell–matrix and cell–cell adhesive interactions have important roles in the normal organisation and stabilisation of the epithelial tissue. The conversion of normal epithelial cells to malignant ones is associated with changes in the expression and function of these adhesion systems that enable a switch to a migratory phenotype in tumour invasion and metastasis.7 As an actin‐binding protein, fascin is important for a diverse set of cell protrusions with functions in cell adhesion, cell interaction and cell migration.14 Overexpression of fascin has only recently been described in epithelial neoplasms. In ESCC, Hashimoto et al12 reported that the immunostaining intensity of fascin expression was usually increased in ESCC compared with that in normal epithelium in 200 specimens. We carried out western blot and quantitative real‐time RT‐PCR besides immunohistochemistry to examine fascin expression. We found that fascin was up regulated both at translational and transcriptional levels in ESCC.
Fascin expression in five ESCC cell lines was also examined using western blot and immunofluorescent staining. We found that 80% (4/5) of ESCC cell lines expressed fascin at a high level. Besides, in the cytoplasm, immunofluorescence for fascin was also clearly observed in the membrane protrusions, which are important in cell adhesion, interaction and migration.6 This suggested that fascin expression was also up regulated in ESCC cell lines and had important roles in cell motility.
Human ESCC typically evolves through a sequence of histopathological lesions, including oesophagitis, mild to severe dysplasia, carcinoma in situ, and finally, invasive cancer. Recent reports about fascin usually focused on the level of fascin expression in cancerous and normal tissues. But it was more urgent to find out when the up regulation of fascin occurred during the process of transformation and development of neoplasms. Maitra et al15 reported that up regulation of fascin seemed to be a late event in the multistep pathogenesis of pancreatic ductal adenocarcinoma, because 89% of low‐grade pancreatic intraepithelial neoplasias had no fascin expression, whereas 40% of high‐grade pancreatic intraepithelial neoplasias expressed fascin. Nevertheless, in our study, obvious up regulation of fascin was observed in dysplasia, the precancerous lesion of ESCC (table 2; fig 4D,E). To our knowledge, this is the first report that showed up regulation of fascin appearing at an early stage of malignant transformation. We also observed that level of fascin expression and frequency of overexpression increased gradually in the progression from normal epithelium, simple hyperplasia, dysplasia, carcinoma in situ, to invasive ESCC (fig 4). This finding did not support the previous reports that fascin was scarcely expressed in precancerous lesions or carcinoma in situ of other different anatomical sites, such as the pancreas and lungs.15,16 As the malignant transformation of different cell types may be somewhat different, the carcinogenesis of ESCC also had its characteristics. The earliest malignant lesion of the oesophagus often resulted from the abnormal regulation and control of cell cycle in the basal layer cells of the epithelium.17,18 The most intensive staining for fascin was often observed in the basal and lower spinous layers of the oesophageal epithelium with precancerous lesions in our study. These probably suggest that up regulation of fascin has a role in formation of precancerous lesions in ESCC. On the other hand, a significant correlation was also seen between up regulation of fascin (both in the cancer focus and the distal resected margins) and lymph‐node metastasis in ESCC (tables 2 and 3). These findings raised the possibility that fascin could be a novel biomarker for early identification of aggressive ESCC.
According to recent reports, tumours overexpressing fascin mostly had markedly more lymph‐node metastasis in comparison with tumours with low fascin expression. The mechanism of this phenomenon could be explained by the high motility of tumour cells overexpressing fascin, based on the finding of different cell lines.6,12 Lymph‐node metastasis was an important negative prognostic indicator of ESCC and was often related to the depth of invasion and distant metastasis.19,20 In our study, no marked differences were found between fascin expression and depth of invasion or distant metastasis in ESCC. However, we could not conclude that there was no association between fascin and these two factors, because of the insufficient ESCC specimens of T1/T2 and M1.
Changes in cell proliferation may be a key factor in regulating tumour growth and progression.21 Several reports showed the relationship between fascin expression and the status of cell proliferation. Pelosi et al16 reported that the overexpression of fascin was markedly associated with a high Ki‐67 labelling index in the case of non‐small cell lung adenocarcinomas. We examined the level of PCNA expression, a biomarker for evaluating the approximative extent of cell proliferative activity,22 using western blot. The markedly higher level of PCNA expression in the group overexpressing fascin than in the group with low fascin expression supported previous findings. Recent work in our laboratory confirmed this relationship between fascin expression and the proliferative status of ESCC cells by targeting fascin expression with the RNA interfering technique in vitro.23 In addition, the dividing heterogeneous cells showed most intensive immunostaining for fascin in the dysplasia. This suggested that the association between fascin expression and cell proliferative status occurred not only in ESCC but also in its precancerous lesion. However, the mechanism by which fascin expression is associated with cell proliferation is still unclear. Further investigations to elucidate the mechanism are under way.
In summary, fascin was overexpressed in ESCC and the high level of fascin expression correlated with the activity of cell proliferation and lymph‐node metastasis. Moreover, the up regulation of fascin occurred early in the progression from normal epithelium to invasive carcinoma. These may implicate fascin as a novel biomarker that allows the tumour to be identified at an early stage in high‐risk people. However, the mechanism of up regulation of fascin in ESCC remains ambiguous. Thus, future work to understand the mechanism of overexpression of fascin in neoplasms will be of particular interest.
Take‐home messages
Fascin was overexpressed in oesophageal squamous cell carcinoma (ESCC), and the high expression level of fascin correlated with cell proliferative activity and lymph node metastasis.
The up regulation of fascin occurred early in the progression from normal epithelium to invasive carcinoma.
Fascin expression increased progressively from normal epithelium, simple hyperplasia, atypical hyperplasia, carcinoma in situ, to invasive ESCC.
Acknowledgements
We thank Professor XJ Yu from the Medical College of Shantou University for his kind assistance in photomicrography.
Abbreviations
ESCC - oesophageal squamous cell carcinoma
IgG - immunoglobulin G
RT‐PCR - reverse transcription‐polymerase chain reaction
PBS - phosphate‐buffered saline
PCNA - proliferating cell nuclear antigen
Footnotes
Funding: This work was supported by grants from the National Natural Science Foundation of China (No 39900069, No 30170428, No 30370641, No 30570849); Guangdong Scientific Fund Key Items (No 37788, No 05104541); and Natural Science Foundation of Guangdong Province (No 010431).
Competing interests: None.
This study was approved by the ethics committee of the First Affiliated Hospital of Shantou University (Shantou City, Guangdong Province, PR China) and written informed consent to use resected samples for research was obtained from all patients undergoing surgery.
References
- 1.Enzinger P C, Mayer R J. Esophageal cancer. N Engl J Med 20033492241–2252. [DOI] [PubMed] [Google Scholar]
- 2.Brooks‐Brunn J A. Esophageal cancer: an overview. Med Surg Nurs 20009248–254. [PubMed] [Google Scholar]
- 3.Shen Z, Cen S, Shen J.et al Study of immortalization and malignant transformation of human embryonic esophageal epithelial cells induced by HPV18 E6E7. J Cancer Res Clin Oncol 2000126589–594. [DOI] [PubMed] [Google Scholar]
- 4.Rong J, Xu L Y, Cai W J.et al Expression of fascin 1 gene in the process of the immortalized esophageal carcinoma carcinogenesis. Ai Zheng 200423243–248. [PubMed] [Google Scholar]
- 5.Bryan J, Edwards R, Matsudaira P.et al Fascin, an echinoid actin‐bundling protein, is a homolog of the Drosophila singed gene product. Proc Natl Acad Sci USA 1993909115–9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamashiro S, Yamakita Y, Ono S.et al Fascin, an actin‐bundling protein, induces membrane protrusions and increases cell motility of epithelial cells. Mol Biol Cell 19989993–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jawhari A U, Buda A, Jenkins M.et al Fascin, an actin‐bundling protein, modulates colonic epithelial cell invasiveness and differentiation in vitro. Am J Pathol 200316269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto Y, Shimada Y, Kawamura J.et al The prognostic relevance of fascin expression in human gastric carcinoma. Oncology 200467262–270. [DOI] [PubMed] [Google Scholar]
- 9.Yoder B J, Tso E, Skacel M.et al The expression of fascin, an actin‐bundling motility protein, correlates with hormone receptor‐negative breast cancer and a more aggressive clinical course. Clin Cancer Res 200511186–192. [PubMed] [Google Scholar]
- 10.Goncharuk V N, Ross J S, Carlson J A. Actin‐binding protein fascin expression in skin neoplasia. J Cutan Pathol 200229430–438. [DOI] [PubMed] [Google Scholar]
- 11.Tong G X, Yee H, Chiriboga L.et al Fascin‐1 expression in papillary and invasive urothelial carcinomas of the urinary bladder. Hum Pathol 200536741–746. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto Y, Ito T, Inoue H.et al Prognostic significance of fascin overexpression in human esophageal squamous cell carcinoma. Clin Cancer Res 2005112597–2605. [DOI] [PubMed] [Google Scholar]
- 13.Chomczynski P. A reagent for the single‐step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques 199315532–4, 5367. [PubMed] [Google Scholar]
- 14.Adams J C. Fascin protrusions in cell interactions. Trends Cardiovasc Med 200414221–226. [DOI] [PubMed] [Google Scholar]
- 15.Maitra A, Iacobuzio‐Donahue C, Rahman A.et al Immunohistochemical validation of a novel epithelial and a novel stromal marker of pancreatic ductal adenocarcinoma identified by global expression microarrays: sea urchin fascin homolog and heat shock protein 47. Am J Clin Pathol 200211852–59. [DOI] [PubMed] [Google Scholar]
- 16.Pelosi G, Pastorino U, Pasini F.et al Independent prognostic value of fascin immunoreactivity in stage I nonsmall cell lung cancer. Br J Cancer 200388537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitamura K, Kuwano H, Yasuda M.et al What is the earliest malignant lesion in the esophagus? Cancer 1996771614–1619. [DOI] [PubMed] [Google Scholar]
- 18.Shi S T, Yang G Y, Wang L D.et al Role of p53 gene mutations in human esophageal carcinogenesis: results from immunohistochemical and mutation analyses of carcinomas and nearby non‐cancerous lesions. Carcinogenesis 199920591–597. [DOI] [PubMed] [Google Scholar]
- 19.Christein J D, Hollinger E F, Millikan K W. Prognostic factors associated with resectable carcinoma of the esophagus. Am Surg 200268258–62 discussion 2623. [PubMed] [Google Scholar]
- 20.Wong R, Malthaner R. Esophageal cancer: a systematic review. Curr Probl Cancer 200024297–373. [DOI] [PubMed] [Google Scholar]
- 21.Wang L D, Zhou Q, Yang W C.et al Apoptosis and cell proliferation in esophageal precancerous and cancerous lesions: study of a high‐risk population in northern China. Anticancer Res 199919369–374. [PubMed] [Google Scholar]
- 22.Kimos M C, Wang S, Borkowski A.et al Esophagin and proliferating cell nuclear antigen (PCNA) are biomarkers of human esophageal neoplastic progression. Int J Cancer 2004111415–417. [DOI] [PubMed] [Google Scholar]
- 23.Xie J J, Xu L Y, Zhang H H.et al Role of fascin in the proliferation and invasiveness of esophageal carcinoma cells. Biochem Biophys Res Commun 2005337355–362. [DOI] [PubMed] [Google Scholar]