Abstract
The case of an 83‐year‐old woman with an uncommon presentation of cutaneous metastases from muscle‐invasive transitional cell carcinoma of the urinary bladder is reported. The band‐like eruption of the metastatic lesion can often be misdiagnosed and treated initially as herpes zoster. A detailed immunohistochemical analysis is also described to differentiate metastatic lesions from other sources.
Cutaneous metastasis from bladder carcinoma is rare. Of the cutaneous metastasis from genitourinary organs, transitional cell carcinoma (TCC) of the bladder accounts for 17% of cases.1 Clinical diagnosis can often be difficult owing to non‐specific appearances of these lesions, which can vary from nodular to sclerodermoid skin lesions. With the availability of antibodies to different cytokeratins (CKs), it is possible to define the tissue‐specific profiles of cytokeratins staining by immunohistochemistry.2 The differential expression of cytokeratins by different epithelial tissues can be used to subclassify epithelial tumours from different organs. We describe an immunohistochemical profile of a herpetiform metastatic lesion from muscle‐invasive TCC of the urinary bladder.
Case report
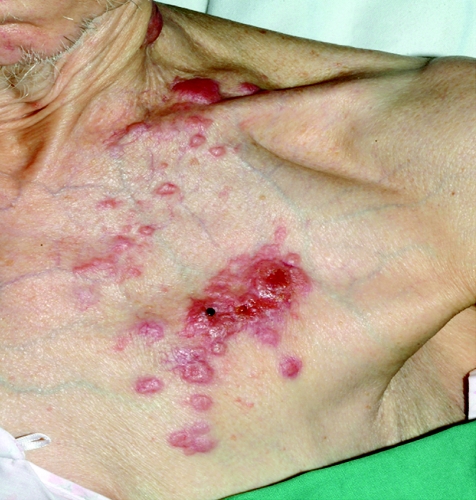
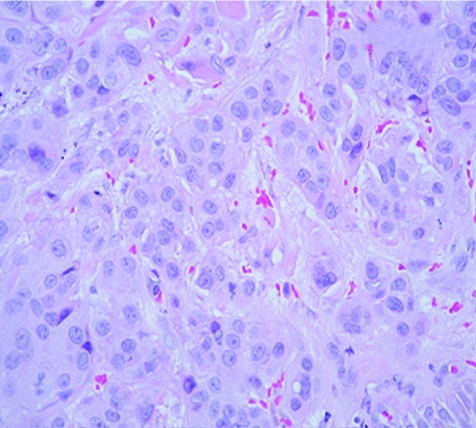
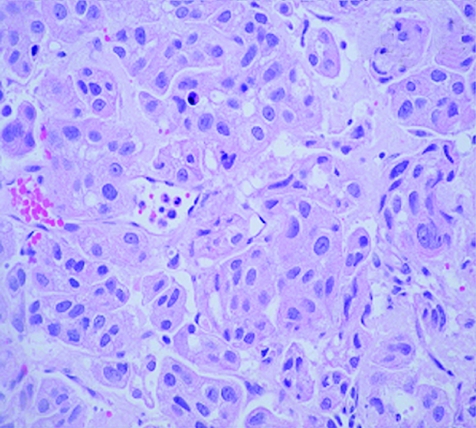
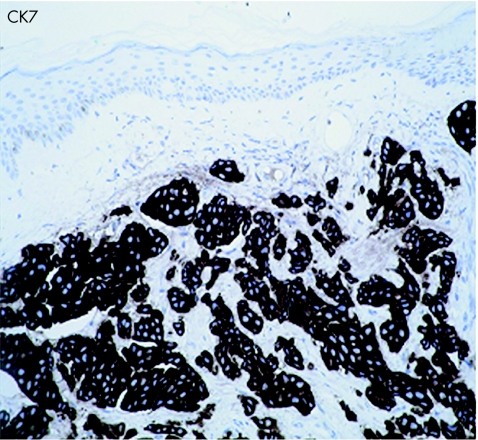
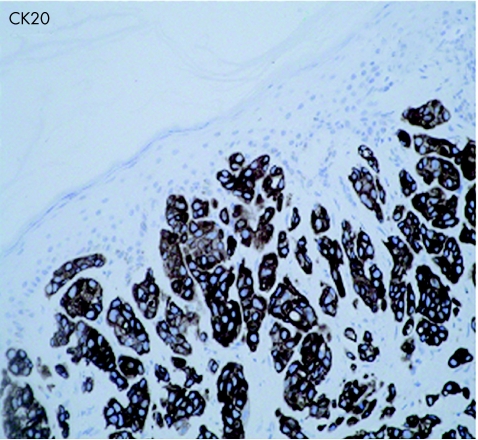
An 83‐year‐old woman presented with a 3‐week history of gross haematuria. She underwent a transurethral resection of a solid‐looking bladder tumour. The radiological investigation, which included a computed tomography scan of the abdomen, showed a large bladder tumour with perivesicle extension. The upper urinary tract was normal. Chest x ray was normal and the histopathological examination showed a poorly differentiated (pT2 G3) muscle‐invasive TCC of the bladder associated with carcinoma in situ. Six weeks later, she presented with multiple reddish nodules on the left side of her neck and chest (fig 1). Biopsy of the lesion showed metastatic carcinoma, with appearance similar to TCC of the urinary bladder (fig 2, 3). Immunocytochemical analysis indicated that these cells were positive for CK7 (fig 4) and CK20 (fig 5). An algorithm (table 1) was used to exclude metastases from most common sites in female subjects. She underwent four fractions of palliative radiotherapy to her pelvis, owing to problems with persistent bleeding and pain, and a single fraction of radiotherapy to the metastasis on her left chest wall owing to ulceration after biopsy. We found no clinical evidence of healing of the ulcer. She died at a follow‐up of 6 months.
Figure 1 Cutaneous metastases from transitional cell carcinoma of urinary bladder.
Figure 2 Haematoxylin and eosin staining of skin lesion (×400).
Figure 3 Haematoxylin and eosin staining of primary bladder carcinoma (×400).
Figure 4 Cytokeratin 7 immunocytochemical staining of skin lesion (×200).
Figure 5 Cytokeratin 20 immunocytochemical staining of skin lesion (×200).
Table 1 Immunoreactivity patterns in different carcinomas.
| Malignancy | Immunoreactivity patterns | ||||
|---|---|---|---|---|---|
| CK7 | CK20 | CEA | TTF1 | CA125 | |
| Ovarian | + | − | − | − | + |
| TCC (urinary) | + | + | +/− | − | − |
| Breast | + | − | + | − | − |
| Lung | + | − | +/− | + | +/− |
| Colon | − | + | + | − | − |
| Thyroid | + | − | +/− | + | +/− |
| RCC | − | − | − | − | − |
CA125, cancer antigen 125; CEA, carcinoembryonic antigen; CK, cytokeratin; RCC, renal cell carcinoma; TCC, transitional cell carcinoma; TTF1, thyroid transcription factor 1; +, present; −, absent.
Discussion
Cutaneous metastasis from primary bladder carcinoma is a rare event and carries a poor prognosis.1 The primary sites for metastatic bladder cancer are the liver, lung and bone. Our patient presented with haematuria and was diagnosed as having TCC of the bladder. Six weeks later, she developed a herpetiform rash on her neck and chest wall. Owing to the diagnostic dilemma and a suspicion of skin metastasis, a punch biopsy of the lesion was carried out, which showed a metastatic, poorly differentiated carcinoma in the dermis. Immunohistochemical analysis showed that the tumour cells were negative for oestrogen receptors and thyroid transcription factor 1, and positive for carcinoembryonic antigen, CK7 and CK20, which was consistent with metastasis from TCC of the bladder.
The site of origin has prognostic and therapeutic significance.2 Immunohistochemical methods to identify the site of origin have been used before. Cytokeratin staining is useful to identify metastasis from the lung, and urothelial and colorectal carcinomas, because tumours tend to maintain their tissue‐specific cytokeratin profile. A morphological overlap is seen in carcinomas that arise in different organs, making it challenging at times to differentiate the neoplasm by conventional histological methods. Determining the origin of metastatic carcinomas can be difficult, if not impossible.
Immunostaining patterns show a considerable overlap between various tumours. We present a list of immunohistochemical markers to discriminate metastasis originating from a variety of sites in females subjects.
The CK7+/CK20+/CA125− immunophenotype is highly characteristic of TCC of the urinary bladder.3,4 Colorectal tumours are CK7−/CK20+/CEA+.5,6 Thyroid tumours are CK7+/CK20−/TTF1+.7 Ovarian tumours are CK7+/CK20−/CEA−/CA125+.5,8 Lung tumours are CK7+/CK20−/TTF1+.2,4,9
Take‐home messages
Exclude cutaneous metastases in patients presenting with herpetiform cutaneous lesions, specailly in eldelry population.
A panel of antibodies should be used for immunohistochemistry in order to reach a proper diagnosis.
Prognosis of patients with cutaneous metastatic disease is poor.
As the CK7+/CK20+ phenotype strongly favours transitional cells,3,10,11 we used this in conjunction with other immunohistochemical markers to diagnose the skin metastasis from TCC in the bladder of our patient.
Conclusion
Cutaneous metastasis may mimic dermatological disorders, and a definitive diagnosis requires that this possibility is kept in mind and a skin biopsy is carried out.1 Metastatic lesions often preserve histological similarities to the primary lesion, but they can be poorly differentiated and anaplastic, making comparisons difficult. As no single marker is specific for an organ site, immunohistochemical staining with positive and negative markers should be carried out.12 These stains also serve to exclude metastasis from other malignancies in these patients.
Abbreviations
TCC - transitional cell carcinoma
Footnotes
Competing interests: None declared.
References
- 1.Mueller T J, Wu H, Greenberg R E.et al Cutaneous metastases from genitourinary malignancies. Urology 2004631021–1026. [DOI] [PubMed] [Google Scholar]
- 2.Rubin B P, Skarin A T, Pisick E.et al Use of cytokeratins 7 and 20 in determining the origin of metastatic carcinoma of unknown primary, with special emphasis on lung cancer. Eur J Cancer Prev 20011077–82. [DOI] [PubMed] [Google Scholar]
- 3.Chang A, Cai J, Miranda G.et al Usefulness of CA 125 as a preoperative prognostic marker for transitional cell carcinoma of the bladder. J Urol 2004172(Pt 1)2182–2186. [DOI] [PubMed] [Google Scholar]
- 4.Harlamert H A, Mira J, Bejarano P A.et al Thyroid transcription factor‐1 and cytokeratins 7 and 20 in pulmonary and breast carcinoma. Acta Cytol 1998421382–1388. [DOI] [PubMed] [Google Scholar]
- 5.Drlicek M, Bodenteich A, Urbanits S.et al Immunohistochemical panel of antibodies in the diagnosis of brain metastases of the unknown primary. Pathol Res Pract 2004200727–734. [DOI] [PubMed] [Google Scholar]
- 6.Lagendijk J H, Mullink H, Van Diest P J.et al Tracing the origin of adenocarcinomas with unknown primary using immunohistochemistry: differential diagnosis between colonic and ovarian carcinomas as primary sites. Human Pathol 199829491–497. [DOI] [PubMed] [Google Scholar]
- 7.Bejarano P A, Nikiforov Y E, Swenson E S.et al Thyroid transcription factor‐1, thyroglobulin, cytokeratin 7, and cytokeratin 20 in thyroid neoplasms. Appl Immunohistochem Mol Morphol 20008189–194. [DOI] [PubMed] [Google Scholar]
- 8.Cameron R I, Ashe P, O'Rourke D M.et al A panel of immunohistochemical stains assists in the distinction between ovarian and renal clear cell carcinoma. Int J Gynecol Pathol 200322272–276. [DOI] [PubMed] [Google Scholar]
- 9.Kummar S, Fogarasi M, Canova A.et al Cytokeratin 7 and 20 staining for the diagnosis of lung and colorectal adenocarcinoma. Br J Cancer 2002861884–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cid Mouteira P, Ortiz Rey J A, Anton Badiola I.et al Coordinated expression of cytokeratin 7 and 20 in transitional carcinoma of the bladder: diagnostic usefulness. Actas Urol Esp 200226279–284. [DOI] [PubMed] [Google Scholar]
- 11.Ordonez N G. Transitional cell carcinomas of the ovary and bladder are immunophenotypically different. Histopathology 200036433–438. [DOI] [PubMed] [Google Scholar]
- 12.Pires Y, Andrade L, Cuello M.et al Immunohistochemical flow chart to differentiate between primary adenocarcinoma and the most frequent extragynecological metastases in the ovary. Rev Med Chile 20021301232–1240. [PubMed] [Google Scholar]