Abstract
Background
The presence of lipid in the cell cytoplasm is useful for supporting the diagnosis of sebaceous gland carcinoma (SGC). Currently this requires histochemical stains that are carried out on frozen sections of unprocessed tissue. Recently, several anti‐adipocytic antibodies that recognise proteins associated with lipid vesicles have been described. These antibodies can be applied to paraffin‐wax sections.
Aim
To assess the ability of anti‐adipocytic antibodies to identify intracytoplasmic lipid in SGC.
Methods
Immunohistochemistry with a monoclonal antibody to adipophilin and polyclonal antibodies to perilipin and TIP47/PP17 was carried out on archival, formalin‐fixed, paraffin‐wax‐embedded sections of 26 samples of SGC. The immunostaining was compared with 22 other eyelid tumours (11 basal cell carcinomas (BCC), 10 squamous cell carcinomas (SCC) and 1 Merkel cell tumour).
Results
Immunohistochemical staining was positive in 23, 10 and 2 cases of 26 SGC with adipophilin, perilipin and TIP47, respectively. The positive staining identified cytoplasmic lipid vesicles. Anti‐adipophilin was positive in five other eyelid tumours (4 BCC and 1 SCC) staining small cytoplasmic granules that can be easily distinguished from the staining in SGC.
Conclusions
Immunohistochemical staining for adipophilin and perilipin is a useful ancillary technique for the demonstration of lipid in SGC that may be applied to paraffin‐wax sections.
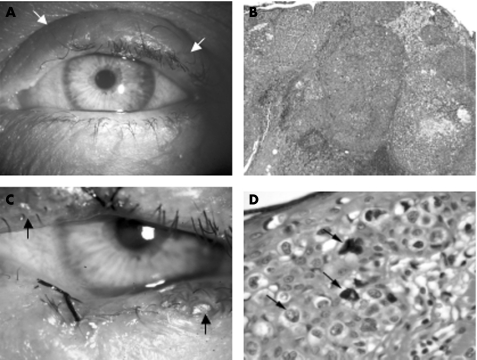
Sebaceous gland carcinoma (SGC) is a rare tumour of the skin. However, it is the second most common malignancy of the eyelid, accounting for 1–5% of all eyelid neoplasms.1,2,3 SGC usually originates in the Meibomian gland, but may also arise from the gland of Zeiss or sebaceous glands of the eyelid skin.3,4 Ocular SGC commonly occurs in elderly patients and shows a preponderance in female sex.3,5,6,7 The prognosis is poor compared with most other malignant eyelid tumours, with local recurrence in up to 18% of patients and metastases in 8–40%.3,5,6,7,8,9,10 The prognosis improves considerably with early diagnosis and surgery.1,11,12,13 However, the clinical appearance of SGC is variable and this tumour is notorious for masquerading as other benign or malignant lesions that may result in delayed diagnosis. Specifically, SGC may be indistinguishable from squamous cell carcinoma (SCC) or basal cell carcinoma (BCC), or may mimic a range of benign conditions including chalazion and blepharoconjunctivitis3 (fig 1A,B).
Figure 1 Clinical and histopathological appearances of nodular and diffuse sebaceous gland carcinoma (SGC). (A) Left eye showing swelling of the upper eyelid (arrows) clinically thought to be a chalazion. Excision biopsy of the lesion showed a nodular SGC. (B) Moderately differentiated SGC with a predominantly nodular growth pattern (haematoxylin and eosin (H&E), ×100). (C) Left eye showing patchy inflammation at upper and lower lid margins (arrows). This is associated with patchy loss of eyelashes. A suture at the centre of the lower lid indicating the previous biopsy site. Biopsy showed diffuse pagetoid spread in the epithelium. (D) Diffuse SGC. Atypical vacuolated cells are evident in the epidermis (H&E, ×400).
SGC can also present a challenge to the histopathologist. This is a rare tumour that may show a nodular or diffuse growth pattern. Previous studies have reported an incorrect initial histological diagnosis in 40–75% of patients.1,8,12,14 Misdiagnosis is more common when sections are interpreted by pathologists inexperienced in tumours occurring in the ocular area rather than an ophthalmic pathologist.11,12 In addition, failure to identify pagetoid spread of tumour cells in the epithelium may also result in incorrect reporting of excision margins.15
The presence of intracytoplasmic lipid in SGC by fat stains such as Oil Red O and Sudan IV may aid the diagnosis of SGC, distinguishing it from other poorly differentiated carcinomas. These stains require fresh or unprocessed tissue as lipid is dissolved during standard processing. However, even short periods of formalin fixation may result in less intense staining with conventional lipid stains.16 The ability to undertake conventional lipid stains depends on there being sufficient tissue for frozen sections in addition to paraffin‐wax sections, which is not always possible with small incisional biopsy specimens.
Recently, antibodies that recognise proteins associated with lipid droplets have been described, including adipophilin, perilipin and TIP47/PP17. Adipophilin is present in milk fat globule membranes and on the surface of lipid droplets in various normal cell types, including the zona fasiculata of the adrenal, Sertoli cells and the glandular breast tissue in lactation.17 Adipophilin is also present in hepatocytes in alcoholic steatosis.17 The perilipins are a family of phosphoproteins found on the surface of intracellular lipid droplets and in the adrenal gland, Leydig cells and both brown and white fat.18 TIP47/PP17 is a cargo protein associated with the trafficking of the mannose‐6‐phosphate receptor between endosomes and the Golgi apparatus.19 TIP47/PP17 has been seen in human milk fat globule membranes, placenta, and has been investigated as an oncodevelopmental marker in cervical carcinoma.20 Our study aims to assess the ability of antibodies to these lipid‐associated proteins to identify lipid in SGC in formalin‐fixed paraffin‐wax‐embedded sections by immunocytochemistry.
Materials and methods
Tissue samples
Archival, formalin‐fixed, paraffin‐wax‐embedded tissue samples were retrieved from the ophthalmic pathology files of the Western Infirmary, Glasgow, UK. All samples were anonymised and full ethical approval in accordance with local policy was obtained for the use of these tissue samples. The samples included 26 SGC, 11 BCC, 10 SCC and 1 Merkel cell tumour.
The pathology was reviewed and samples of SGC were categorised according to their degree of differentiation (well, moderately or poorly differentiated) and predominant growth pattern (nodular or diffuse). Wherever available, Oil Red O sections were also reviewed.
Immunohistochemistry
Table 1 shows the information on the three antibodies used in this study.
Table 1 Anti‐adipocytic antibodies.
| Antibody | Antigen | Dilution | Source |
|---|---|---|---|
| Mouse Monoclonal | Adipophilin | 1:5 | Progen, Heidelberg, GermanyBiotechnik GmbH, Heidelberg, Germany |
| Guinea‐pig polyclonal | Perilipin | 1:100 | Progen |
| Guinea‐pig polyclonal | TIP47/PP17 | 1:50 | Progen |
Immunohistochemical staining was carried out on 3 μm sections that were immunostained using the DakoCytomation Chemate Envision system (DAKO, Ely, Cambridge, UK). Briefly, sections were washed with Tris‐buffered saline containing Tween buffer (tris‐buffered saline (TBS)/TB), then blocked with normal goat serum (1:20) for 20 min. Sections were then incubated with primary antibody for 30 min, followed by a further wash in TBS or TB. After this, the sections were incubated with Dako Envision for 30 min. After a final wash in TBS or TB, colour was developed with diaminobenzidene for 10 min and sections were counterstained with haematoxylin. For antigen retrieval, slides were microwaved under pressure in 1 mmol/l ethylenediamine tetra‐acetic acid, pH 8.0 for 5 min. Appropriate control material (according to manufacturer's instructions) was used for each run. For negative controls, the primary antibody was omitted.
Interpretation
The slides were evaluated by light microscopy and the presence and location of positive staining for each antibody was recorded. Staining that was weak or difficult to interpret was regarded as equivocal.
Statistical analysis
The sensitivity and specificity were calculated for each antibody and for Oil red‐O staining, where available, as previously described.21 Sensitivity was calculated as positive SGC/negative SGC+positive SGC. Specificity was calculated as negative non‐SGC/positive non‐SGC+negative non‐SGC. Equivocal results were not included in the calculations.
Results
Table 2 summarises the results.
Table 2 Staining of eyelid tumours for Oil Red O and anti‐adipocytic antibodies.
| Tumour type | Oil Red‐O | McAb adipophilin | PcAb perilipin | PcAb TIP47/PP17 |
|---|---|---|---|---|
| SGC | ||||
| WD, N | 2/2 | 2/2 | 0/2 | 1/2 |
| MD, N | 1/5 | 4/6 | 5/6 | 1/6 |
| MD, D | 1/6 | 7/7 | 2/7 | 0/7 |
| PD, N | 1/4 | 5/5 | 2/5 | 0/5 |
| PD, D | 3/5 | 5/6 | 1/6 | 0/6 |
| All | 8/22 | 23/26 | 10/26 | 2/26 |
| SCC | N/A | 1/10 | 0/10 | 0/10 |
| BCC | N/A | 4/11 | 0/11 | 0/11 |
| MCC | N/A | 0/1 | 0/1 | 0/1 |
BCC, basal cell carcinoma; D, diffuse growth pattern; McAb, monoclonal antibody; MCC, Merkel cell tumour; MD, moderately differentiated; N, nodular growth pattern; PcAb, polyclonal antibody; PD, poorly differentiated; SCC, squamous cell carcinoma; SGC, sebaceous gland carcinoma; WD, well differentiated.
Histological review of SGC
The diagnosis of SGC was confirmed in all 26 samples. Of these, 11 samples were poorly differentiated, 13 moderately differentiated and 2 well differentiated. The growth pattern was nodular in 13 samples and diffuse in 13 samples with Pagetoid involvement of the epithelium (fig 1C,D).
Oil Red O staining was available in 22 samples and was positive in 8 (4 poorly, 2 moderately and 2 well differentiated). Oil red O staining was not carried out on the other eyelid tumours.
Adipophilin
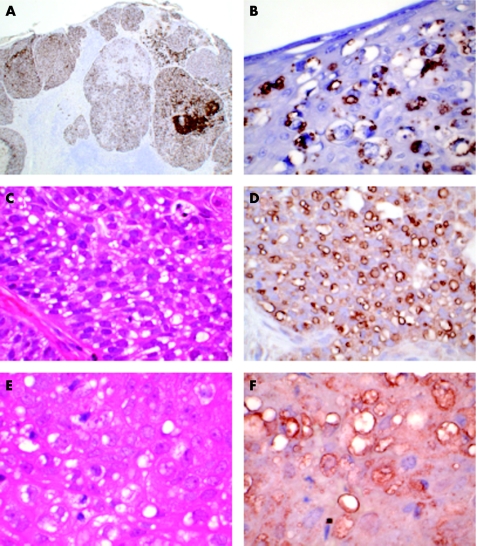
Moderate to strong positive immunostaining for adipophilin was identified in 23 of the 26 samples of SGC and seemed to stain the membrane of intracytoplasmic vesicles considered to represent lipid droplets (fig 2A,B). The remaining three samples were negative. These consisted of two moderately differentiated nodular SGC and poorly differentiated diffuse SGC. Staining for Oil Red O was positive in one of these three samples.
Figure 2 Immunohistochemical staining for adipophilin, perilipin and TIP47/PP17 in sebaceous gland carcinoma (SGC). (A) Immunohistochemical staining for adipophilin of SGC shown in fig 1B. There is widespread strong positivity, with numerous cytoplasmic vacuoles (anti‐adipophilin, ×100). (B) Immunohistochemical staining for adipophilin of SGC shown in fig 1D. There is strong positivity staining of vacuoles highlighting the Pagetoid infiltration of the epidermis (anti‐adipophilin, ×400). (C) Moderately differentiated nodular SGC showing numerous intracytoplasmic vacuoles (haematoxylin and eosin (H&E), ×400). (D) Immunohistochemical staining for perilipin outlines numerous small lipid vacuoles within the tumour cells (anti‐perilipin, ×400). (E) Moderately differentiated nodular SGC showing numerous intracytoplasmic vacuoles (H&E, ×400). (F) Immunohistochemical staining for TIP47/PP17 outlines numerous small lipid vacuoles in the tumour cells (anti‐TIP47/PP17, ×400).
Positive immunostaining for adipophilin was seen in four BCCs and one SCC. The pattern of staining, however, differed from that seen in SGC, taking the form of focal (often perinuclear) intracytoplasmic granules. The remaining 17 samples (7 BCC, 9 SCC and 1 Merkel cell tumour) were negative.
Therefore, immunostaining for adipophilin has a sensitivity of 88.5% and a specificity of 77%.
Perilipin
Moderate to strong positive immunostaining for perilipin was identified in 10 of the 26 samples of SGC (7 moderately differentiated—5 nodular and 2 diffuse; and 3 poorly differentiated—2 nodular and 1 diffuse), again staining the membrane of intracytoplasmic vesicles (fig 2C,D). Four samples showed weak staining that was interpreted as equivocal. The remaining 12 samples were negative.
The other eyelid tumours were all negative for anti‐perilipin immunostaining. Thus, the specificity of perilipin was 100%, although the sensitivity was only 45.5%.
TIP47/PP17
Moderate to strong positive immunostaining was evident in two samples of nodular SGC (1 well differentiated and 1 moderately differentiated). Staining was in the form of intracytoplasmic vesicles as with adipophilin and perilipin (fig 2E,F). Equivocal staining was observed in two further samples of nodular SGC. Staining was negative in the other 22 samples of SGC. All non‐SGC were negative with this antibody.
Therefore, anti‐TIP47/PP17 had a sensitivity of 8.3%, but a specificity of 100% (95% confidence interval 83.9% to 100%).
Anti‐adipocytic antibodies as an immunohistochemical panel
Anti‐perilipin was positive in two samples that were negative for anti‐adipophilin.
Thus, in combination, anti‐adipophilin and anti‐perilipin have a sensitivity of 96%. The two samples that were anti‐TIP47/PP17 positive were also anti‐adipophilin positive. Nine samples of SGC showed positive immunostaining with more than one antibody (7 samples with anti‐adipophilin and anti‐perilipin; 2 samples with anti‐adipophilin and anti‐TIP47). One sample stained with all three antibodies. Staining with more than one antibody occurred in both moderately and poorly differentiated tumours and in nodular and diffuse tumours.
Discussion
The morphological diagnosis of SGC is supported by the presence of intracytoplasmic lipid in the tumour cells by fat stains such as Sudan IV or Oil Red O. These histochemical stains require unprocessed tissues because the graded alcohols used in processing dissolve tissue lipid. However, there exist inherent limitations to the use of unprocessed tissue for this purpose. There may be no clinical suspicion of SGC at the time of biopsy such that unprocessed tissue is not available when the diagnosis is suspected on histological examination, or the biopsy may be a small incisional biopsy. Even if residual formalin‐fixed tissue is available, it is well recognised that even though formalin fixation does not dissolve tissue lipids short periods of formalin, fixation can reduce the intensity of Oil Red O staining considerably. In this study, we have shown that anti‐adipocytic antibodies can identify lipid vesicles in the cytoplasm of the cells of SGC. The three antibodies used in this study identify proteins associated with fat vesicles; the antigens, unlike the lipid itself, are preserved during tissue processing. Immunohistochemistry for two of these fat‐associated proteins was superior to Oil Red O staining of frozen sections and the sensitivity was improved by using both anti‐adipophilin and anti‐perilipin immunostaining. Oil red O staining was positive in only 36% (8/22) of SGC, whereas staining for adipophilin and perilipin was positive in 88% (23/26) and 38%(10/26), respectively. Staining for either adipophilin or perilipin was positive in 96% (25/26) of cases. The third antibody, TIP47/PP17, was less sensitive than Oil Red O, with positive staining in only 8% (2/26) of SGC. In addition to confirming the diagnosis of SGC, anti‐adipophilin highlighted the intraepithelial component of SGC with Pagetoid spread and may have a useful role for confirming excision margins. No association of positive staining with degree of differentiation or pattern of spread was found. Similar to Oil Red O, these antibodies showed positive staining in other lipid‐containing cells including normal sebaceous units such that the entire histopathological picture is required to make a diagnosis of SGC.
Histologically, SGC must be distinguished from other more common eyelid tumours including poorly differentiated SCC and BCC.22 BCC with sebaceous differentiation can present particular diagnostic difficulties. It is worth noting, however, that these tumours are extremely rare and that this diagnosis must be made with extreme caution in the eyelid, where SGC is more likely.22 Merkel cell tumour, an uncommon and aggressive tumour, should also be considered in the differential diagnosis.23 In this study, there was focal positive immunostaining for adipophilin in four BCC and one SCC. The staining, however, was weak and focal, with small intracytoplasmic granules often in a perinuclear location. This staining was easily distinguishable from the widespread and moderate to strong positive staining seen in SGC. As the pattern of staining is different, this probably represents a cross reaction with some other antigen rather than the presence of a small amount of cytoplasmic lipid. It is surprising, however, that this occurred with anti‐adipophilin, the only monoclonal antibody in the study. No staining of the other eyelid tumours was observed with antibodies to perilipin or TIP47/PP17.
Several previous studies have used immunohistochemistry to differentiate SGC from BCC and SCC. SGC is usually positive with antibodies to epithelial membrane antigen (EMA)16 and androgen receptor,24 but negative for carcinoembryonic antigen (CEA).16 EMA may, however, be focal or negative in poorly differentiated tumours.24 Furthermore, some skin adnexal tumours may share this immunoprofile.25 SCC is usually positive with antibodies to CEA and negative for EMA. BCC is negative for EMA and CEA, but there may be focal positivity for androgen receptor in up to 60% of samples. Expression of CK7 is also helpful as this is positive in most cases of ocular SGC, but is usually negative in SCC and BCC.26,27
In conclusion, antibodies to adipophilin and perilipin that identify proteins associated with lipid vesicles are a useful ancillary technique for the identification of lipid in SGC of the eyelid. This immunohistochemical technique is applicable to paraffin wax sections. It is more sensitive and avoids the need for unprocessed tissue and frozen sections required for traditional histochemical stains such as Oil Red O. These antibodies may have an important role either alone or as an addition to an immunohistochemical panel for the diagnosis of SGC.
Take‐home messages
Identification of intracytoplasmic lipid is helpful in the diagnosis of sebaceous gland carcinoma.
Oil Red O and Sudan IV require fresh or unprocessed tissue and results may be less than optimal after short periods of formalin fixation.
Immunohistochemical staining for lipid‐associated proteins adipophilin and perilipin carried out on paraffin wax‐embedded sections is a more sensitive way of identifying intracytoplasmic lipid in sebaceous gland carcinoma than conventional histochemical stains.
Acknowledgements
We thank Mr Jim Ralston, Pathology Department, Western Infirmary, Glasgow, UK, and Mr David Murray, Pathology Department, Royal Infirmary, Glasgow, UK, for their technical expertise.
Abbreviations
BCC - basal cell carcinoma
CEA - carcinoembryonic antigen
EMA - epithelial membrane antigen
SCC - squamous cell carcinoma
SGC - sebaceous gland carcinoma
TBS - tris‐buffered saline
Footnotes
Competing interests: None.
References
- 1.Yeatts R P, Waller R R. Sebaceous carcinoma of the eyelid: pitfalls in diagnosis. Ophthal Plast Reconstr Surg 1985135–42. [DOI] [PubMed] [Google Scholar]
- 2.Kass L G, Hornblass A. Sebaceous carcinoma of the ocular adnexa. Surv Ophthalmol 198933477–490. [DOI] [PubMed] [Google Scholar]
- 3.Shields J A, Demirci H, Marr B P.et al Sebaceous carcinoma of the ocular region: a review. Surv Ophthalmol 200550103–122. [DOI] [PubMed] [Google Scholar]
- 4.Nelson B R, Hamlet K R, Gillard M.et al Sebaceous carcinoma. J Am Acad Dermatol 1995331–15. [DOI] [PubMed] [Google Scholar]
- 5.Rao N A, Hidayat A A, McLean I W.et al Sebaceous carcinomas of the ocular adnexa: a clinicopathologic study of 104 cases, with five‐year follow‐up data. Hum Pathol 198213113–122. [DOI] [PubMed] [Google Scholar]
- 6.Ni C, Searl S S, Kuo P K.et al Sebaceous cell carcinomas of the ocular adnexa. Int Ophthalmol Clin 19822223–61. [DOI] [PubMed] [Google Scholar]
- 7.Callahan E F, Appert D L, Roenigk R K.et al Sebaceous carcinoma of the eyelid: a review of 14 cases. Dermatol Surg 2004301164–1168. [DOI] [PubMed] [Google Scholar]
- 8.Zurcher M, Hintschich C R, Garner A.et al Sebaceous carcinoma of the eyelid: a clinicopathological study. Br J Ophthalmol 1998821049–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doxanas M T, Green W R. Sebaceous gland carcinoma. Review of 40 cases. Arch Ophthalmol 1984102245–249. [DOI] [PubMed] [Google Scholar]
- 10.Boniuk M, Zimmerman L E. Sebaceous carcinoma of the eyelid, eyebrow, caruncle, and orbit. Trans Am Acad Ophthalmol Otolaryngol 196872619–642. [PubMed] [Google Scholar]
- 11.Muqit M M, Roberts F, Lee W R.et al Improved survival rates in sebaceous carcinoma of the eyelid. Eye 20041849–53. [DOI] [PubMed] [Google Scholar]
- 12.Shields J A, Demirci H, Marr B P.et al Sebaceous carcinoma of the eyelids: personal experience with 60 cases. Ophthalmology 20041112151–2157. [DOI] [PubMed] [Google Scholar]
- 13.Snow S N, Larson P O, Lucarelli M J.et al Sebaceous carcinoma of the eyelids treated by Mohs micrographic surgery: report of nine cases with review of the literature. Dermatol Surg 200228623–631. [DOI] [PubMed] [Google Scholar]
- 14.Ni C, Kuo P K. Meibomian gland carcinoma: a clinicopathological study of 156 cases with long‐period follow‐up of 100 cases. Jpn J Ophthalmol 197923388–401. [Google Scholar]
- 15.Khan J A, Doane J F, Grove A S., Jr Sebaceous and meibomian carcinomas of the eyelid. Recognition, diagnosis, and management. Ophthal Plast Reconstr Surg 1991761–66. [DOI] [PubMed] [Google Scholar]
- 16.Johnson J S, Lee J A, Cotton D W.et al Dimorphic immunohistochemical staining in ocular sebaceous neoplasms: a useful diagnostic aid. Eye 199913104–108. [DOI] [PubMed] [Google Scholar]
- 17.Heid H W, Moll R, Schwetlick I.et al Adipophilin is a specific marker of lipid accumulation in diverse cell types and diseases. Cell Tissue Res 1998294309–321. [DOI] [PubMed] [Google Scholar]
- 18.Blanchette‐Mackie E J, Dwyer N K, Barber T.et al Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J Lipid Res 1995361211–1226. [PubMed] [Google Scholar]
- 19.Wolins N E, Rubin B, Brasaemle D L. TIP47 associates with lipid droplets. J Biol Chem 20012765101–5108. [DOI] [PubMed] [Google Scholar]
- 20.Than G N, Turoczy T, Sumegi B.et al Overexpression of placental tissue protein 17b/TIP47 in cervical dysplasias and cervical carcinoma. Anticancer Res 200121639–642. [PubMed] [Google Scholar]
- 21.Bland M.An introduction to medical statistics. 3rd edn. Oxford: Oxford University Press, 2004
- 22.Misago N, Suse T, Tetsuji U.et al Basal cell carcinoma with sebaceous differentiation. Am J Dermatopathol 200426298–303. [DOI] [PubMed] [Google Scholar]
- 23.Metz K A, Jacob M, Schmidt U.et al Merkel cell carcinoma of the eyelid: histological and immunohistochemical features with special respect to differential diagnosis. Graefes Arch Clin Exp Ophthalmol 1998236561–566. [DOI] [PubMed] [Google Scholar]
- 24.Bayer‐Garner I B, Givens V, Smoller B. Immunohistochemical staining for androgen receptors: a sensitive marker of sebaceous differentiation. Am J Dermatopathol 199921426–431. [DOI] [PubMed] [Google Scholar]
- 25.Ansai S, Hashimoto H, Aoki T.et al A histochemical and immunohistochemical study of extra‐ocular sebaceous carcinoma. Histopathology 199322127–133. [DOI] [PubMed] [Google Scholar]
- 26.Schirren C G, Stefani F H, Sander C A.et al Ocular sebaceous carcinoma and basal cell carcinoma show different profiles of cytokeratin intermediate filaments [abstract]. J Cutan Pathol 199724123 [Google Scholar]
- 27.Martinka M, Trotter M, White V. Cytokeratin 7; aid to differential diagnosis of ocular sebaceous carcinoma, squamous cell carcinoma and basal cell carcinoma [abstract]. J Cutan Pathol 200027564 [Google Scholar]