Abstract
The development of sensitive reagents and detection systems, together with the introduction of heat‐induced antigen retrieval, has rapidly entrenched immunohistology as an indispensable adjunct to routine histological examination, contributing to diagnosis, prognosis and treatment. New antibodies continue to be produced and new applications for “old” antibodies are described. The production of antibodies enabling the detection of genetic abnormalities, including mutations, gene amplifications and specific chromosomal translocations associated with novel chimeric proteins, promises to yield further insights into the genesis and behaviour of tumours. The ability to stain for target molecules that regulate tumour growth and proliferation is essential for selecting tumours for treatment with monoclonal antibodies. The mechanism of antigen retrieval remains debated. The absence of optimal controls continues to hinder standardisation of immunostaining and invalidates current attempts at quantification of immunostaining.
Compared with other technological developments in anatomical pathology, immunohistochemistry is one technique that has rapidly become established as an invaluable adjunct to morphological diagnosis. As recently as 1982, the Journal of Clinical Pathology published an article describing the use of monoclonal antibodies for the histopathological diagnosis of human malignancy.1 Today, most diagnostic laboratories routinely carry out immunostaining with consistent high‐quality results that contribute to diagnosis, prognosis and treatment. Although the terms immunohistochemistry and immunocytochemistry are often used interchangeably, they fail to emphasise the most important attribute of the technique. Immunohistochemistry is morphology based, and in many instances interpretation is morphology dependent. Hence, we advocated the term immunohistology as more appropriate.2
New antibodies
New antibodies continue to be produced and sold commercially almost on a daily basis, but their claimed sensitivity and specificity must be tested in the diagnostic arena before they are routinely used. Disclaimers that the antibody is “for research purposes only” continue to be included in the product inserts of distributors. With the exception of Cluster Designation (CD), the naming of antibodies continues to be chaotic. Antibodies are named with the initials of people, hybridoma numbers, genes (the nomenclature of genes is by no means systematic), proteins, functions and their combinations. Here, we review a selection of “new” antibodies and recount some “old” ones with new applications.
Rabbit monoclonal antibodies
The development of mouse monoclonal antibodies represented a major milestone in diagnostic immunohistology, as it provided unique properties of uniformity, purity and, importantly, infinite availability, so that new antibodies have continued to be generated for diagnostic application and, more recently, for therapeutic purposes. One major drawback to mouse hybridoma technology is the poor antibody response in mice to some immunogens. Although polyclonal antisera do not have many of the unique properties of monoclonal antibodies, they tend to exhibit higher affinity, as they are directed against different epitopes or against several amino acid sequences of an epitope in any given antigen. Rabbits are known to produce high titres of high‐affinity antibodies even with antigens that are not immunogenic to mice. Rabbit polyclonal antisera of greater sensitivity than monoclonal antibodies can be raised to short synthetic polypeptide sequences. Rabbits, for unknown reasons, also seem to generate higher‐affinity antibodies to human epitopes, so that, theoretically, rabbit monoclonal antibodies promise to combine the best attributes of both monoclonal antibodies and antisera. Initial attempts at mouse–rabbit heterohybridomas were not successful and it was only with the generation of the c‐myc/v‐abl transgenic rabbit that it became possible to produce rabbit plasmacytoma cell lines to create stable rabbit–rabbit hybridomas.
Several rabbit monoclonal antibodies are now available, including those to oestrogen receptor, progesterone receptor, cyclin D1, Ki‐67, CD3, CD5, CD23 and synaptophysin. One comparative study suggested that all these rabbit monoclonal antibodies show increased sensitivity, with no apparent loss of specificity compared with corresponding mouse monoclonal antibodies.3 Antigen retrieval did not seem to be required for the demonstration of the antigen with some antibodies, attesting to the robustness of the new rabbit antibodies. The availability of “universal” linking reagents is an added convenience when interchanging between mouse and rabbit antibodies.
Cyclin‐dependent kinase inhibitors
p16INK4a, p21WAF1, p27Kip1 and p57Kip2, effective in fixed paraffin‐wax‐embedded sections, are four of the many checkpoint proteins associated with the control of the cell cycle and apoptosis. Whereas Ki‐67 and other cell proliferation markers assess tumour proliferation and aggressiveness, these cyclin‐dependent kinase inhibitors provide insights into cell cycle arrest and may represent favourable prognostic indicators. Several studies on a variety of tumours have shown the loss of p57Kip2 expression to be associated with poor prognosis, and overexpression of p21WAF1 and p27Kip1 to be indices for favourable prognosis, but these results need further validation.
p57Kip2 has a more important role in the separation of complete molar pregnancy from diploid hydropic miscarriage and triploid partial mole. p57Kip2 is paternally imprinted (maternally expressed) and immunoexpressed in the normal placenta at frequencies of up to 100%. Nuclear staining is seen in villous cytotrophoblasts and implantation site interstitial trophoblasts, as well as in villous stromal cells and maternal decidual cells. The protein is present in partial mole but absent in complete mole. The absence in complete mole is a finding that is expected, given that the diploid complete mole lacks a copy of the maternal genome, whereas the partial mole has a haploid copy of the maternal genome and two haploid copies of the paternal genome.4 Cell proliferation markers are raised in complete moles.5 When combined with Ki‐67 indices, the following profiles permit distinction of the three entities considered in differential diagnosis—hydropic miscarriage: low Ki‐67 and high p57Kip2; partial mole: moderate to high Ki‐67 and high p57Kip2; and complete mole: high Ki‐67 and low p57Kip2 (unpublished data).
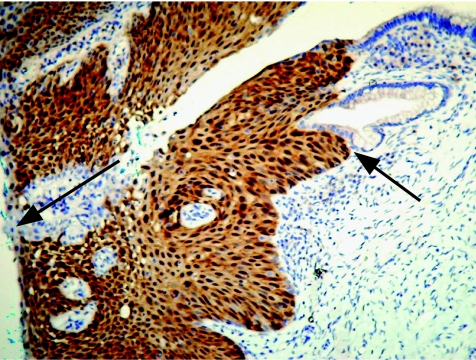
p16INK4a immunostaining is mostly used to identify high‐grade cervical squamous dysplasia (fig 1) and glandular atypia. High‐risk human papillomavirus (HPV) subtypes, particularly types HPV16, HPV18, HPV31 and HPV45, integrate with host DNA, overexpressing viral E6 and E7 genes. HPV E6 protein binds and degrades p53, and E7 protein inactivates the tumour suppressor retinoblastoma protein (pRb). As p16INK4a undergoes a negative feedback control by pRb, inactivation of pRb resulting from HPV infection or mutations may result in overexpression of p16INK4a. This has been shown by immunostaining in both tissue sections and cytological preparations of several cancerous tissues, including cervical squamous and glandular carcinomas and dysplasias6,7 and other lesions that may harbour integrated high‐risk HPV or have functional inactivation of the Rb gene. The antibody to p16INK4a shows nuclear and cytoplasmic localisation, with a sharp distinction between positive and negative cells.
Figure 1 Cervical intraepithelial neoplasia 2 stained for p16, showing both cytoplasmic and nuclear localisation of the antigen. Staining is crisp and clearly demarcated from epithelial and glandular cells that do not express the antigen (arrows).
p63
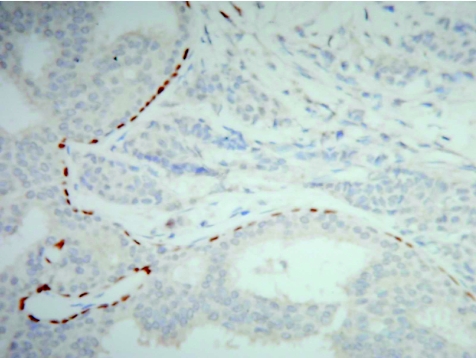
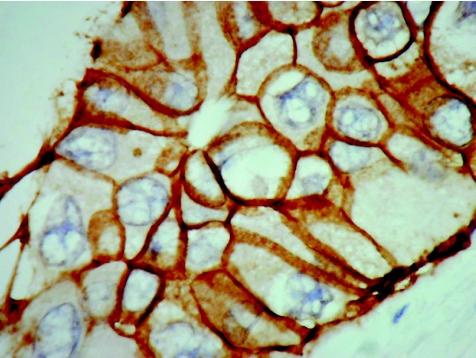
The p63 gene is a member of the p53 gene family that is necessary to maintain a stem cell epithelial population. Although it shows a striking homology with p53, it exerts few similar functions and does not seem to have a tumour suppressor function. p63 is essential for the development of epithelia of the limbs, craniofacial structures, breast and prostate. It is expressed in the basal cells of several epithelia, including the skin, uterine cervix, urogenital tract, prostate and breast, and is considered to be a possible marker of stem or reserve cells. The antigen is expressed in the nucleus and current applications relate mostly to the expression of basal cells in the prostate and breast, in the prostate to identify benign glands8 and in the breast to delineate myoepithelial cells in benign ductal proliferations (fig 2).9 The expression of p63 in almost 90% of metaplastic breast cancers compared with absence of the protein in invasive non‐metaplastic carcinomas and sarcomas was used to support derivation of such tumours from myoepithelial cells.10
Figure 2 Ductal carcinoma in situ of the breast showing a distinct layer of preserved myoepithelial cells identified by the nuclear immunoexpression of p63. No staining is seen in the adjacent invasive nests of carcinoma cells (right).
Other antigens related to cell cycle, apoptosis and proliferation
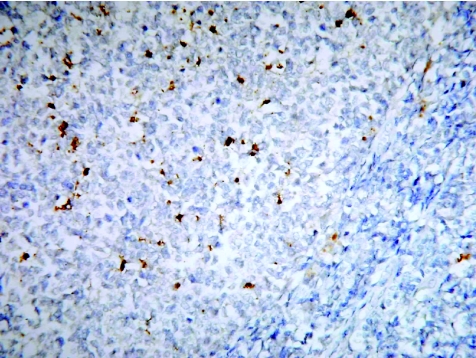
The interest in proteins related to proliferation and cell death, as both prognostic and potential therapeutic markers, has resulted in a plethora of antibodies to such proteins. Among these are the inflammation‐activated, nuclear factor (NF)κB‐regulated proteins, inducible nitric oxide synthase and cyclo‐oxygenase (COX)‐2. Nitric oxide, produced by inducible nitric oxide synthase, has been shown to inhibit apoptosis by inhibiting caspase activity,11 whereas COX‐2 inhibition prevents cell proliferation, promotes apoptosis and is a potential chemopreventive target.12 Changes in the expression of other apoptosis‐related proteins, such as death receptors (Fas) and Bcl‐2 family members, are also seen in a variety of tumours. The characterisation of apoptotic cells through the detection of fragmented DNA strand ends has largely been through the application of the terminal deoxynucleotidyl transferase‐mediated deoxyuridine triphosphate nick end labelling technique, which is prone to misinterpretation because necrotic cells also generate fragmented DNA. The activation of caspase by cleavage of procaspase is a hallmark of almost all apoptotic systems. Caspase 3 is a central effector caspase that mediates its own cleavage, that of other downstream caspases and caspase substrates; therefore, staining for cleaved caspase 3 is a sensitive and specific indicator of apoptosis (fig 3).13
Figure 3 Reactive germinal centre of a lymph node stained for caspase 3 to show apoptotic cells.
The detection of telomerase catalytic protein is of considerable prognostic interest because of the role of telomeres in the control of cell proliferation. The proliferative activity of normal somatic cells is limited by the gradual loss of tandem repeats at the ends of chromosomes, known as telomeres. Telomerase functions as a reverse transcriptase that can synthesise the telomeric ends of chromosomes at each cell division. In adults, telomerase is only active in the germ line, certain stem cells and activated lymphocytes, and is undetectable in differentiated cells, including most somatic cells. By contrast, telomerase activity has been found in as many as 85% of human cancers, including breast, lung, liver, pancreas, colorectal, laryngeal and prostate.14 Telomerase thus provides the cell with unlimited proliferative potential or immortality, but also leads to the accumulation of other genetic abnormalities, ultimately resulting in genomic instability and cell death. Despite the pivotal role of telomerase in cell proliferation, there seem to be few papers describing the immunohistological relevance of this marker in neoplastic disease. This may largely be due to the difficulty of demonstrating nuclear telomerase. In contrast, cytoplasmic activity is obtained with most antigen‐retrieval solutions, but this may represent an artefact or the nascent enzyme. The development of a new enhancing buffer for nuclear telomerase promises to provide more appropriate visualisation and localisation of telomerase.15
P504S/α‐methylacyl‐CoA racemase
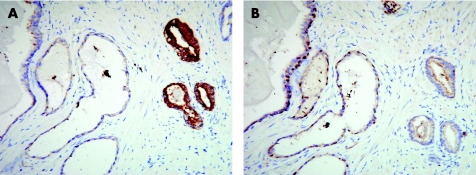
A mitochondrial and peroxisomal enzyme, α‐methylacyl‐CoA racemase (AMACR), is associated with the metabolism of branched‐chain fatty acid and bile acid intermediates. P504S mRNA is markedly raised in prostate cancer compared with benign prostate cancer, a feature that can also be shown by immunostaining. P504S/AMACR immunoreactivity was found in as many as 94% of carcinomas, enabling the identification of small foci of prostate carcinoma.16 More recent experience indicates that weak P504S/AMACR reactivity may be seen in benign prostate glands, high‐grade prostate intraepithelial neoplasia and atypical adenomatous hyperplasia. Diagnostic accuracy is increased by combining P504S immunostaining with the labelling of basal cells by antibodies to high‐molecular‐weight cytokeratin (34β‐E12, CK5 or CK6) or p63, both antigens are present in benign but not in malignant glands. The combination of these antibodies has been successfully used as a cocktail.17 P504S expression may be raised in several other cancers, including colorectal, lung and, less frequently, endometrial and breast cancer, and melanoma.18 In our experience, staining for basal cells alone can be difficult to interpret, especially in samples with diathermic artefact and when staining is discontinuous, so that it is helpful to use a basal cell marker in combination with P504S or in sequential sections (fig 4). The polyclonal P504S may produce some staining of benign glands, and the monoclonal antibody that has recently become available promises greater specificity.
Figure 4 Prostatic adenocarcinoma showing cytoplasmic staining in the neoplastic glands for P504S (α‐methylacyl‐CoA racemase) in contrast with the negative adjacent benign atrophic glands (A), which show a preserved basal cell layer with nuclear staining for p63 in the consecutive section (B). Basal cells are absent in the malignant glands.
D2‐40
D2‐40 is a monoclonal antibody raised against M2A, an O‐linked sialoglycoprotein expressed specifically on the endothelium of lymphatic vessels and as an oncofetal antigen in fetal germ cells. Until now, the antibodies CD31 and CD34 were used for labelling endothelial cells, but they did not discriminate between those of blood vessels and lymphatics.19,20 D2‐40 is sensitive and specific, and provides a clear distinction between lymphatic and blood vessel endothelium and their corresponding tumours. Interestingly, D2‐40 is consistently positive in Kaposi's sarcoma at all stages of presentation,21,22 suggesting that this tumour originates from a cell type with the potential of undergoing lymphatic differentiation. The antigen is also expressed in cerebellar haemangioblastoma, enabling a distinction from its morphological mimic, metastatic clear‐cell renal carcinoma. D2‐40 is also expressed in normal and reactive mesothelium and is reported to be a highly sensitive and specific marker of epithelioid mesothelioma.23 The ever‐increasing list of mesothelioma markers confirms the need for extended panels to identify this neoplasm because of the low sensitivity and specificity of the markers, especially for identifying the sarcomatoid subtype.
Podoplanin
Podoplanin is a 43‐kDa mucin‐type transmembrane glycoprotein that is localised to the podocytes of normal rat glomeruli and is specifically expressed by lymphatic, but not vascular, endothelial cells in culture. Podoplanin deficiency results in congenital lymphoedema and impaired vascular patterning. Podoplanin is expressed in lymphangiomas, Kaposi's sarcomas, in a subgroup of angiosarcomas with probable lymphatic differentiation24 and in epithelioid mesothelioma, paralleling the findings with D2‐40. Both markers did not label sarcomatoid mesothelioma and, interestingly, the epithelioid component of biphasic synovial sarcoma also stained for both antigens.23
CD22
The CD22 (BL‐CAM) antigen is a membrane glycoprotein of about 135 kDa and shows restricted expression on B lymphocytes, initially in the cytoplasm of developing B cells and subsequently on the surface of mature B cells, where it parallels surface immunoglobulin (Ig)D. It is displayed on cells of the follicle centre, mantle and marginal zones. It may be useful in distinguishing classic Hodgkin's lymphoma from diffuse large B cell lymphoma and nodular lymphocyte‐predominant Hodgkin's lymphoma, the last two of which express CD22.25 Hairy‐cell leukaemia and variants strongly express CD22. Anti‐CD22 treatment produced a high response rate in refractory hairy‐cell leukaemia, and trials on other B cell neoplasms are ongoing.26
C4d
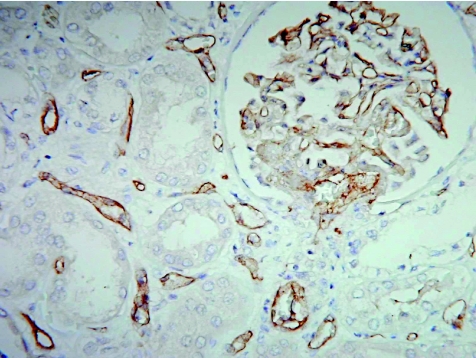
Complement C4d is the stable remnant of classic complement activation and its presence implicates humoral attacks on the endothelial cells that escape detection by other methods. Staining for C4d has thus become an important tool for identifying humoral rejection in the transplantation of the kidney, heart and liver. Circulating autoantibodies encounter the grafted endothelium as the first target. Bound antibodies and products of the classic complement cascade are rapidly eliminated from the endothelial cells. This is in contrast with deposits of antibody–immune complexes that are retained on inert basement membranes and connective tissues that are not directly exposed to blood. On activation, C4 and C3 form covalent bonds with target molecules to generate the C4d molecule, the split product of which can be stained on the peritubular capillary endothelium in acute (fig 5)27 and chronic renal allograft rejection.28 The use of a polyvalent antiserum to C4d enables staining in paraffin wax sections. Dendritic reticulum cells in tonsils serve well as controls, as they retain entrapped C4d.
Figure 5 C4d staining of peritubular capillaries and glomerular endothelial cells in acute humoral allograft rejection.
ZAP‐70
ZAP‐70 is a tyrosine kinase that participates in early B cell differentiation. In chronic lymphocytic leukaemia, expression of this marker has been shown to be a poor prognostic factor associated with a non‐mutated configuration of the IgVH genes.29 Although immunohistological assessment for ZAP‐70 can be readily carried out with commercially available antibody clones, several other lymphomas, including Burkitt's, mantle cell, marginal zone and diffuse large B cell lymphoma, may also express the marker.30 More importantly, normal and neoplastic T cells will show strong staining for ZAP‐70, so that it is necessary, especially in bone marrow sections, to distinguish staining of chronic lymphocytic leukaemia from benign and neoplastic T cells. Given the problems of weak staining, antigen instability, lack of a gold standard and absence of an acceptable method of reporting, which are associated with the flow cytometry assay of ZAP‐70, immunohistological expression is an alternative method of assessment.31
Miscellaneous antibodies
Several other antibodies are worth mentioning in some detail. New antibodies to infectious agents including parvovirus B19,32 herpes type 8 that is seen consistently in Kaposi's sarcoma,33 polyomavirus simian virus 40 associated with inflammatory kidney disease and specific tumours, such as mesothelioma and lymphoma, and some tumours in the brain and bone are now available.34
Galectin‐3 has been implicated in various physiological and pathological processes, including neoplastic transformation.35 Some authors have suggested that it may be a marker for malignant thyroid tumours of epithelial origin from thyroid adenomas and non‐neoplastic conditions36; however, this antigen may also be expressed in a large number of non‐neoplastic thyroid conditions.37 In this regard some “old” antibodies have found new roles in the distinction of thyroid neoplasms. CK19 has been used to identify papillary carcinoma and its variants, but weak staining may be observed in non‐neoplastic conditions.37,38 HBME1, a marker described for mesothelioma, may prove to be a useful discriminator of papillary and follicular thyroid carcinoma, being expressed in 60–70% of cases.39
Identifying genomic abnormalities
Immunohistochemistry has traditionally been used to demonstrate tissue proteins, largely as markers of a particular cell or tissue type. Increased specificity and sensitivity permits extension of the technique to the detection of some genetic abnormalities including genetic mutations, specific chromosomal translocations that result in novel chimeric proteins, gene amplification and molecular therapeutic targets for treatment. Gown40 coined the term “genogenic immunohistochemistry” for these applications. Several such genogenic or genomic applications are currently routinely practised, affording an effective substitute for the more expensive and time‐consuming genetic studies conventionally used for the same abnormalities.
Gene mutations
Mismatch repair gene proteins
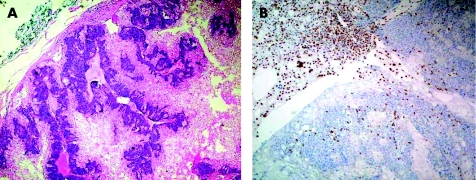
Immunohistology is conventionally used for the detection of cellular proteins. In gene mutations, it is used to show the loss of protein expression, reflecting the final pathway that results from mutation, promoter methylation or other genetic mechanisms. The demonstration of loss of mismatch repair gene proteins in colorectal carcinoma is one such example. Hereditary non‐polyposis colon cancer forms a small subset of colorectal cancers that do not arise from the multistep process of carcinogenesis in familial adenomatous polyps. Instead, they arise by a process of alterations in DNA mismatch repair genes, commonly hMLH1 and hMSH2, giving rise to the status referred to as microsatellite instability (MSI). Patients with MSI have colorectal cancers and other tumours, which can be recognised by their failure to express the mismatch repair gene proteins, such as MLH1 (fig 6) and MSH2, in contrast with normal nuclear immunoexpression in non‐tumorous epithelium and in inflammatory and stromal cells. More recently, the detection of MSI has been used as a prognostic marker in sporadic colorectal cancers. Between 12% and 15% of sporadic colorectal carcinomas showed MSI.41 A recent review of 32 eligible reported studies with survival statistics, comprising 7642 cases of colorectal carcinoma, including 1277 with MSI, suggested that those with MSI had significantly better prognosis than those with intact mismatch repair.41 MSI‐positive tumours also showed better response to fluoropyrimidine‐based chemotherapy.42 Antibodies sensitive to MSH6 and PMS2, other MSI gene proteins, are also commercially available and can be used in fixed paraffin‐wax‐embedded sections.
Figure 6 Sebaceous adenoma showing normal nuclear immunoexpression of MLH‐1 in inflammatory cells and stroma, but no staining in the epithelial cells of the tumour. MSH‐2 and MSH‐6 proteins were expressed normally in the tumour and connective tissue cells. This patient was later found to have endometrial carcinoma, which also showed no MLH‐1 in the tumour, and a family history of astrocytoma and colonic adenomatous polyps in her siblings.
E‐cadherin
The loss of E‐cadherin, a cell–cell adhesion protein,43 results in the characteristic single dyscohesive cell infiltration seen in some cancers, particularly lobular carcinoma of the breast and signet ring carcinoma of the stomach. Mutations in the E‐cadherin gene were shown in 56% of lobular carcinomas,44 but not in any other form of breast cancer. A variety of genetic alterations that result in genetic or epigenetic changes to the E‐cadherin gene result in a loss of protein expression in virtually all lobular cancers.45 Germ line mutation in the E‐cadherin gene is recognised in a familial form of diffuse gastric cancer that may be associated with lobular breast carcinoma.46 Variable and reduced staining of the extracellular domain of the protein is an independent prognostic factor in multivariate analysis, especially in patients with advanced gastric cancer.47 We have shown the loss of this protein at the invasive front of both ductal carcinoma of the breast48 and transitional cell carcinoma of the urinary bladder.49 Such losses occur with the acquisition of spindled morphology in epithelial tumour cells, signalling a loss of cell adhesion and acquisition of invasiveness.48
INI1 expression in malignant rhabdoid tumour and typical teratoid or rhabdoid tumour
Rhabdoid morphology, represented by large epithelioid cells with eosinophilic globular inclusions of intermediate filaments, eccentric vesicular nuclei and prominent nucleoli, is a phenotype that is seen in a variety of tumour types.50 The recent finding of somatic or germ line mutations and deletions in the INI1/hSNF5 tumour suppressor gene on chromosome 22q11.2 suggests that the loss of INI1/hSNF5 function, which is known to affect the actin cytoskeleton, may explain the rhabdoid appearance.51 The distinctive rhabdoid phenotype seems to arise through similar molecular mechanisms regardless of histogenesis, and INI1/hSNF5 is the first gene recognised to be associated with a characteristic cytomorphological cell type. Rhabdoid tumours represent both a specific entity and a secondary morphological phenotype of a variety of high‐grade tumour types that include melanoma, carcinomas, neuroblastomas, primitive neuroectodermal tumours (PNETs) and sarcomas.50 Two tumours that are encountered in childhood are generally accepted as true rhabdoid tumours—namely, malignant rhabdoid tumour (renal and extrarenal) and atypical teratoid or rhabdoid tumour of the central nervous system.52 Both types show a predilection for infants and young children, are highly aggressive, and display a polyphenotypic immunoprofile and characteristic deletions and mutations in the INI1/hSNF5 gene. The development of a commercial antibody to the INI1/BAF47 protein provides a method to detect such abnormalities in the gene through the loss of nuclear staining. It was recently shown to be the most useful ancillary technique for separating malignant rhabdoid tumour and atypical teratoid or rhabdoid tumours from other tumours with rhabdoid cytomorphology, with the loss of INI1 expression being found in the first two types of tumours.52
Chromosomal translocations
Some cancers are characterised by recurrent chromosomal translocations, often as the only karyotypic abnormality identified, suggesting that they are necessary for the oncogenic process. These chromosomal translocations result in gene fusions encoding novel chimeric proteins of diagnostic and prognostic relevance to which antibodies have been produced.
Anaplastic large‐cell lymphoma kinase protein (CD246)
Anaplastic large‐cell lymphoma kinase (ALK) protein is the best known of the chimeric proteins and is the result of the t(2;5) translocation in CD30+ anaplastic large‐cell lymphoma. ALK, which is normally not expressed, is important for diagnosis and is the most important favourable prognostic indicator for this form of lymphoma. The alteration entails the ALK locus on chromosome 2, most commonly as a translocation to the nucleophosmin gene on chromosome 5, with nuclear, nucleolar and cytoplasmic immunoexpression of the ALK protein. Variant translocations in the ALK and other partner genes on chromosomes 1, 2, 3 and 17 can occur and result in the up regulation of ALK, but the immunolocalisation of ALK varies according to the translocation, often occurring in the cytoplasm or cell membrane and not in the nucleus.53 The classic t(2;5) is readily detected by reverse transcriptase‐polymerase chain reaction (RT‐PCR), but cases with variant translocations will be negative by standard RT‐PCR with primers that are specific to the ALK and nucleophosmin genes.54 As such, immunohistology has largely supplemented molecular analysis for the diagnosis of anaplastic large‐cell lymphoma.
Wilms's tumour‐I overexpression in t(11;22) translocation in desmoplastic small round‐cell tumour
The desmoplastic small round‐cell tumour described in children need not be desmoplastic and can occur in an older age group, prompting us to name such tumours “polyphenotypic” small‐cell tumours, to highlight their more important and consistent attribute.55 Although this lesion shares the t(11;22) translocation seen in Ewing's sarcoma and PNET, it differs fundamentally with respect to the breakpoint on chromosome 11 (t(11;22) (p13;q12)), which results in the unique fusion peptide EWS–WT‐1.56 A portion of this fusion peptide corresponds to the C‐terminal portion of the WT‐1 gene product, allowing an immunohistological demonstration of WT‐1 overexpression as a marker for such tumours. With the exception of Wilms's tumour, the desmoplastic small round‐cell tumour is unique among the small blue round‐cell tumours in children, showing positive nuclear immunostaining for the WT‐1 gene product.55,56
FLI‐1 overexpression in t(11;22) translocation in Ewing's sarcoma or PNET
The t(11;22)(q24;q12) translocation is found in about 90% of cases of Ewing's sarcoma or PNET. The translocation results in the production of the unique EWS–FLI‐1 product and overexpression of the FLI‐1 protein, which can be shown immunohistologically. FLI‐1 is observed in almost 75% of cases of Ewing's sarcoma or PNET, but not in other small blue round‐cell tumours that are difficult to separate histologically.57
Bcl‐10 and mucosa‐associated lymphoid tissue‐1 overexpression in lymphomas of mucosa‐associated lymphoid tissues
Lymphomas of mucosa‐associated lymphoid tissues (MALTs) are generally indolent tumours. In the stomach, such lymphomas respond well to antibiotics58 directed at the eradication of the instigating stimulus Helicobacter.59 Three chromosomal translocations are specifically associated with MALT lymphoma and their detection is important in diagnosis and prognosis. Among gastric MALT lymphomas, about 30% show a t(11;18)/API1–MALT1 and another 5% show a t(1;14)/Bcl‐10–IgH translocation. A third translocation t(14;18)/IgH–MALT occurs more often in non‐gastrointestinal MALT lymphoma. The fusion of the genes involved in these forms of translocation results in different patterns of expression of MALT1 and Bcl‐10. Compared with non‐neoplastic lymphoid cells that were either negative for MALT1 and Bcl‐10 or showed weak to moderate immunostaining, predominantly in the cytoplasm, MALT lymphomas with t(14;18) translocation showed strong homogenous cytoplasmic staining of both MALT1 and Bcl‐10 in the lymphoma cells.60 In cases with t(1;14) there was weak cytoplasmic staining for MALT1 with strong nuclear immunoexpression of Bcl‐10; whereas those with t(11;18) showed weak cytoplasmic MALT1 and moderate nuclear Bcl‐10 staining.60 The accumulation of cytoplasmic Bcl‐10 in t(14;18)/IgH–MALT1 was proposed to result from the MALT1 interaction with Bcl‐10 that stabilises the Bcl‐10 in the cytoplasm, but no explanation was available for the moderate nuclear staining of Bcl‐10 observed with t(11;18)/API2–MALT1.60 The differential staining patterns observed suggested that immunostaining may be used for the screening of these translocations. This would be of prognostic relevance as about 30% of cases of gastric MALT lymphomas fail to respond to antibiotics used for eradication of Helicobacter and most of these show one of the translocations described, commonly t(11;18) (q21;q21) or t(1;14) (p22;q32).61 Both antibodies promise to be useful, but their sensitivity and specificity need to be proved.
Transcription factor 3 in alveolar soft‐part sarcoma and a subset of renal carcinomas in children
The gene fusion resulting from the specific chromosomal translocation in alveolar soft‐part sarcoma was recently identified. The specific der(17)t(X;17) (p11.2;q25) results in fusion between the transcription factor 3 gene (TFE3) on chromosome Xp11.2 and the novel alveolar soft‐part sarcoma gene locus on chromosome 17q25. The TFE3 gene is also associated with translocations in chromosome Xp11.2 in a subset of renal cell carcinomas in children and young adults. In this subset, the TFE3 gene is disrupted on chromosome X and fused to the PRCC gene on chromosome 1, leading to the formation of different fusion proteins, but is still identifiable with antibodies to the TFE3 gene product. Nuclear localisation of TFE3 has a sensitivity of 97.5% and a specificity of 99.6% for alveolar soft‐part sarcoma and renal tumours bearing TFE3 gene fusion products in children.62
NFκB activity
NFκB comprises a family of closely related transcription factors that include five genes NFκB1 (p50/p105), NFκB2 (p52/p100), RelA (p65), c‐Rel and RelB. The derived proteins have a well‐recognised function in the regulation of immune responses and inflammation. More recently, they are recognised to have a role in oncogenesis through the suppression of apoptosis.63,64 Activation of NFκB can result through several pathways triggered by cytokines, growth factors and tyrosine kinases, as well as members of other receptors, including tumour necrosis factor, insulin growth factor and epidermal growth factor.
The NFκB dimer is typically triggered by inflammatory cytokines with resultant nuclear translocation, where it acts as a transcription factor. Alternatively, activation occurs through tumour necrosis factor. Activation of NFκB is tightly regulated and transient, with regulatory mechanisms returning NFκB to its inactive state. Activation up regulates the transcription of several genes responsible for the suppression of apoptosis through proteins like FLIP, Bcl‐2 and p53. NFκB also promotes cell cycle progression through the expression of the cyclins D1, D2, D3 and E, c‐myc and c‐mycb. It also induces the expression of intercellular adhesion molecule‐1 and E‐selectin, as well as matrix metalloproteinases that are associated with tumour invasion. Angiogenic factors including vascular endothelial growth factor are promoted and expression of COX‐2 is induced. Tumours with constitutive NFκB activation usually show increased resistance to chemotherapy and NFκB may induce the expression of the multidrug‐resistant P‐glycoprotein. On the other hand, inhibition of NFκB improves the apoptotic response to radiation therapy and it has been suggested that NFκB is required for taxol‐induced cell death.64
With the many roles that NFκB has in development, progression and treatment of cancer, this molecule is of potential prognostic and therapeutic interest. Antibodies to the different subunits of the protein will enable assessment for nuclear translocation of NFκB and will be particularly useful in the evaluation of inflammatory and neoplastic lesions.
Therapeutic targets for cancer treatment
A contemporary trend in cancer treatment is the targeting of specific molecules expressed by the cancer cells, most commonly molecules on the cell surface that participate in the regulation of growth and proliferation. Humanised or chimeric monoclonal antibodies have been produced to a handful of these target molecules and the best response to treatment generally occurs in tumours expressing large amounts of the target molecules.
HER2/neu in breast cancer
The immunohistological detection of HER2/neu overexpression in breast cancer is now a well‐established procedure. HER2/neu is a transmembrane glycoprotein belonging to the epidermal growth factor receptor (EGFR) group and is expressed at low levels in a variety of normal epithelia, including that of mammary duct cells. In as many as 25% of invasive ductal carcinomas, this protein is overexpressed as a result of amplification of the HER2/neu gene. This overexpression has been confirmed to be an independent prognostic marker in node‐positive and, more recently, also in node‐negative patients. More importantly, HER2/neu positive status predicts positive response to adriamycin‐based treatment and poor response to tamoxiphen, even in oestrogen receptor‐positive tumours. Patients with metastatic breast cancer that shows HER2/neu overexpression respond to immunotherapy with the new humanised anti‐HER2/neu antibody, trastuzumab. Immunostaining for HER2/neu is reproducible and shows good correlation with fluorescence in situ hybridisation (FISH) and PCR assays, with concordance rates ranging between 78% and 98%.65 A semiquantitative scoring system of 0, 1+, 2+ and 3+ is recommended, with cases scoring 2+ being considered equivocal and subjected to FISH assay. One large study of more than 3200 breast cancer cases with both FISH and immunohistological evaluation showed a positive predictive value of 92% for positive immunohistology for positive FISH, and a negative predictive value of 97% for negative immunohistology for negative FISH.66 Our own experience suggests that the presence of a tram track pattern produced by the staining of opposing cell membranes is a reliable method of assigning a 3+ score (fig 7). Membrane or cytoplasmic staining in the absence of this pattern should be scored 2+, with most such cases failing to show amplification by FISH (unpublished data).
Figure 7 Infiltrating ductal carcinoma of the breast, grade 3, stained for HER2/neu to show a distinct tram track pattern of membranous staining corresponding to a score of 3+.
c‐Kit (CD117) in gastrointestinal stromal tumours
Imatinib (Glivec, Gleeved, STI571, North Ryde, Australia) was initially developed for the treatment of chronic myeloid leukaemia (CML). Imatinib inhibits the kinase activity of BCR–ABL, the oncogenic product of the Philadelphia chromosome that is diagnostic of CML. It represents the first of a growing list of kinase‐targeted treatments for use against human cancers. Imatinib is not specific for the ABL tyrosine kinase and has activity against several related kinases, including c‐Kit and the two receptors for platelet‐derived growth factors PDGFRA and PDGFRB.
As many as 94% of gastrointestinal stromal tumours (GISTs) express the CD117 antigen and harbour activating mutations in the KIT gene. A further 5–7% have mutations in the PDGFRA gene. The kinase isoforms derived from these mutations are oncogenic and have a critical role in the development and progress of these tumours. Fortunately, most of these mutant isoforms are sensitive to imatinib and >80% of patients with GISTs benefit from treatment with imatinib. Cures from this treatment, however, do not seem to occur, as residual KIT‐positive tumour cells are almost always present in specimens from imatinib‐treated patients and surgery remains the main therapeutic modality.67 Immunohistological detection of CD117 is an effective method of identifying tumours treatable with imatinib.68
In addition to CML and GIST, three other tumours have shown selective response to imatinib. Chronic myelomonocytic leukaemia is characterised by a t(5;12) translocation resulting in fusion of the TEL and PDGFRB genes that generate the oncogenic form of PDGFRB kinase. Another myeloproliferative disorder, the hypereosinophilic syndrome, is driven by an oncogenic form of PDGFRA that results from fusion of the FIP1L1 and PDGFRA genes. Both these conditions respond well to low‐dose imatinib. Lastly, dermatofibrosarcoma protuberans, which has a t(17;22) translocation, results in the overproduction of PDGFB, the ligand of PDGFRB kinase, and may be responsive to imatinib, particularly in recurrence or metastasis. Imatinib has been tried against other tumours, but success is exceptional.
CD20 in aggressive B cell lymphoma
The mouse–human chimeric antibody rituximab (Rituxam, Genentech Inc, San Francisco, California, USA) has been used against aggressive B cell lymphomas such as mantle‐cell lymphoma and diffuse large‐cell lymphoma.69 Whereas these lymphomas express CD20 that is detectable by immunostaining, CD20 may be lost after rituximab treatment and in recurrent tumours. The immunohistological identification of this antigen is thus important in case of recurrence.
CD33 in acute myeloid leukaemia
Gemtuzumab ozogamicin (Myotarg, Wyeth, Cambridge, Massachusetts, USA) is the humanized anti‐CD33 antibody (hP67.6), that is bound to N‐acetyl‐γ‐calicheamicin, which damages the leukaemia cells by becoming internalised after CD33 binds to the cell membrane.70 CD33 is expressed in about 90% of blasts in acute myeloid leukaemia.
EGFR in refractory colorectal carcinoma
Cetuximab (Erbitux, Merck, Darmstadt, Germany) has received approval from the Food and Drug Administration for the treatment of irinotecan refractory or intolerant metastatic colorectal cancer. The EGFR pharmDx kit is approved by the Food and Drug Administration for the detection of EGFR expression to aid patients who are eligible for treatment with erbitux, which is believed to interfere with the growth of cancer cells by binding to EGFR and competing with the normal ligand.71
Tyrosine kinase HER1/EGFR in non‐small‐cell carcinoma of the lung
Gefitinin (Iressa, AstraZenaca, Macclesfield, UK) and erlotinib (Tarceva, Genentech Inc, San Francisco, California, USA) target the adenosine triphosphate clefts in the tyrosine kinase HER1/EGFR that is expressed in 40–80% of non‐small‐cell carcinoma of the lung and many other cancers. Somatic mutations of the EGFR were found in 15 of 58 unselected non‐small‐cell lung cancers in Japan and in 1 of 61 cases from the US. These mutations lead to increased growth factor signalling and confer susceptibility to the inhibitor, with one study with erlotinib showing a response rate of 12.3%72 and another study with gefitinib showing 27.5%.73 Antibodies to EGFR are available, but validation of immunohistological staining with therapeutic response to the humanised antibodies is not yet available.
Somatostatin receptors in pituitary adenomas and gastroenteropancreatic endocrine tumours
Somatostatin receptors (SSTRs) are composed of five isoforms; SSTR2 and SSTR5 are expressed by pituitary adenomas and SSTR2 by gastroenteropancreatic tumours. The somatostatin analogue, sandostatin (Octreotide, Novatis Pharmaceuticals, North Ryde, Australia), binds to SSTR2 and SSTR5 and inhibits tumour cell growth. SSTR detection may predict the response to somatostatin and in addition to detection by RT‐PCR, isoform‐specific antibodies are now available for immunostaining.74
Technological developments
Tissue microarrays
Tissue microarray technology represents advancement on the concept of the “sausage roll” and multi‐tissue blocks that were initially introduced for the testing and optimisation of antibodies.75 In comparison with the multi‐tissue blocks, tissue microarrays allow for the rapid immunohistological analysis of many more miniaturised samples arranged in an orderly fashion. The technology has opened the range of applications to quality control, long‐term tissue banking and microdissection of cells for molecular analysis.76
Heat‐induced antigen retrieval
Heat‐induced antigen retrieval continues to be the most effective method for the demonstration of a vast range of antigens in fixed processed tissues. A variety of methods, including wet autoclaving, boiling, steaming, pressure cooking and microwave irradiation,77 are used, but none offers accurate control of time and temperature, which are two of the most important variables in heat‐induced antigen retrieval. One commercial instrument (Mega T/T, Milestone, Sorisole, Italy) allows for accurate computer control of these two variables and shows that accurate superheating at 120°C results in greater immunostaining for most tissue antigens.78
Although heat seems to be a common denominator in antigen retrieval, the mechanism of this empirical observation remains debated and largely relates to our somewhat antiquated and limited understanding of fixation using formalin and its action on protein structure.79 Experimental systems in which heat generation is minimal and exposure to ultrasound, which does not generate heat, have both produced a noticeable enhancement of immunostaining79, raising questions about the role of kinetics in antigen retrieval. The most recent explanation for antigen retrieval implicates the Mannich reaction as an important factor in the process.80
Quantitation and quality control
The development of sensitive reagents and detection systems and the antigen retrieval procedure have contributed appreciably to our ability to stain consistently for a range of tissue antigens in the diagnostic setting. However, standardisation of immunostaining is not possible. A “total test” approach should be adopted when analysing the immunostaining procedure. Pre‐analytical variables such as the duration of fixation, delays in fixation and state of preservation of the tissue sample are beyond our control. By contrast, analytical variables, such as tissue processing, antigen retrieval, staining procedure and detection systems, can be controlled in the laboratory and hence standardised between laboratories. However, it should be noted that external controls for the test sample, even if known to contain quantified amounts of the antigen of interest, have been subjected to different pre‐analytical variables that influence antigen preservation. This deficiency largely negates their value as ideal test controls that should be subjected to identical pre‐analytical and analytical factors.81 Until external controls that satisfy these requirements become available, the next best controls are those within the test tissue sample. These are non‐lesional normal tissues that contain the antigen of interest; however, in the context of the tumour antigen, normal cells are also not optimal controls. The absence of ideal control tissues that have been processed in a manner identical to that of the test sample calls into question attempts to quantify immunostains, as it is not possible to standardise immunostaining between laboratories and between different specimens in the same laboratory, as each specimen is subjected to different pre‐analytical variables. Post‐analytical variables include interpretation and reporting and these are readily amenable to standardisation, as previously discussed.82
Conclusions
This short review of recent developments in immunohistology has by necessity been selective and subjective. The rapid adoption of immunostaining as an integral part of the investigation of a large proportion of routine surgical biopsy specimens confirms the contributions of this powerful tool, particularly to the diagnosis, prognostication and treatment of tumours.83 The recent development of antibodies to the products of specific genomic alterations provides a rapid method of assessing molecular abnormalities that promise to yield greater insights into tumour genesis, identification and therapeutic response. Such antibodies represent the most important area of development of immunohistology in recent times and enable the visualisation of specific molecular changes and correlations with morphological features.
Abbreviations
ALK - anaplastic large‐cell lymphoma kinase
AMACR - α‐methylacyl‐CoA racemase
CML - chronic myeloid leukaemia
COX - cyclo‐oxygenase
EGFR - epidermal growth factor receptor
FISH - fluorescence in situ hybridisation
GIST - gastrointestinal stromal tumour
HPV - human papillomavirus
MALT - mucosa‐associated lymphoid tissue
MSI - microsatellite instability
PDGFR - platelet‐derived growth factor receptor
PNET - primitive neuroectodermal tumour
pRb - retinoblastoma protein
RT‐PCR - reverse transcriptase‐polymerase chain reaction
SSTR - somatostatin receptor
TFE3 - transcription factor 3 gene
WT‐1 - Wilms's tumour 1 gene
Footnotes
Competing interests: None declared.
References
- 1.Gatter K C, Abdulaziz Z, Beverley P.et al Use of monoclonal antibodies for the histopathological diagnosis of human malignancy. J Clin Pathol 1982351253–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leong A S ‐ Y, Wick M R, Swanson P E.Immunohistology and electron microscopy of anaplastic and pleomorphic tumors. Cambridge: Cambridge University Press, 1987
- 3.Rossi S, Laurino L, Furlanetto A.et al Rabbit monoclonal antibodies. A comparative study between a novel category of immunoreagents and the corresponding mouse monoclonal antibodies. Am J Clin Pathol 2005124295–302. [DOI] [PubMed] [Google Scholar]
- 4.Crisp H, Burton J L, Stewart R.et al Refining the diagnosis of hydatidiform mole: image ploidy analysis and p57KIP2 immunohistochemistry. Histopathology 200343363–373. [DOI] [PubMed] [Google Scholar]
- 5.Kale A, Soylemez F, Ensari A. Expressions of proliferation markers (Ki‐67, proliferating cell nuclear antigen, and silver‐staining nucleolar organizer regions) and of p53 protein in gestational trophoblastic disease. Am J Obstet Gynecol 2001184567–574. [DOI] [PubMed] [Google Scholar]
- 6.Negri G, Egarter‐Vigl E, Kasal A.et al p16INK4a is a useful marker for the diagnosis of adenocarcinoma of the cervix uteri and its precursors. Am J Surg Pathol 200327187–193. [DOI] [PubMed] [Google Scholar]
- 7.Bibbo M, Klump W J, DeCecco J.et al Procedure for immunocytochemical detection of p16INK4a in thin layer, liquid‐based specimens. Acta Cytol 20024625–29. [DOI] [PubMed] [Google Scholar]
- 8.Wu H H, Lapkus O, Corbin M. Comparison of 34betaE12 and p63 in 100 consecutive prostate carcinoma diagnosed by needle biopsies. Appl Immunohistochem Mol Morphol 200412285–289. [DOI] [PubMed] [Google Scholar]
- 9.Barbareschi M, Pecciarini L, Giulia Cangi M.et al p63, a p53 homologue, is a selective nuclear marker of myoepithelial cells of the human breast. Am J Surg Pathol 2001251054–1060. [DOI] [PubMed] [Google Scholar]
- 10.Koker M M, Kleer C G. P63 expression in breast cancer: a highly sensitive and specific marker of metaplastic carcinoma. Am J Surg Pathol 200428506–512. [DOI] [PubMed] [Google Scholar]
- 11.Jaiswal M, LaRusso N F, Shapiro R A.et al Nitric oxide‐mediated inhibition of DNA repair potentiates oxidative DNA damage in cholangiocytes. Gastroenterology 2001120190–199. [DOI] [PubMed] [Google Scholar]
- 12.Gasparini G, Longo R, Sarmiento R.et al Inhibitors of cyclo‐oxygenase 2: a new class of anticancer agents? Lancet Oncol 20034605–615. [DOI] [PubMed] [Google Scholar]
- 13.Gown A M, Willingham M C. Improved detection of apoptotic cells in archival paraffin sections: immunohistochemistry using antibodies to cleaved caspase 3. J Immunohistochem Cytochem 200250449–454. [DOI] [PubMed] [Google Scholar]
- 14.Dhaene K, Van Marck E, Parwaresch R. Telomeres, telomerase and cancer: an update. Virchows Arch 20004371–16. [DOI] [PubMed] [Google Scholar]
- 15.Leung T ‐ M D, Ma C ‐ H, Niu H.et al Nuclear telomerase is less accessible to antibody probing than known nuclear antigens: retrieval with new immunostaining buffer. Histochem Cell Biol 2005123105–112. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Z, Wu C ‐ L, Wodaet al P504S/α‐methylacyl‐CoA racemase. A useful marker for diagnosis of small foci of prostate carcinoma on needle biopsy. Am J Surg Pathol 2002261169–1174. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Z, Li C, Fischer A.et al Using an AMACR (P504S)/34βE12/p63 cocktail for the detection of small focal prostate carcinoma in needle biopsy specimens. Am J Clin Pathol 2005123231–236. [DOI] [PubMed] [Google Scholar]
- 18.Nassar A, Amin M B, Sexton D G.et al Utility of alpha‐methylacyl coenzyme A racemase (P504S antibody) as a diagnostic immunohistochemical marker for cancer. Appl Immunohistochem Mol Morph 200513252–255. [DOI] [PubMed] [Google Scholar]
- 19.Suthipintawong C, Leong A S ‐ Y, Vinyuvat S. A comparative study of immunomarkers for lymphangiomas and hemangiomas. Appl Immunohistochem 19953239–244. [Google Scholar]
- 20.Leong A S ‐ Y, Cooper K, Leong F J W.Manual of diagnostic antibodies for immunohistology. 2nd edn. London: Greenwich Medical Media, 2003
- 21.Kahn H J, Bailey D, Marks A. Monoclonal antibody D2‐40, a new marker of lymphatic endothelium, reacts with Kaposi's sarcoma and a subset of angiosarcomas. Mod Pathol 200215434–440. [DOI] [PubMed] [Google Scholar]
- 22.Fukunaga M. Expression of D2‐40 in lymphatic endothelium of normal tissues and in vascular tumors. Histopatholology 200546396–402. [DOI] [PubMed] [Google Scholar]
- 23.Odonez N G. D2‐40 and podoplanin are highly specific and sensitive immunohistochemical markers of epithelioid malignant mesothelioma. Hum Pathol 200536372–380. [DOI] [PubMed] [Google Scholar]
- 24.Breiteneder‐Geleff S, Soleiman A, Kowalski H.et al Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol 1999154385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Browne P, Petrosyan K, Hernandez A.et al The B cell transcription factors BSAP, Oct‐2, and BOB.1 and the pan‐B cell markers CD20, CD22, and CD79a are useful in the differential diagnosis of classic Hodgkin lymphoma. Am J Clin Pathol 2003120767–777. [DOI] [PubMed] [Google Scholar]
- 26.Kreitman R J, Squires D R, Stetler‐Stevenson M.et al Phase I trial of recombinant immunotoxin RFB4 (dsFv)‐PE38*BL22) in patients with B cell malignancies. J Clin Oncol 2005236719–6729. [DOI] [PubMed] [Google Scholar]
- 27.Nickeleit V, Zeiler M, Gudat F.et al Detection of the complement degradation product C4d in renal allografts: diagnostic and therapeutic implications. J Am Soc Nephrol 200213242–251. [DOI] [PubMed] [Google Scholar]
- 28.Regele H, Bohmig G A, Habicht A.et al Capillary deposition of complement split product C4d in renal allografts is associated with basement membrane injury in peritubular and glomerular capillaries: a contribution of humoral immunity to chronic allograft rejection. J Am Soc Nephrol 2002132371–2380. [DOI] [PubMed] [Google Scholar]
- 29.Crespo M, Bosch F, Villamor N.et al ZAP‐70 expression as a surrogate for immunoglobulin‐variable‐region mutations in chronic lymphocytic leukemia.N Engl J Med 20033481764–1775. [DOI] [PubMed] [Google Scholar]
- 30.Carreras J, Villamor N, Colomo L.et al Immunohistochemical analysis of ZAP‐70 expression in B‐cell lymphoid neoplasms. J Pathol 2005205507–513. [DOI] [PubMed] [Google Scholar]
- 31.Wiestner A, Rosenwald A, Barry T S.et al ZAP‐70 expression identifies a chronic lymphocytic leukemia subtype with unmutated immunoglobulin genes, inferior clinical outcome and distinct gene expression profile. Blood 20031014944–4951. [DOI] [PubMed] [Google Scholar]
- 32.Knosel T, Meisel H, Borgmann A.et al Parvovirus B19 infection associated with unilateral cervical lymphadenopathy, apoptotic sinus histiocytosis, and prolonged fatigue. J Clin Pathol 200558872–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss R A, Whitby D, Talbot S.et al Human herpesvirus type 8 and Kaposi's sarcoma. J Natl Cancer Inst Monogr 19982351–54. [DOI] [PubMed] [Google Scholar]
- 34.Barbinto‐Brodano G, Sabbioni S, Martini F.et al Simian virus 40 infection in humans and association with human diseases: results and hypotheses. Virology 20043181–9. [DOI] [PubMed] [Google Scholar]
- 35.Danguy A, Camby I, Kiss R. Galectins and cancer. Biochem BiophysActa20021572285–293. [DOI] [PubMed] [Google Scholar]
- 36.Breesley M F, McLaren K M. Cytokeratin 19 and galectin‐3 immunohistochemistry in the differential diagnosis of solitary thyroid nodules. Histopathology 200241236–243. [DOI] [PubMed] [Google Scholar]
- 37.Casey M B, Lohse C M, Lloyd R V. Distinction between papillary thyroid hyperplasia and papillary thyroid carcinoma by immunohistochemical staining for cytokeratin 19, galectin 3 and HBME1. Endocr Pathol 20031455–60. [DOI] [PubMed] [Google Scholar]
- 38.Sahoo S, Hoda S A, Rosai J.et al Cytokeratin 19 immunoreactivity in the diagnosis of papillary thyroid carcinoma: a note of caution. Am J Clin Pathol 2001116696–702. [DOI] [PubMed] [Google Scholar]
- 39.Cheung C C, Ezzat S, Freeman J L.et al Immunohistochemical diagnosis of papillary thyroid carcinoma. Mod Pathol 200114338–342. [DOI] [PubMed] [Google Scholar]
- 40.Gown A M. Genogenic immunohistochemistry: a new era in diagnostic immunohistochemistry. Curr Diagn Pathol 20028193–200. [Google Scholar]
- 41.Popat S, Hubner R, Houlston R S. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 200523609–618. [DOI] [PubMed] [Google Scholar]
- 42.Ribic C M, Sargent D J, Moore M J.et al Tumor microsatellite‐instability status as a predictor of benefit from fluorouracil‐based adjuvant chemotherapy for colon cancer. N Engl J Med 2003349247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wood B, Leong A S ‐ Y. Cell adhesion proteins—biology, detection and applications. Pathology 200335101–105. [PubMed] [Google Scholar]
- 44.Berx G, Cleton J ‐ A M, Strumane K.et al E‐cadherin is inactivated in a majority of human lobular breast cancer by truncation mutations throughout its extracellular domain. Oncogene 1996131919–1925. [PubMed] [Google Scholar]
- 45.Droufakou S, Deshmane V, Roylance R.et al Multiple ways of silencing E‐cadherin gene expression in lobular carcinoma of the breast. Int J Cancer 200192404–408. [DOI] [PubMed] [Google Scholar]
- 46.Keller G, Vogelsang H, Becker I.et al Diffuse type gastric and lobular breast carcinoma in a familial cancer patient with an E‐cadherin germline mutation. Am J Pathol 1999155337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graziano F, Mandolesi A, Ruzzo A.et al Predictive and prognostic role of E‐cadherin protein expression in patients with advanced gastric carcinomas treated with palliative chemotherapy. Tumour Biol 200425106–110. [DOI] [PubMed] [Google Scholar]
- 48.Sormunen R, Leong A S ‐ Y, Vaaraniemi J.et al Immunolocalisation of fodrin, E‐cadherin and beta‐catenin adhesion complex in infiltrating ductal carcinoma of the breast—comparison with an in vitro model. J Pathol 1999187416–423. [DOI] [PubMed] [Google Scholar]
- 49.Yin H, Leong A S ‐ Y. Histological grading of non‐invasive papillary urothelial tumors. Validation of the 1998 WHO/ISUP system by immunophenotyping and follow‐up. Am J Clin Pathol 2004121679–687. [DOI] [PubMed] [Google Scholar]
- 50.Leong F J, Leong A S ‐ Y. Malignant rhabdoid tumours in adults. A unique morphologic phenotype of heterogeneous lineage differentiation. Pathol Res Pract 1996192796–807. [DOI] [PubMed] [Google Scholar]
- 51.Medjkane S, Novikov E, Versteege I.et al The tumor suppressor hSNF5/INI1 modulates cell growth and actin cytoskeleton organization. Cancer Res 2004643406–3413. [DOI] [PubMed] [Google Scholar]
- 52.Perry A, Fuller C E, Judkins A R.et al INI1 expression is retained in composite rhabdoid tumors, including rhabdoid meningiomas. Mod Pathol 200518951–958. [DOI] [PubMed] [Google Scholar]
- 53.Stein H, Foss H D, Durkop H.et al CD30+ anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood 2000963681–3695. [PubMed] [Google Scholar]
- 54.Falini B, Bigerna B, Fizzotti M.et al ALK expression defines a distinct group of T/null lymphomas (“ALK lymphomas”) with a wide morphological spectrum. Am J Pathol 1998153875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leong A S ‐ Y, Wick M E, Swanson P.Immunohistology and electron microscopy of anaplastic and pleomorphic tumors. Cambridge: Cambridge University Press, 1997
- 56.Leong A S ‐ Y. Immunohistology of small round cell tumors in childhood. J Histotechnol 199922239–246. [Google Scholar]
- 57.Folpe A L, Goldblum J R, Shehata B M.et al Morphologic and immunophenotypic diversity in Ewing family tumors: a study of 66 genetically confirmed cases. Am J Surg Pathol 2005291025–1033. [PubMed] [Google Scholar]
- 58.Du M Q, Isaccson P G. Gastric MALT lymphoma: from aetiology to treatment. Lancet Oncol 2002397–104. [DOI] [PubMed] [Google Scholar]
- 59.Hussell T, Isaacson P G, Crabtree J E.et al Helicobacter pylori‐specific tumor‐infiltrating T cells provide contact dependent help for growth of malignant B cells in low‐grade gastric lymphoma of mucosa‐associated lymphoid tissue. J Pathol 1996178122–127. [DOI] [PubMed] [Google Scholar]
- 60.Ye H, Gong L, Liu H.et al MALT lymphoma with t(14;18)(q32;q21)/IgH‐MALT1 is characterised by strong cytoplasmic MALT1 and Bcl10 expression. J Pathol 2005205293–301. [DOI] [PubMed] [Google Scholar]
- 61.Isaacson P G, Du M ‐ Q. Gastrointestinal lymphoma: where morphology meets molecular biology. J Pathol 2005205255–274. [DOI] [PubMed] [Google Scholar]
- 62.Argani P, Lal P, Hutchinson B.et al Aberrant nuclear immunoreactivity for TFE3 in neoplasms with TFE3 gene fusions. A sensitive and specific immunohistochemical assay. Am J Surg Pathol 200327750–761. [DOI] [PubMed] [Google Scholar]
- 63.Dehalle S, Blasius R, Dicato M.et al A beginner's guide to NK‐KB signalling pathways. Ann N Y Acad Sci 200410301–13. [DOI] [PubMed] [Google Scholar]
- 64.Dolcet X, Llobet D, Pallares J.et al NF‐KB in development and progression of human cancer. Virch Arch 2005446475–482. [DOI] [PubMed] [Google Scholar]
- 65.Press M F, Slamon D J, Flom K J.et al Evaluation of HER‐2/neu gene amplification and overexpression: comparison of frequently used assay methods in a molecularly characterised cohort of breast cancer specimens. J Clin Oncol 2002203095–3105. [DOI] [PubMed] [Google Scholar]
- 66.Yaziji H, Goldstein L C, Barry T S.et al HER‐2 testing in breast cancer using parallel tissue‐based methods. JAMA 20042911972–1977. [DOI] [PubMed] [Google Scholar]
- 67.Nowain A, Bhakta H, Pais S.et al Gastrointestinal stromal tumors: clinical profile and pathogenesis, treatment strategies and prognosis. J Gastroenterol Hepatol 200520818–824. [DOI] [PubMed] [Google Scholar]
- 68.Berman J, O'Leary T J. Gastrointestinal stromal tumor workshop. Hum Pathol 200132578–582. [DOI] [PubMed] [Google Scholar]
- 69.Hernandez‐Ilizaliturri F J, Gowda A, Czuczman M S. Development of targeted therapies for B‐cell non‐Hodgkin lymphoma and multiple myeloma. Clin Adv Hematol Oncol 20042606–618. [PubMed] [Google Scholar]
- 70.Linenberger M L. CD33‐directed therapy with gemtuzumab ozogamicin in acute myeloid leukemia: progress in understanding cytotoxicity and potential mechanisms of drug resistance. Leukemia 200519176–182. [DOI] [PubMed] [Google Scholar]
- 71.Goldstein N S, Armin M. Epidermal growth factor receptor immunohistochemical reactivity in patients with American Joint Committee on Cancer Stage IV colon adenocarcinoma: implications for a standardized scoring system. Cancer 2001921331–1346. [DOI] [PubMed] [Google Scholar]
- 72.Sandler A. Clinical experience with the HER1/EGFR tyrosine kinase inhibitor erlotinib. Oncology 200316(Suppl 12)17–22. [PubMed] [Google Scholar]
- 73.Fukuoka M, Yano S, Giaccone G.et al Multi‐institutional randomised phase II trial of gefitinib for previously treated patients with advanced non‐small cell lung cancer. J Clin Oncol 2003212237–2246. [DOI] [PubMed] [Google Scholar]
- 74.Volante M, Bozzalla‐Cassione F, Papotti M. Somatostatin receptors and their interest in diagnostic pathology. Endocr Pathol 200415275–291. [DOI] [PubMed] [Google Scholar]
- 75.Leong A S ‐ Y. ed. Applied immunohistochemistry for the surgical pathologist. London: Edward Arnold, 199414
- 76.Packeisen J, Korsching E, Herbst H.et al Demystified … tissue microarray technology. Mol Pathol 200356198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leong A S ‐ Y. Microwaves in immunohistology—the past 12 years. J Cell Pathol 19961111–120. [Google Scholar]
- 78.Leong A S ‐ Y, Lee E S, Yin H.et al Antigen retrieval with controlled superheating at 120°C. Appl Immunohistochem Mol Morphol 200210263–268. [DOI] [PubMed] [Google Scholar]
- 79.Leong A S ‐ Y.Microwave technology for light microscopy and ultrastructural studies. Bangkok: Amarin Printing and Publishing, 2005
- 80.Sompuran S R, Vani K, Messana E.et al A molecular mechanism of formalin fixation and antigen retrieval. Am J Clin Pathol 2004121190–199. [DOI] [PubMed] [Google Scholar]
- 81.Leong A S ‐ Y. Quantitation in immunohistology—fact or fiction? A discussion of factors that influence results. Appl Immunohistochem Mol Morphol 2004121–7. [DOI] [PubMed] [Google Scholar]
- 82.Leong A S ‐ Y. Pitfalls in diagnostic immunohistology. Adv Anat Pathol 20041186–93. [DOI] [PubMed] [Google Scholar]
- 83.Leong A S ‐ Y. Immunohistological markers for tumor prognostication. Curr Diagn Pathol 20017176–186. [Google Scholar]