Abstract
Aim
To examine eosinophil infiltration and degranulation in 50 oesophageal biopsy specimens from 30 patients (21 men, 9 women; mean 39 years) with eosinophilic oesophagitis, by haematoxylin and eosin staining and immunohistochemistry.
Methods
Immunohistochemistry was carried out using a monoclonal antibody for human eosinophilic major basic protein (MBP). Eosinophils were counted in three high power fields (×40) and degranulation, as quantified by extracellular MBP immunostaining, was scored on a scale of 1–4. Morphological changes (basal cell hyperplasia, elongation of papillae and dilatation of intercellular spaces) were scored on a 1–4 scale on sections stained with haematoxylin and eosin.
Results
Numbers of intraepithelial eosinophils were significantly higher with MBP immunostaining than with haematoxylin and eosin staining (mean 109.6 v 80.6; p<0.001), whereas numbers of eosinophils were considerably correlated (r = 0.794). Eosinophil degranulation was higher in the distal oesophagus. Additionally, basic morphological changes were markedly associated with eosinophil infiltration. Extracellular deposition of eosinophil‐MBP and eosinophil infiltration in subepithelial connective tissue, present in the biopsy specimens, were detected by immunohistochemistry.
Conclusion
Numbers of eosinophils and degranulation are underestimated by haematoxylin and eosin staining. Immunohistochemistry detected up to two times more eosinophils than routine haematoxylin and eosin staining. Moreover, eosinophil‐MBP immunoreactivity in extracellular regions indicates the release of toxic eosinophil granule proteins and gives further evidence for a causative role of eosinophils with regard to structural changes in eosinophilic oesophagitis. Immunohistochemistry may serve as a useful diagnostic tool to support the morphological differential diagnosis of eosinophilic oesophagitis and gastro‐oesophageal reflux disease.
Accumulation and activation of eosinophils are considered to have an important role in allergic diseases such as allergic rhinitis, asthma, atopic dermatitis and primary biliary cirrhosis.1,2,3,4 Activated eosinophils induce epithelial damage by releasing cytotoxic eosinophil granule proteins such as major basic protein (MBP) and eosinophilic cationic protein (ECP).5,6,7 They are present in intestinal inflammatory disorders such as ulcerative colitis, Crohn's disease, coeliac disease and eosinophilic gastroenteritis.8,9,10,11,12
Eosinophilic oesophagitis is a rapidly increasing disease entity both in paediatric and adult gastroenterology.13,14 Prominent and diffuse oesophageal eosinophilia has been a feature of a few reported cases of eosinophilic oesophagitis, representing an unusual variant in the spectrum of eosinophilic gastroenteritis.15,16,17 In 1993, Attwood et al18 described oesophageal eosinophilia with dysphagia as a distinct clinicopathological syndrome. Today, eosinophilic oesophagitis has become an important differential diagnosis in children and in adults with oesophageal symptoms.19,20 In adults, it causes acute or intermittent solid‐food dysphagia accompanied by food impaction or oesophageal symptoms, with no response to antacid or antiacid secretory treatment. Disease onset mainly occurs between the second and fourth decades and is predominant in male subjects.14,21
The histologic hallmark of eosinophilic esophagitis, a rapidly increasing disease entity in paediatric and adult gastroenterology, is an intense eosinophil infiltration in oesophageal squamous epithelium with concomitant regenerative epithelial changes.18
Many endoscopic signs such as whitish pinpoint exudates or papules, granularity, loss of vascular pattern, linear furrow and fold pattern, rings, corrugation, linear sheering of mucosa after dilatation, focal stricture (often proximal oesophagus) and long‐segment stricture (narrow or small‐calibre oesophagus) were observed in eosinophilic oesophagitis.20 According to Croese et al,22 mucosal furrows are a sentinel endoscopic feature of eosinophilic oesophagitis. Straumann et al23 characterised the endoscopic manifestation of eosinophilic oesophagitis and found a loss of the capillary pattern in oesophageal mucosa and described the crepe‐paper mucosa as a rare and pathognomonic sign for eosinophilic oesophagitis.
The histological diagnostic hallmark of eosinophilic oesophagitis is an intense eosinophil infiltration in oesophageal squamous epithelium (>20 eosinophils per high power field (HPF) in the squamous epithelium).18 The important findings associated with eosinophil infiltration are basal‐zone hyperplasia, elongation of papillae, dilatation of intercellular spaces, eosinophil infiltration and fibrosis of lamina propria.
Deposition of eosinophil granule proteins and concomitant tissue damage have been documented in human eosinophilic inflammation.24 Eosinophil degranulation is difficult to detect by conventional haematoxylin and eosin staining. Therefore, we used a monoclonal antibody against MBP, the most abundant eosinophil granule protein, as a marker of eosinophil infiltration and degranulation, to detect the presence of cytotoxic MBP in oesophageal biopsy specimens in comparison with haematoxylin and eosin staining of specimens from adult patients with eosinophilic oesophagitis.
Materials and methods
Cases of eosinophilic oesophagitis were systematically identified from the pathology database of the Institute of Pathology, Klinikum Bayreuth, Bayreuth, Germany, from the first half of the year 2004. In all, 166 sections from 50 oesophageal biopsy specimens obtained from 30 adults (21 men and 9 women; mean age 39 years (range 19–85)) with eosinophilic oesophagitis were examined. The criteria were the presence of epithelial proliferative changes (basal‐zone hyperplasia and elongation of papillae) and a minimum of 20 eosinophils per HPF (×40) in the distal oesophagus. Furthermore, we examined 10 distal oesophageal biopsy specimens from a control group of 10 adults (8 men and 2 women; mean age 53 years (range 37–73)) with gastro‐oesophageal reflux disease (GERD). Furthermore, we examined 13 instead of 10 distal oeseophageal biopsies.
Tissue preparation and histochemistry
All oesophageal biopsy specimens were fixed in 4% buffered formalin, processed routinely and embedded in paraffin wax. Tissue sections (5 μm) were routinely stained with haematoxylin and eosin.
Immunolocalisation of eosinophils in oesophageal epithelium
Mouse monoclonal antibodies to human MBP (Cymbus Biotechnology Ltd, Hants, UK; clone BMK13; 1:200) were applied to consecutive tissue sections. Immunohistochemical studies were conducted with a streptavidin–biotin complex technique (Biogenex, Mainz, Germany) with alkaline phosphatase as the detection enzyme and 3‐hydroxy‐2‐naphthylacid 2,4‐demethylanilid as the colour substrate. The sections were pretreated with pronase (2 mg/ml in phosphate‐buffered saline, pH 7.3, for 30 min at room temperature).
Histomorphological analysis and immunohistochemistry
Sections of biopsy specimens stained with haematoxylin and eosin were examined according to the following criteria:
1. Thickness of basal zone of the squamous epithelium and papillary length (0, normal; +, lower; ++, middle; +++, upper third of squamous epithelium)
2. Dilatation of intercellular spaces (0, normal; +, lower; ++, middle; +++, upper third of squamous epithelium) and presence of perinuclear vacuoles
3. Presence of eosinophilic aggregates in superficial squamous epithelium
4. Number and distribution of intraepithelial eosinophils.
The distribution of eosinophils in the epithelium was classified as diffuse, focal or superficially attenuated. Accompanying lamina propria was not regularly sampled, but when present, eosinophil content and fibrosis were semiquantitatively estimated and noted. For each biopsy specimen, immunohistochemistry was carried out as described earlier. Afterwards, immunostained sections were examined again according to the following criteria:
1. Number of intraepithelial eosinophils in immunostained sections corresponding to sections stained with haematoxylin and eosin
2. Presence of eosinophil aggregates
3. Semiquantitative assessment of intraepithelial eosinophil degranulation (0/+, no or few extracellular granules; ++, moderate; +++, intense degranulation)
4. Semiquantitative analysis of eosinophil content and degranulation in the lamina propria, when present.
Twelve oesophageal biopsy specimens from nine patients with eosinophilic oesophagitis contained subepithelial tissue, permitting qualitative and semiquantitative analysis of connective tissue, eosinophil infiltration and degranulation. The remaining 48 biopsy specimens from 31 patients consisted exclusively of squamous epithelium.
Histological features were tabulated only for specimens in which three consecutive well‐oriented papillae could be identified. Intraepithelial eosinophils were counted in three HPF areas with the most dense eosinophilic infiltrates. Data were expressed as the maximal numer of eosinophils per HPF and mean number of eosinophils per three HPFs (Olympus BX50, UPlanFl 40×, ocular magnification ×10; area of the microscopic field 0.55 mm2).
Statistical analysis
Data were statistically analysed with SPSS V.11. Wilcoxon's signed‐rank test and Kruskall–Wallis test were used to prove differences in ordinal variables and the χ2 test for those in proportions. Correlation coefficients were calculated in accordance with Spearman (interval data) and Kendall (interval and ordinal data). All tests were two tailed and significance was determined at α = 0.05 (p⩽0.05).
Results
Comparison of eosinophil infiltration by haematoxylin and eosin staining and by MBP immunostaining
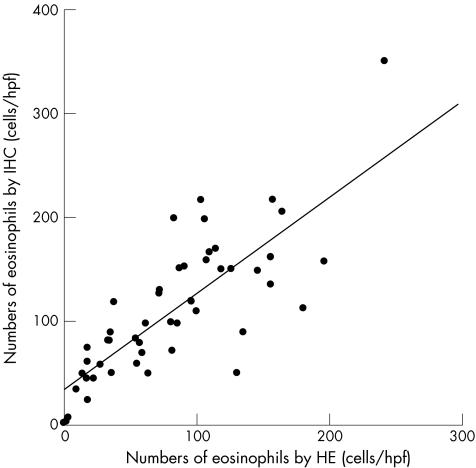
The study included 50 oesophageal biopsy specimens obtained from 30 patients (21 men and 9 women; mean age, 39 years (range 19–85)) with eosinophilic oesophagitis. Density of the eosinophil tissue infiltration was higher by immunohistochemical staining than by haematoxylin and eosin staining, independent of localisation. Especially in the distal oesophagus, the number of eosinophils was distinctly enhanced by immunostaining (p<0.001) when compared with counterparts that were routinely stained with haematoxylin and eosin (fig 1A,B). Additionally, a marked correlation of eosinophil infiltrates in sections stained with haematoxylin and eosin with those in immunohistochemical stained sections was observed (p<0.05; r = 0.794; fig 2). Table 1 summarises the results.
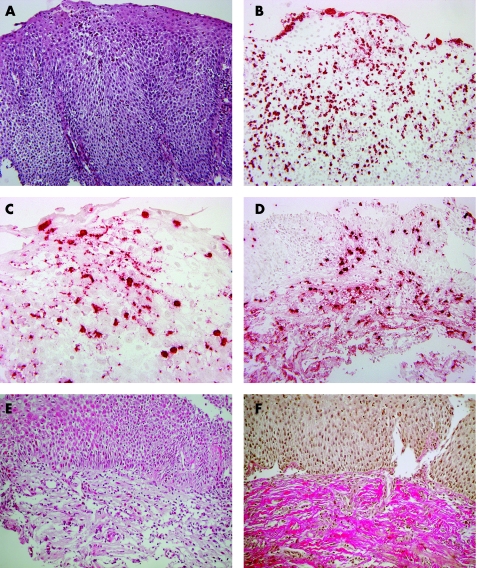
Figure 1 Oesophageal mucosa in eosinophilic oesophagitis. (A) Haematoxylin and eosin staining, magnification ×200. (B) Immunohistochemistry with major basic protein antibody, magnification ×200. (C) Degranulation of eosinophils, immunohistochemistry, magnification ×400. (D) Eosinophil infiltrate in the lamina propria of oesophageal mucosa, immunohistochemistry, magnification ×100. (E) Eosinophil infiltrate and fibrosis of lamina propria, haematoxylin and eosin, magnification ×100. (F) Fibrosis of lamina propria, Elastica van Gieson, magnification ×100.
Figure 2 The relationship between the numbers of eosinophils (cells/high power fields(HPF)) in oesophageal epithelium by haematoxylin and eosin (HE) staining and by immunohistochemical (IHC) staining.
Table 1 Numbers of eosinophils (cells/high power fields) in oesophageal epithelium.
| Staining (n) | Haematoxylin and eosin staining mean (range) | Immunohistochemistry mean (range) | p Value (Wilcoxon's signed‐rank test) | |
|---|---|---|---|---|
| Distal oesophagus (24) | 91.5 (18–244) | 125.7 (57–350) | 0.000** | |
| Middle oesophagus (9) | 62.1 (3–182) | 80.3 (6–159) | 0.161 | |
| Proximal oesophagus (9) | 63.3 (0–157) | 71.2 (1–153) | 0.260 | |
| Oesophagus* (8) | 88 (54–131) | 133.7(1–350) | 0.093 | |
| Total (50) | 80.6 (0–244) | 109.6 (1–350) | 0.000** | |
| GERD*** (13) | 27.5 (2–80) | 35.6 (4–109) | 0.173 | |
GERD, gastro‐oesophageal reflux disease.
*Not otherwise specified localisation.
**Statistically
***Only distal oesophagus.
Detection of eosinophil MBP immunoreactivity in extracellular regions indicated the release of eosinophil contents not visible in routine haematoxylin and eosin staining (fig 1C). Only 2 (4%) biopsy specimens showed intense degranulation (+++), 12 (24%) showed moderate degranulation (++), 22 (44%) showed few and 14 (28%) showed no extracellular granules (table 2). Biopsy specimens from patients with GERD showed no degranulation.
Table 2 Degranulation of eosinophils in oesophageal squamous epithelium.
| Degranulation (n) | ++++++ | ++++ | ++ | O | |
|---|---|---|---|---|---|
| Distal oesophagus (24) | – | 8 | 13 | 3 | |
| Middle oesophagus (9) | – | 2 | 2 | 5 | |
| Proximal oesophagus (9) | 1 | 1 | 2 | 5 | |
| Oesophagus* (8) | 1 | 1 | 5 | 1 | |
| Total (50) | 2 | 12 | 22 | 14 |
+++, >50% eosinophils with degranulation; ++, <50% eosinophils with degranulation; +, <10% eosinophils with degranulation; O, no degranulation.
*Not otherwise specified localisation.
Distribution of eosinophils was similar in both stainings and showed a diffuse pattern in 40 (80%), apical emphasis in 2 (4%), and focally accentuated or diffuse pattern in 8 (16%) of 50 biopsy specimens. Eosinophil aggregates in the superficial epithelium were seen in haematoxylin and eosin staining as well as in immunostaining. Eosinophil aggregates were seen in 18 (36%) biopsy specimens, whereas 32 (64%) had no aggregates.
Correlation of morphological changes with eosinophil infiltration in oesophageal squamous epithelium
Basal‐cell hyperplasia and elongation of papillae showed strong (+++) increase in 29 (58%) and moderate (++) increase in 15 (30%) biopsy specimens. Few biopsy specimens (5 (10%)) had minor or no (1 (2%)) basal‐cell hyperplasia. Dilatation of intercellular spaces was intense in 33 (66%), moderate in 10 (20%) and slight in 7 (14%) biopsy specimens. Vacuolisation of epithelial cells was noted in 28 (56%) biopsy specimens; 22 (44%) had no perinuclear vacuoles. Basal‐cell hyperplasia, elongation of papillae, dilatation of intercellular spaces and vacuolisation of epithelial cells were more extensively, but not markedly, observed in the distal oesophagus. Additionally, significant correlation of morphological changes with the number of eosinophils detected with MBP immunostaining or haematoxylin and eosin staining was observed (p<0.05). Basal‐cell hyperplasia, papillary height and dilatation of intercellular spaces correlated significantly with eosinophil degranulation (p<0.05).
Correlation of morphological changes and eosinophil infiltration in oesophageal lamina propria
Subepithelial tissue was histologically and qualitatively analysed in 12 biopsy specimens from nine patients; the remaining 48 biopsy specimens contained exclusively epithelial structures, as biopsy samples contained almost exclusively squamous epithelium owing to the use of standard forceps in the biopsy procedure. Besides an increase in fibrous tissue in all 12 biopsy specimens, 6 specimens showed marked (+++) eosinophil infiltration and interstitial deposition of eosinophil MBP (fig 1E) and 4 specimens showed increase in fibrosis without detectable eosinophils (fig 1F).
Discussion
The oesophagus is normally devoid of eosinophils, and therefore the finding of eosinophils in oesophageal squamous epithelium denotes pathology.20,25,26 Eosinophils in the oesophagus are observed in GERD, drug reactions and eosinophilic oesophagitis. Eosinophil infiltration with >20 eosinophils per HPF, as judged by haematoxylin and eosin staining, is considered to be a histomorphological threshold of eosinophilic oesophagitis. Nevertheless, precise criteria or a specific cut‐off point for the diagnosis of eosinophilic oesophagitis have not been established.19,20 Eosinophils morphologically resembling lymphocytes and mast cells will not be counted as eosinophils in conventional histology. Moreover, it is almost impossible to identify degranulated eosinophils with haematoxylin and eosin staining.27
In our study, the density of eosinophils in immunostained samples was considerably higher when compared with those stained with standard haematoxylin and eosin. Immunostaining detected up to two times more eosinophils than routine haematoxylin and eosin staining. Increased numbers of eosinophils and degranulated eosinophils in the oesophageal mucosa were underestimated in conventional haematoxylin and eosin staining. Perhaps immunohistochemistry helps to discover “minimal‐change” eosinophilic oesophagitis and to distinguish eosinophilic oesophagitis from GERD. In addition, the distribution and density of eosinophils in the oesophageal mucosa were inhomogeneous and more intense in the distal than in the proximal oesophagus. This phenomenon is contradictory to the understanding of eosinophilic oesophagitis based on immunoallergic mechanisms affecting the whole oesophagus. A possible explanation is a shorter passage time for food (antigens) in the proximal oesophagus in association with delayed passage in the distal oesophagus. Straumann et al23 reported an inhomogeneous distribution of eosinophils in the oesophagus. Possibly, eosinophil infiltration fluctuates over time, or the biopsies from the proximal oesophagus in our study were taken from normal mucosal regions. Nevertheless, the reason for the inhomogeneous distribution pattern of eosinophils in eosinophilic oesophagitis remains unresolved.
Besides dense eosinophil infiltration of oesophageal squamous epithelium, basal‐cell hyperplasia, elongation of papillae and dilatation of intercellular spaces are the main features of eosinophilic oesophagitis (fig 1A). It is tempting to speculate that the epithelial changes in eosinophilic oesophagitis are directly linked to eosinophilic infiltration and degranulation. We found that epithelial proliferative changes were more extensive in the distal oesophagus and correlated markedly with degranulation and the number of eosinophils in both MBP immunostaining and routine haematoxylin and eosin staining. A causative role of eosinophilia in promoting epithelial proliferation is supported in Mishra et al's28 animal studies on interleukin (IL)‐5‐deficient mice. In a long‐term study on eosinophilic oesophagitis in adult patients, Straumann et al21,29 reported that the chronic inflammation in eosinophilic oesophagitis may lead to irreversible structural changes, with a concomitant risk of impairment of function and persistent dysphagia. In our study, analysis of subepithelial tissue in 12 biopsy specimens from nine patients showed an increase in fibrous tissue (fig 1F), and interstitial deposition of eosinophil‐MBP was found in six biopsy specimens (fig 1E). These results may be convenient to the hypothesis on the activation of eosinophils as causative agents of lamina propria fibrosis and consecutive structural and functional changes.3,6,30,31,32,33,34
The underlying mechanism that causes eosinophils to infiltrate the oesophageal squamous epithelium in eosinophilic oesophagitis is unknown. Mishra et al35 showed that the T helper (Th)2 cytokine, IL‐5, that is required for pulmonary eosinophilia, is also necessary and sufficient for the induction of eosinophil trafficking to the oesophagus.35 Recent investigations suggest that aeroallergens may contribute to the pathogenesis of oesophageal inflammation in a subset of patients with primary eosinophilic oesophagitis and GERDs.28
Intraepithelial eosinophils are a marker for GERD too.36,37 As these patients show an adequate clinical response to medical management of GERD, they should be differentiated from patients with eosinophilic oesophagitis. Moreover, diffuse and intense infiltration of oesophageal epithelium by eosinophils, eosinophil aggregates, and extensive concomitant morphological and structural changes such as in eosinophilic oesophagitis are unusual in connection with gastro‐oesophageal reflux alone. In a small series of 10 biopsy specimens obtained from patients with GERD, eosinophils were present, but degranulation of eosinophils as noted with MBP immunostaining in eosinophilic oesophagitis could not be found. On the basis of this observation, it is tempting to speculate that eosinophils act as effector cells in eosinophilic oesophagitis and have a regulatory character in GERD. Perhaps, degranulation serves to distinguish eosinophilic oesophagitis and reflux oesophagitis independently of the imprecise criteria currently in use.
Although the density of eosinophil infiltration and degranulation is underestimated in routine HE [haematoxylin and eosin] stains, the latter is sufficient to establish a diagnosis of EE [eosinophilic oesophagitis] in routine process. Perhaps, degranulation might help to distinguish EE from reflux oesophagitis independently of the imprecise criteria used now.
In conclusion, our results indicate that the density of eosinophil infiltration and degranulation in biopsy specimens from patients with eosinophilic oesophagitis are underestimated in conventional haematoxylin and eosin staining. Immunohistochemistry detected up to two times more eosinophils than routine haematoxylin and eosin staining and can perhaps be used to discover minimal‐change eosinophilic oesophagitis. In addition, distribution and density of eosinophils in the oesophageal mucosa was inhomogeneous and different in the proximal and the distal oesophagus. This would be contradictory to an immunoallergic mechanism affecting the whole oesophagus in eosinophilic oesophagitis. A marked correlation of eosinophil infiltration and degranulation with morphological and structural abnormalities of the oesophageal mucosa in eosinophilic oesophagitis suggests that in eosinophilic oesophagitis the structural abnormalities are likely to be promoted by eosinophils. Although it is not surprising that MBP immunostains identify noticeably higher numbers of eosinophils than routine haematoxylin and eosin stains, degranulation gives evidence of the eosinophils' character of effector cells in eosinophilic oesophagitis and may help to distinguish eosinophilic oesophagitis from GERD.
Abbreviations
ECP - eosinophilic cationic protein
GERD - gastro‐oesophageal reflux disease
HPF - high power field
MBP - major basic protein
Footnotes
Competing interests: None.
References
- 1.Peters M S, Schroeter A L, Kephart G M.et al Localization of eosinophil granule major basic protein in lung tissues of patients with bronchial asthma. Lancet 1983211–16. [DOI] [PubMed] [Google Scholar]
- 2.Alam R, Busse W W. The eosinophil—quo vadis? J Allergy Clin Immunol 200411338–42. [DOI] [PubMed] [Google Scholar]
- 3.Birkland T P, Cheavens M D, Pincus S H. Human eosinophils stimulate DNA synthesis and matrix production in dermal fibroblasts. Arch Dermatol Res 1994286312–318. [DOI] [PubMed] [Google Scholar]
- 4.Yamazaki K, Nakadate I, Suzuki K. Eosinophilia in primary biliary cirrhosis. Am J Gastroenterol 199691516–522. [PubMed] [Google Scholar]
- 5.Gleich G J, Frigas E, Loegering D A. Cytotoxic properties of the eosinophil major basic protein. J Immunol 19791232925–2927. [PubMed] [Google Scholar]
- 6.Gleich G J, Glitz D G, Abu‐Ghazaleh R I. Eosinophil granule proteins: structure and function. In: Gleich GKA, ed. Eosinophils in allergy and inflammation. New York: Marcel Dekker, 19941–20.
- 7.Gleich G J, Kita H, Adolphson C R. Eosinophils. In: Frank MMAK, Claman HN, Unanue ER, eds. Samter's immunologic diseases. Boston: Little, Brown, 1995205–245.
- 8.Talley N J, Kephart G M, McGovern T W.et al Deposition of eosinophil granule major basic protein in eosinophilic gastroenteritis and coeliac disease. Gastroenterology 1992103137–145. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho A T, Elia C C, de Souza H S.et al Immunohistochemical study of intestinal eosinophils in inflammatory bowel disease. J Clin Gastroenterol 200336120–125. [DOI] [PubMed] [Google Scholar]
- 10.Forbes E, Murase T, Yang M.et al Immunopathogenesis of experimental ulcerative colitis is mediated by eosinophil peroxidase. J Immunol 20041725664–5675. [DOI] [PubMed] [Google Scholar]
- 11.Bischoff S C, Mayer J, Nguyen Q T.et al Immunohistological assessment of intestinal eosinophil activation in patients with eosinophilic gastroenteritis and inflammatory bowel disease. Am J Gastroenterol 1999943521–3529. [DOI] [PubMed] [Google Scholar]
- 12.Rothenberg M E. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol 200411311–28. [DOI] [PubMed] [Google Scholar]
- 13.Bates B. ‘Explosion' of eosinophilic oesophagitis in children. Pediatr News 2000344 [Google Scholar]
- 14.Potter J W, Saelan K, Staff D.et al Eosinophilic oesophagitis in adults: an emerging problem with unique esophageal features. Gastrointest Endosc 200459355–361. [DOI] [PubMed] [Google Scholar]
- 15.Dobbins J W, Sheahan D G, Behar J. Eosinophilic gastroenteritis with esophageal involvement. Gastroenterology 1977721312–1316. [PubMed] [Google Scholar]
- 16.Landres R, Kuster G, Strum W. Eosinophilic oesophagitis in a patient with vigorous achalasia. Gastroenterology 1978741298–1301. [PubMed] [Google Scholar]
- 17.Lee R G. Marked eosinophilia in esophageal mucosal biopsies. Am J Surg Pathol 19859475–479. [DOI] [PubMed] [Google Scholar]
- 18.Attwood S E, Smyrk T C, Demeester T R.et al Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci 199338109–116. [DOI] [PubMed] [Google Scholar]
- 19.Walsh S V, Antonioli D A, Goldman H.et al Allergic oesophagitis in children. A clinicopathological entity. Am J Surg Pathol 199923390–396. [DOI] [PubMed] [Google Scholar]
- 20.Fox V L, Nurko S, Furuta G T. Eosinophilic oesophagitis: it's not just kid's stuff. Gastrointest Endosc 200256260–270. [DOI] [PubMed] [Google Scholar]
- 21.Straumann A, Spichtin H ‐ P, Grize L.et al Natural history of primary eosinophilic oesophagitis: a follow‐up of 30 adult patients for up to 11.5 years. Gastroenterology 20031251660–1669. [DOI] [PubMed] [Google Scholar]
- 22.Croese J, Fairley S K, Masson J W.et al Clinical and endoscopic features of eosinophilic oesophagitis in adults. Gastrointest Endosc 200358516–522. [DOI] [PubMed] [Google Scholar]
- 23.Straumann A, Spichtin H ‐ P, Bucher K A.et al Eosinophilic oesophagitis: red on microscopy, white on endoscopy. Digestion 200470109–116. [DOI] [PubMed] [Google Scholar]
- 24.Erjefalt J S. Allergen‐induced eosinophil cytolysis is a primary mechanism for granule protein release in human upper airways. Am J Respir Crit Care Med 1999160304–312. [DOI] [PubMed] [Google Scholar]
- 25.Kato M, Kephart G M, Talley N J.et al Eosinophil infiltration and degranulation in normal human tissue. Anat Rec 1998252418–425. [DOI] [PubMed] [Google Scholar]
- 26.Rothenberg M E, Mishra A, Collins M H.et al Pathogenesis and clinical features of eosinophilic oesophagitis. J Allergy Clin Immunol 2001108891–894. [DOI] [PubMed] [Google Scholar]
- 27.Levy A M, Yamazaki K, Van Keulen V P.et al Increased eosinophil infiltration and degranulation in colonic tissue from patients with collagenous colitis. Am J Gastroenterol 2001961522–1528. [DOI] [PubMed] [Google Scholar]
- 28.Mishra A, Hogan S P, Brandt E B.et al An etiological role for aeroallergens and eosinophils in experimental oesophagitis. J Clin Invest 200110783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Straumann A, Rossi L, Simon H ‐ U.et al Fragility of the esophageal mucosa: a pathognomonic endoscopic sign of primary eosinophilic oesophagitis? Gastrointest Endosc 200357407–412. [DOI] [PubMed] [Google Scholar]
- 30.Pincus S H, Ramesh K S, Wyler D J. Eosinophils stimulate fibroblast DNA synthesis. Blood 198770572–574. [PubMed] [Google Scholar]
- 31.Rochester C L, Ackerman S J, Zheng T.et al Eosinophil‐fibroblast interactions. Granule major basic protein interacts with IL‐1 and transforming growth factor‐ß in the stimulation of lung fibroblast IL‐6‐type cytokine production. J Immunol 19961564449–4456. [PubMed] [Google Scholar]
- 32.Sanderson C J. Interleukin‐5, eosinophils, and disease. Blood 1992793101–3109. [PubMed] [Google Scholar]
- 33.Xu X, Rivkind A, Pikarsky A.et al Mast cells and eosinophils have a potential profibrogenic role in crohn disease. Scand J Gastroenterol 200439440–447. [DOI] [PubMed] [Google Scholar]
- 34.Zagai U, Skold C M, Trulson A.et al The effect of eosinophils on collagen gel contraction and implications for tissue remodelling. Clin Exp Immunol 2004135427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mishra A, Hogan S P, Brandt E B.et al IL‐5 promotes eosinophil traffickig to the esophagus. J Immunol 20021682464–2469. [DOI] [PubMed] [Google Scholar]
- 36.Brown L F, Goldman H, Antonioli D A. Intraepithelial eosinophils in endoscopic biopsies of adults with reflux oesophagitis. Am J Surg Pathol 19848899–905. [DOI] [PubMed] [Google Scholar]
- 37.Cury E K, Schraibman V, Faintuch S. Eosinophilic infiltration of the esophagus: gastroesophageal reflux versus eosinophilic oesophagitis in children—discussion on daily practice. J Pediatr Surg 200439e4–e7. [DOI] [PubMed] [Google Scholar]