Abstract
Primary effusion lymphoma (PEL) was once defined as a body cavity‐based lymphoma without identifiable contiguous tumour mass, but is now recognised as an independent clinicopathological entity. The case of a 67‐year‐old Japanese woman with PEL is reported, in which the clinical findings showed a pericardial effusion and multiple erythema on the hypogastrium and inguinal region. The histopathological findings showed a diffuse infiltration of large neoplastic B cells from the dermis to the subcutis. After the disappearance of pericardial effusion without any treatment, she received several rounds of chemotherapy to resolve the skin eruption, but she finally died from multiple organ failure. No tumour mass was observed during the course of her disease.
Primary effusion lymphoma (PEL) is a rare neoplasm of B cells, which usually presents as serous effusions without any detectable tumour masses. Initially defined as a subtype of lymphoma based in body cavities but lacking an identifiable contiguous tumour mass,1 PEL is now recognised as an independent clinicopathological entity, and has previously been reported in association with human herpes virus 8 (HHV‐8).2 Typically, PEL occurs in immunodeficient patients,3 but there is also a case of PEL in an HHV‐8‐negative immunocompetent woman.4 We present a new example of PEL, not yet found in the literature, that includes skin involvement and the diffuse infiltration of large neoplastic B cells from the dermis to the subcutis.
Case report
A 67‐year‐old Japanese woman came to the Tsuyama Central Hospital, Tsuyama, Japan, presenting with multiple erythema of the left inguinal region in November 2002. Since 1998, she had been treated with prednisolone and actarit, a slow‐acting antirheumatic drug for rheumatoid arthritis. She first had slight chest pain in April 2002 and a detectable pericardial effusion was noted on an echocardiogram in May 2002.
Fine‐needle aspiration of the pericardial effusion showed heteromorphic cells with large atypical nuclei that stained positive for CD20, but negative for CD3 and CD45RO; both stains are immunohistochemically specific for B cells. Thoracic, abdominal and pelvic computed tomography (CT) scanning and whole‐body scanning showed pericardial effusion without lymphadenopathy, organomegaly or definable tumour masses. On the basis of these findings, the patient was diagnosed with PEL. The pericardial effusion disappeared spontaneously late in June 2002 without any treatment. In October 2002, an erythema was noted on the left inguinal region. By November 2002, the erythema had slowly progressed to affect the hypogastrium and both inguinal regions.
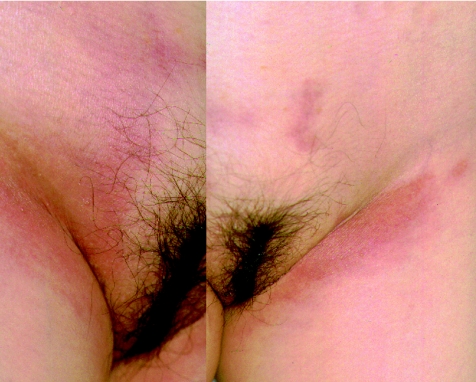
Physical examination showed multiple indurative erythema of the hypogastrium and bilateral inguinal regions (fig 1). Haemogram and serum chemistry were normal, whereas the concentration of serum interleukin 2 was raised at 1410 U/ml (normal 190–650 U/ml). Whole‐body CT scanning showed pericardial effusion without definable tumour masses. She was negative for HIV infection tested using enzyme immunoassay, but serological tests showed a previous infection with Epstein–Barr virus (EBV).
Figure 1 Indurative erythema on the patient's hypogastrium and bilateral inguinal regions.
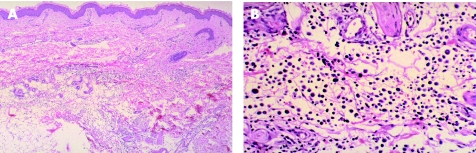
Skin biopsy specimens from the right inguinal region showed diffuse neoplastic lymphocytic infiltration extending from the dermis to the subcutis (fig 2A). The infiltrating cells were large and irregular in shape, with prominent nuclei (fig 2B). These cells stained positively for CD20 and CD79A but not for CD3, indicating that the infiltrates were predominantly composed of B cells.
Figure 2 (A) Infiltration of diffuse large neoplastic lymphocytes from dermis to subcutaneous tissue (haematoxylin eosin (H&E) stain, ×40). (B) The infiltrated cells were large and irregular in shape with prominent nuclei (H&E stain, ×200).
Amplification of the gene for immunoglobulin heavy chain (IgH) was carried out by semi‐nested polymerase chain reaction (PCR) on DNA extracted from the skin biopsy specimen by the method originally reported by Wan et al.5 Only a single band of about 100 bp was detected in this case. This result indicated that the monoclonal proliferation of cells with IgH gene rearrangement indicated the B cell origin of lymphoma cells. The skin biopsy specimen was negative for the HHV‐8 sequence using PCR primers specific for HHV‐8 (laboratory testing service by BML, Tokyo, Japan). The EBV genome was not detected by an in situ hybridisation technique for EBV‐encoded small RNAs from the skin biopsy specimen.
The patient was treated with three cycles of treatment with cyclophosphamide, doxorubicin, vincristine and prednisolone, and showed notable improvement of the skin eruption and pericardial effusion. However, after five cycles of the treatment, the indurative erythema of the right inguinal region recurred. Although the patient was treated with several regimens—MEPP (mitoxantrone hydrochloride, etoposide, cisplatin and prednisolone) , DeVIC (dexamethasone, etoposide, ifosfamide and carboplatin), dexamethasone pulse treatment and low‐dose CPT‐11 (irinotecan hydrochloride)—the lesion spread to the abdomen, and to the whole of the left upper and right lower extremities. The patient died from multiple organ failure, probably due to sepsis, 16 months after the appearance of pericardial effusion. No tumour mass was observed, including on the skin and in other organs, during the course of her disease. Autopsy findings showed conspicuous lower‐extremity oedema, and this lesion contained numerous diffuse large lymphoma cells from the dermis to the subcutis. No lymphadenopathy or bone marrow infiltration of the lymphoma cells was evident. The foci of the abscess due to a Candida infection were observed in the liver and spleen without presence of lymphoma cells.
Discussion
Typically, most cases of PEL arise in association with decreased immunocompetence—for example, in the setting of HIV infection—with most patients being young to middle‐aged homosexual men.2,6 PEL also occurs in high frequency in elderly men from areas with a high prevalence of HHV‐8 infection, such as the Mediterranean.7,8 However, some cases have been reported in HIV‐negative patients who received an organ transplant, suggesting that reduced immune competence may have a role in the development of PEL in the absence of viral pathogens.9 In addition, there was one reported case of PEL with classic Kaposi's sarcoma in an HIV‐negative and EBV‐negative woman.10
Our patient had PEL that affected the skin, with B cell lymphoma infiltration but without any evidence of HIV or HHV‐8 infection. The recurrence of pericardial effusion around the same time and morphological and immunohistochemical evaluation and PCR analysis of the IgH gene rearrangement confirmed the skin lymphoma to be a secondary manifestation of the PEL. The initial site of infiltration seemed to be the pericardium without any evidence of lymphadenopathy. Interestingly, the initial lesion resolved spontaneously. In some cases,9 which were complicated with liver cirrhosis or chronic hepatitis, PEL spontaneously regressed without any treatment of the malignant lymphoma. However, our patient had neither liver dysfunction nor hepatitis C virus infection. After 6 months, the pericardial effusion reappeared and the skin lesions developed in the hypogastrium and both inguinal regions. In clinical and autopsy findings, there was no evidence of mass lesion or bone marrow involvement of the lymphoma cells. To our knowledge, this is the first case of cutaneous involvement in a patient with PEL. This case suggests that long‐period steroid treatment for rheumatoid arthritis combined with slow‐acting antirheumatic drugs may have contributed to the development of PEL.
Abbreviations
EBV - Epstein–Barr virus
HHV‐8 - human herpes virus 8
IgH - immunoglobulin heavy chain
PCR - polymerase chain reaction
PEL - primary effusion lymphoma
Footnotes
Competing interests: None declared.
Written consent was obtained from this patient for publication of the images in print.
References
- 1.Cesarman E, Chang Y, Moore P S.et al Kaposi's sarcoma‐associated herpesvirus‐like DNA sequences in AIDS‐related body‐cavity‐based lymphomas. N Engl J Med 19953321186–1191. [DOI] [PubMed] [Google Scholar]
- 2.Nador R G, Cesarman E, Chadburn A.et al Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma‐associated herpes virus. Blood 199688645–656. [PubMed] [Google Scholar]
- 3.Banks P M, Warnke R A. Primary effusion lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. World Health Organization classification of tumours: tumours of haematopoietic and lymphoid tissues 3rd edn. Lyon: IARC Press, 2001179–180.
- 4.Tanaka S, Katano H, Tsukamoto K.et al HHV8‐negative primary effusion lymphoma of the peritoneal cavity presenting with a distinct immunohistochemical phenotype. Pathol Int 200151293–300. [DOI] [PubMed] [Google Scholar]
- 5.Wan J H, Trainor K J, Brisco M J.et al Monoclonality in B cell lymphoma detected in paraffin wax embedded sections using the polymerase chain reaction. J Clin Pathol 199043888–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Said W, Chien K, Takeuchi S.et al Kaposi's sarcoma‐associated herpesvirus (KSHV or HHV8) in primary effusion lymphoma: ultrastructural demonstration of herpesvirus in lymphoma cells. Blood 1996874937–4943. [PubMed] [Google Scholar]
- 7.Teruya‐Feldstein J, Zauber P, Setsuda J E.et al Expression of human herpesvirus‐8 oncogene and cytokine homologues in an HIV‐seronegative patient with multicentric Castleman's disease and primary effusion lymphoma. Lab Invest 1998781637–1642. [PubMed] [Google Scholar]
- 8.Cobo F, Hernandez S, Hernandez L.et al Expression of potentially oncogenic HHV‐8 genes in an EBV‐negative primary effusion lymphoma occurring in an HIV‐seronegative patient. J Pathol 1999189288–293. [DOI] [PubMed] [Google Scholar]
- 9.Shimazaki M, Fujita M, Tsukamoto K.et al An unusual case of primary effusion lymphoma in a HIV‐negative patient not pathogenetically associated with HHV8. Eur J Haematol 20037162–67. [DOI] [PubMed] [Google Scholar]
- 10.Said J W, Tasaka T, Takeuchi S.et al Primary effusion lymphoma in women: report of two cases of Kaposi's sarcoma herpes virus‐associated effusion‐based lymphoma in human immunodeficiency virus‐negative women. Blood 1996883124–3128. [PubMed] [Google Scholar]