Abstract
Background
Mammary carcinogenesis is a multistep process entailing the transition from normal breast to benign proliferative breast disease (ductal hyperplasia) to ductal carcinoma in situ to infiltrating ductal carcinoma.
Hypothesis
These transitions are associated with changes in the mononuclear inflammatory cell infiltrate.
Materials and methods
A total of 53 mastectomy specimens of normal breast, benign proliferative breast disease, ductal carcinoma in situ and infiltrating ductal carcinoma were evaluated for mononuclear inflammatory cell infiltrate by using immunohistological methods and monoclonal antibodies including CD20, CD68, CD3 and granzyme B, histiocytes, T cells and cytotoxic T cells.
Results
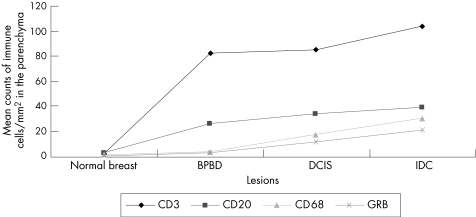
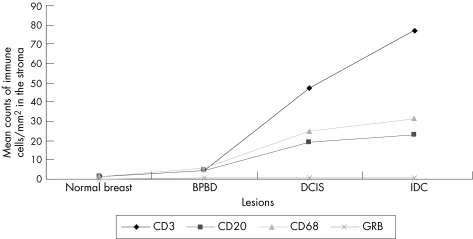
Transitions from normal breast to the subsequent tissue with lesions (normal skin v benign proliferative breast disease v ductal carcinoma in situ v infiltrating ductal carcinoma) were associated with significantly (p<0.01) increased mean (SD) density of mononuclear inflammatory cell infiltrate at the parenchyma (3.2 (1.0) v 26.4 (7.8) v 33.6 (7.9) v 39.1 (4.7) for CD20+ B cells; 2.8 (1.0) v 81.5 (14.0) v 84.0 (14.9) v103.7 (3.9) for CD3; 1.3 (2.0) v 3.8 (4.0) v 12.7 (23) v 22.1 (25.0) for CD68+ macrophages; 2.0 (1.0) v 58.3 (5.0) v 60.0 (10.0) v 74.1 (28.0) for granzyme B+ cytotoxic T cells) and at the stroma (0.7 (1.0) v 3.0 (5.0) v 13.3 (20) v 16.7 (30.0) for CD20+ B cells; 1.0 (2.06) v 4.0 (2.5) v 16.7 (5.0) v 21.7 (15) for CD68+ macrophages; 1.4 (0.6) v 4.2 (1.2) v 46.6 (16.7) v 77.0 (5.0) for CD3+ cells and 0 (0) v 0.5 (1.0) v 0.7 (1.0) v 0.7 (1.0) for granzyme B+ cytotoxic T cells).
Conclusions
The increased mononuclear inflammatory cell infiltrate during mammary carcinogenesis may reflect non‐specific or specific immunological processes.
Mammary carcinogenesis includes several lesions such as ductal hyperplasia, atypical ductal hyperplasia, cancerisation of the lobules, ductal carcinoma in situ and infiltrating ductal carcinoma.1 It also includes different types of progression routes such as lobular, inflammatory, papillary, medullary, mucinous, tubular, infiltrating cribriform, infiltrating papillary, adenoid cystic, carcinomas with neuroendocrine differentiation, myoblastoid, signet ring cell, secretory or juvenile, apocrine, metaplastic or sarcomatoid, squamous cell and low‐grade adenosquamous carcinomas. Lymphocytic infiltration is usually seen in hereditary ductal carcinoma in situ and infiltrating ductal carcinoma.2,3,4,5,6 In premalignant lesions that occur in prophylactically removed breast tissue from patients who are at a high familial risk of breast cancer, lobulitis, with a predominance of T cells, was found in 51% of patients with prophylactically removed breast tissue. These findings imply an immune reaction to unidentified antigen, possibly as part of an early carcinogenic event.6
The immune defence against tumours is largely a cell‐mediated one and relies on the integrity of T cell subsets such as cytotoxic or suppressor T lymphocytes, which are characterised by the presence of cytotoxic cytoplasmic granules such as granzyme B.7 Granzyme B is an exogenous serine proteinase that can activate several procaspases, resulting in apoptotic death of the target cells. Therefore, the existence of granzyme B is associated with increased apoptotic activity in the cells.8 Moreover, B cells can mount humoral immune response with antitumour effects. In this study, we hypothesised that the transition from normal breast to benign proliferative breast disease (ductal hyperplasia) to ductal carcinoma in situ to infiltrating ductal carcinoma is associated with changes in the mononuclear inflammatory cell infiltrate.
Materials and methods
Tissue specimens
This retrospective study included 53 mastectomy specimens obtained from 53 patients with breast cancer. Tissues, fixed in formalin and embedded in paraffin wax, were obtained from the Department of Pathology, Assuit University Hospitals, Assuit, Egypt. Specimens included the entire continuum of the normal tissues and those with the lesions, including benign proliferative breast disease, ductal carcinoma in situ and infiltrating ductal carcinoma. None of the patients included in our study had autoimmune disease. The status of BRCA1 or BRCA2 genes was unknown.
Immunohistochemical analysis
Sections were immunostained as previously described.1 Sections mounted on glass slides were deparaffinised and rehydrated; endogenous peroxidase activity was blocked; non‐specific protein binding was blocked and sections were then incubated with mouse monoclonal antibodies for 30 min at room temperature (Clone L26, PC3/188A, PG‐M1 and granzyme B‐7 for CD20, CD3, CD68 and granzyme B, respectively; DAKO Corporation, Denmark; California, USA). A catalysed signal amplification system (K1500, DAKO, California, USA) was used as described previously. The slides were independently evaluated by the authors and counts were combined to give a final figure (table 1).
Table 1 Antibodies used in the immunohistochemical evaluation of the breast lesions.
| Antibody | Specificity | Retrieval | Dilution | Incubation time | Control (lymph node) | Distribution of staining signals |
|---|---|---|---|---|---|---|
| CD68 | Histiocytes | Citrate, pH = 6 | Ready to use | 30 min at 37°C | Sinusoidal | Cytoplasmic, granular, diffuse |
| CD3 | Pan T cells | Trypsin | 1:100 | 30 min at 37°C | Paracortical | Membranous |
| CD20 | Pan B cells | Citrate | 1:200 | Overnight at 4°C | Follicular | Membranous |
| Granzyme B | Cytotoxic T cells | Citrate, pH = 6 | 1:100 | 30 min at 37°C | Paracortical | Cytoplasmic granular, diffuse |
Table 2 Immunophenotypic profile of the immune cells in the normal breast, benign proliferative breast disease, ductal carcinoma in situ and infiltrating ductal carcinoma in both parenchyma and stroma.
| CD3+ | CD20+ | CD68+ | Granzyme B+ | |||||
|---|---|---|---|---|---|---|---|---|
| Parenchyma | Stroma | Parenchyma | Stroma | Parenchyma | Stroma | Parenchyma | Stroma | |
| Normal | 2.8 (1.0) | 1.4 (0.6) | 3.2 (1.0) | 0.7 (0.3) | 1.3 (0.7) | 1.0 (0.6) | 0.5 (0.3) | 0.0 (0.0) |
| Benign proliferative breast disease | 81.5 (14.0) | 4.2 (1.2) | 26.4 (7.8) | 3.0 (1.2) | 3.8 (1.2) | 4.0 (0.6) | 2.3 (1.4) | 0.5 (0.3) |
| Ductal carcinoma in situ | 84.0 (14.9) | 46.6 (16.7) | 33.6 (7.9) | 13.3 (6.7) | 12.7 (3.7) | 16.7 (1.7) | 8.5 (6.9) | 0.7 (0.3) |
| Infiltrating ductal carcinoma | 103.7 (3.9) | 77.0 (5.0) | 39.1 (4.7) | 16.7 (8.8) | 22.1 (4.1) | 21.7 (4.4) | 16.3 (4.9) | 0.7 (0.3) |
Values are mean (standard error of mean).
Positive controls
Specimens consisted of lymph nodes with reactive lymphoid hyperplasia (table 1).
Negative controls
Additional sections, running in parallel but with the omission of the primary antibody, served as negative controls.1
Counting of the immune cells
The following strategy was used to count the immune cells as a function of numbers per square millimetre:
The cells were counted in serial sections and in at least 10 different fields.
The cells were counted in two sites, including tumour parenchyma and stroma.
Two observers counted the cells.
The areas of highest density were chosen.
Necrotic areas were avoided.
Results were expressed as mean (standard error of mean).5,9,10,11
Statistical analysis
Data were analysed with SPSS for Windows using analysis of variance and significance was set at p<0.05.
Results
We found a marked increase in the density of mononuclear inflammatory cell infiltrate, with the transition from normal breast tissue to tissue with lesions. This infiltrate included CD20+, CD68+, CD3+ and granzyme B+ cells. The CD3+ cells were the predominant cell population. Among the others, a relatively small fraction of T lymphocytes (CD3 cells) had cytotoxic activity: granzyme B+ cells. Within the tumour, most of the mononuclear inflammatory cell infiltrate was in the stroma and the preinvasive lesions were large enough for counting the cells in the epithelial groups. Figs 1–6 show a summary of these results.
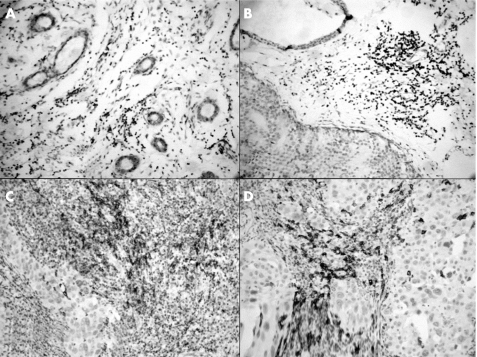
Figure 1 Immunohistochemical staining for CD20 (B cells) in the (A) normal breast, (B) benign proliferative breast disease, (C) ductal carcinoma in situ and (D) infiltrating ductal carcinoma.
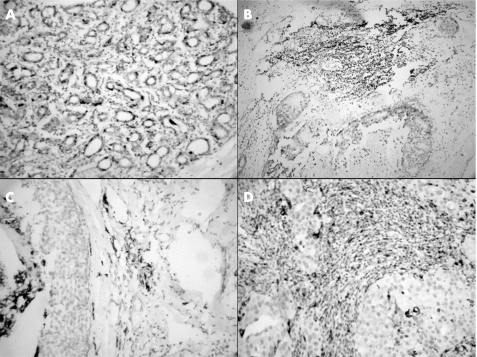
Figure 2 Immunohistochemical staining for CD68 in the (A) normal breast, (B) benign proliferative breast disease, (C) ductal carcinoma in situ and (D) infiltrating ductal carcinoma.
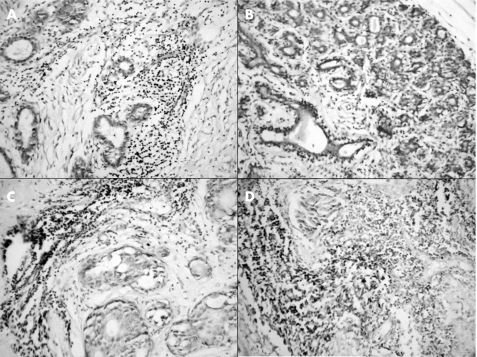
Figure 3 Immunohistochemical staining for CD3 (T cells) in the (A) normal breast, (B) benign proliferative breast disease, (C) ductal carcinoma in situ and (D) infiltrating ductal carcinoma.
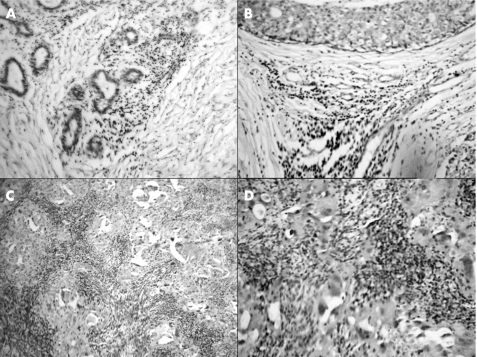
Figure 4 Immunohistochemical staining for granzyme B (cytotoxic T cells) in the (A) normal breast, (B) benign proliferative breast disease, (C) ductal carcinoma in situ and (D) infiltrating ductal carcinoma.
Figure 5 Mean (standard error of mean) values of the immune cells in the normal breast, benign proliferative breast disease (BPBD), ductal carcinoma in situ (DCIS) and infiltrating ductal carcinoma (IDC) in the parenchyma. Values of the immune cells represent their numbers per square millimetre. GRB, granzyme B.
Figure 6 Mean (standard error of mean) values of the immune cells in the normal breast, benign proliferative breast disease (BPBD), ductal carcinoma in situ (DCIS) and infiltrating ductal carcinoma (IDC) in the stroma. Values of the immune cells represent their numbers per square millimetre. GRB, granzyme B.
Discussion
In our series, the presence of mononuclear inflammatory cell infiltrate in the breast tissues with lesions concurs with previous reports2,3,4 and also reflects ongoing immune responses against the neoplastic cells. The presence of mononuclear inflammatory cell infiltrate also suggests that the genetically damaged ductal cells have sufficient immunogenicity to recruit immunocytes. The marked increase in immune cells with the transition from normal breast tissue to that with lesions may be due to an increase in the load of associated antigens on these damaged ductal cells. These antigens possibly include cancer testis antigens12,13 and mutated genes, such as p53, BRCA1 or BRCA2,14,15,16 which can induce local activation and proliferation of the immunocytes.17 Interestingly, breast cancer cells express some major cancer testis antigens12,13 and some viral antigens.18,19 Moreover, the neoplastic ductal cells may produce more cytokines and adhesion molecules, which recruit more immunocytes.5,20,21,22BRCA1 is a tumour suppressor gene that normally inhibits signalling by oestrogen receptor α. In the breast, BRCA1 is normally expressed at puberty and pregnancy. It interacts with cyclin‐dependent kinases and inhibits tumour growth in cell culture. Patients with a BRCA1 mutation have a 35–80% lifetime risk of breast cancer such as medullary carcinoma. Interestingly, the immune cell infiltrate is marked in cancers related to BRCA1 or BRCA2, normal breast, and ductal carcinoma in situ. The status of these BRCA1 or BRCA2 in our study mandates further investigation. The relatively similar immune profile for benign proliferative breast disease and ductal carcinoma in situ suggests that the immune response in mammary carcinogenesis consists of a common immune‐mediated cellular effector pathway. It also suggests that some cases of benign proliferative breast disease may represent precursors for ductal carcinoma in situ.
The presence of both B and T lymphocytes in the neoplastic lesions of the breast is in agreement with previous reports,2,3,4 and suggests the participation of both cell‐mediated and humoral immunity in mammary carcinogenesis. Most of the mononuclear inflammatory cell infiltrate was composed of CD3+ T cells, which coincides with the findings of others3 and reflects increased T cell proliferation. Alternatively, the increased density of CD20+ B cells in breast cancer also concurs with previous reports5 and suggests a relatively intact signalling pathway through the B cell receptor in B lymphocytes. It also indicates that the local antibody response has a role in mammary carcinogenesis.23
Cytotoxic T lymphocytes can recognise and destroy tumour cells through the release of cytoplasmic granules that contain granzyme B.8 The notably higher density of granzyme B+ cells in ductal carcinoma in situ or infiltrating ductal carcinoma as compared with normal breast and benign proliferative breast disease suggests possible pathogenetic and prognostic roles for granzyme B. In our series, examination of expressions of Bcl‐2 and antiapoptotic proteins showed down regulation of Bcl‐2 with the transition from normal tissue to tissue with lesions.1 The expression of Bcl‐2 in both the normal ductal epithelial cells and adjacent mononuclear inflammatory cell infiltrate suggests that Bcl‐2 has a role in regulating the apoptosis of normal breast. Alternatively, the lack of Bcl‐2 expression in infiltrating ductal carcinoma may explain the presence of apoptosis in these lesions. Alternatively, Bcl‐2 expression in benign proliferative breast disease may contribute to the genesis of ductal carcinomas by delaying or preventing p53‐induced apoptosis in dysplastic ductal epithelial cells. It can therefore favour the persistence and propagation of the DNA mutations by its cytoprotective effects. Further correlation of these findings with the status of mononuclear inflammatory cell infiltrate during mammary carcinogenesis mandates further investigation. Also, it would be interesting to analyse the more functional elements, such as the degree of apoptosis or the underlying mechanism (eg, expression of human leucocyte antigen).
To summarise, mammary carcinogenesis is associated with changes in mononuclear inflammatory cell infiltrate. The possible pathogenetic and prognostic ramifications of our findings are open for further investigation.
Footnotes
Competing interests: None declared.
References
- 1.Hussein M R, Ismael H H. Alterations of p53, Bcl‐2, and hMSH2 protein expression in the normal breast, benign proliferative breast disease, in situ and infiltrating ductal breast carcinomas in the upper Egypt. Cancer Biol Ther 20043983–988. [DOI] [PubMed] [Google Scholar]
- 2.Alam S M, Clark J S, Leech V.et al T cell receptor gamma/delta expression on lymphocyte populations of breast cancer patients. Immunol Lett 199231279–283. [DOI] [PubMed] [Google Scholar]
- 3.Chin Y, Janseens J, Vandepitte J.et al Phenotypic analysis of tumor‐infiltrating lymphocytes from human breast cancer. Anticancer Res 1992121463–1466. [PubMed] [Google Scholar]
- 4.Baxevanis C N, Dedoussis G V, Papadopoulos N G.et al Tumor specific cytolysis by tumor infiltrating lymphocytes in breast cancer. Cancer 1994741275–1282. [DOI] [PubMed] [Google Scholar]
- 5.Marsigliante S, Biscozzo L, Marra A.et al Computerised counting of tumour infiltrating lymphocytes in 90 breast cancer specimens. Cancer Lett 199913933–41. [DOI] [PubMed] [Google Scholar]
- 6.Hermsen B B, von Mensdorff‐Pouilly S, Fabry H F.et al Lobulitis is a frequent finding in prophylactically removed breast tissue from women at hereditary high risk of breast cancer. J Pathol 2005206220–223. [DOI] [PubMed] [Google Scholar]
- 7.Kontani K, Sawai S, Hanaoka J.et al Involvement of granzyme B and perforin in suppressing nodal metastasis of cancer cells in breast and lung cancers. Eur J Surg Oncol 200127180–186. [DOI] [PubMed] [Google Scholar]
- 8.Gross T, Wagner A, Ugurel S.et al Identification of TIA‐1+ and granzyme B+ cytotoxic T cells in lichen sclerosus et atrophicus. Dermatology 2001202198–202. [DOI] [PubMed] [Google Scholar]
- 9.Davidorf F H, Lang J R. Lymphocytic infiltration in choroidal melanoma and its prognostic significance. Trans Ophthalmol Soc UK 197797394–401. [PubMed] [Google Scholar]
- 10.Saito T, Tanaka R, Kouno M.et al Immunohistological analysis of infiltrating lymphocyte subpopulations in gliomas and metastatic brain tumors. No To Shinkei 198739339–345. [PubMed] [Google Scholar]
- 11.Becker I, Roggendorf W. Immunohistological investigation of mononuclear cell infiltrates in meningiomas. Acta Neuropathol (Berlin) 198979211–216. [DOI] [PubMed] [Google Scholar]
- 12.Mashino K, Sadanaga N, Tanaka F.et al Expression of multiple cancer‐testis antigen genes in gastrointestinal and breast carcinomas. Br J Cancer 200185713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egland K A, Kumar V, Duray P.et al Characterization of overlapping XAGE‐1 transcripts encoding a cancer testis antigen expressed in lung, breast, and other types of cancers. Mol Cancer Ther 20021441–450. [PubMed] [Google Scholar]
- 14.Bautista S, Theillet C. p53 mutations in breast cancer: incidence and relations to tumor aggressiveness and evolution of the disease. Pathol Biol (Paris) 199745882–892. [PubMed] [Google Scholar]
- 15.Coughlin S S, Khoury M J, Steinberg K K. BRCA1 and BRCA2 gene mutations and risk of breast cancer. Public health perspectives. Am J Prev Med 19991691–98. [DOI] [PubMed] [Google Scholar]
- 16.McDermott R S, Beuvon F, Pauly M.et al Tumor antigens and antigen‐presenting capacity in breast cancer. Pathobiology 200270324–332. [DOI] [PubMed] [Google Scholar]
- 17.Jager E, Jager D, Knuth A. Strategies for the development of vaccines to treat breast cancer. Recent Results Cancer Res 199815294–102. [DOI] [PubMed] [Google Scholar]
- 18.de Ricqles D, Olomucki A, Gosselin F.et al Breast cancer and T‐cell‐mediated immunity to proteins of the mouse mammary tumour virus (MMTV). Eur Cytokine Netw 19934153–160. [PubMed] [Google Scholar]
- 19.Deshpande C G, Badve S, Kidwai N.et al Lack of expression of the Epstein‐Barr Virus (EBV) gene products, EBERs, EBNA1, LMP1, and LMP2A, in breast cancer cells. Lab Invest 2002821193–1199. [DOI] [PubMed] [Google Scholar]
- 20.Camp B J, Dyhrman S T, Memoli V A.et al In situ cytokine production by breast cancer tumor‐infiltrating lymphocytes. Ann Surg Oncol 19963176–184. [DOI] [PubMed] [Google Scholar]
- 21.Adams D H, Hubscher S G, Fisher N C.et al Expression of E‐selectin and E‐selectin ligands in human liver inflammation. Hepatology 199624533–538. [DOI] [PubMed] [Google Scholar]
- 22.Treilleux I, Blay J Y, Bendriss‐Vermare N.et al Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res 2004107466–7474. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda M, Petersson M, Lenkei R.et al Alterations in the signal‐transducing molecules of T cells and NK cells in colorectal tumor‐infiltrating, gut mucosal and peripheral lymphocytes: correlation with the stage of the disease. Int J Cancer 199561765–772. [DOI] [PubMed] [Google Scholar]