Abstract
Aims
To evaluate the nuclear morphometric features of breast columnar cell lesions (CCLs) observed on mammotome core biopsies, to determine if there are significant measurable differences between those with atypia and those without. Correlation with follow‐up open excision specimens was made.
Methods
Mammotome core biopsies performed on patients that contained CCLs were derived from the departmental case files. Histological material was reviewed and foci of CCLs demarcated for nuclear morphometric assessment, which was accomplished using an imaging system. Nuclear parameters studied were nuclear area and perimeter, circularity factor and feret's diameter. Statistical analysis used the GraphPad Prism software, with p<0.05 indicating significance.
Results
On examination of core biopsies of 40 patients with CCLs, 8 lesions were benign, 4 showed atypical lobular hyperplasia, 8 showed CCLs with nuclear atypia, 19 disclosed atypical ductal hyperplasia (ADH) and 1 showed ductal carcinoma in situ (DCIS). The nuclear area, perimeter and feret's diameter of CCLs with atypia were significantly greater than those without (p = 0.04, 0.03 and 0.019, respectively), whereas no difference was observed in the circularity factor. Follow‐up open excision biopsy specimens in 24 patients showed upgrading to DCIS in 40% of cases diagnosed initially with ADH on core biopsy compared with 20% of CCLs with atypia.
Conclusions
Nuclear morphometry in CCLs confirms nuclear size as the key parameter in the assessment of nuclear atypia. Whether it can be potentially used as an adjunctive tool depends on the establishment of appropriate cut‐offs.
Columnar cell lesions (CCLs) of the breast encompass a spectrum of benign to atypical entities, with varying degrees of nuclear atypia and architectural complexity, that have in common variably dilated terminal duct lobular units lined by columnar epithelial cells with prominent apical cytoplasmic snouts.1,2,3 This group of lesions, usually non‐palpable, is often encountered in biopsy specimens of mammographically detected microcalcifications, especially in recent years, due to successful implementation of breast screening programmes in many countries.1,2
CCLs have been previously described by terms such as atypical cystic lobules,4 columnar change with prominent apical snouts and secretions,2 small ectatic ducts lined with atypical ductal cells with apocrine snouts5 and enlarged lobular units with columnar changes.6 Although they do not represent a new category of breast disease, CCLs present an emerging challenge in breast pathology and patient management. An issue of concern is the lack of consistency in the diagnosis of CCLs with marked cytological atypia.7 This has implications for patient management, as CCLs with atypia have been found to be associated more often with atypical hyperplasia, ductal carcinoma in situ (DCIS) and invasive carcinoma as compared with CCLs without atypia, and are commonly recommended for open excision biopsy if found on core biopsy.2,3,8,9
In light of this, we examined the nuclear morphometric features of histological specimens from 40 screen‐detected patients with CCLs on mammotome core biopsy, with the aim of determining whether they serve as useful quantitative parameters for improved characterisation of CCLs, in particular, distinguishing CCLs with atypia from CCLs without atypia. In addition, we evaluated whether CCLs that occur in association with DCIS or atypical hyperplasia can be distinguished morphometrically from CCLs in completely benign biopsy specimens. To further investigate the clinical implications of CCL with atypia diagnosed on core biopsy, a retrospective follow‐up of the outcomes of subsequent open excision was also conducted.
Materials and methods
Patients and histological material
The study population comprised 40 women diagnosed with CCLs on mammotome core biopsy between 2002 and 2005, obtained from the files of the Department of Pathology, Singapore General Hospital, Singapore. The average age of the patients at the time of biopsy was 50 (range 41–63) years, with a median age of 49 years. Twenty four women subsequently underwent open excision biopsy after the initial mammotome biopsy. Table 1 summarises the clinicopathological characteristics of the patients.
Table 1 Patient characteristics on core biopsy (n = 40).
| Age (years) | |
| Mean (SD) | 50.0 (7.1) |
| Median | 49 |
| Histopathology on mammotome core biospy | |
| Benign (innocuous CCL) | 8 |
| Atypical lobular hyperplasia | 4 |
| Atypical ductal hyperplasia | 19 |
| CCLs with nuclear atypia | 8 |
| DCIS | 1 |
| No of cases with open excision follow‐up | 24 |
CCL, columnar cell lesion; DCIS, ductal carcinoma in situ.
Tissues obtained from the biopsies were fixed in 10% buffered formaldehyde (pH 7.0), embedded in paraffin wax, serially sectioned at 4 μm and stained with haematoxylin and eosin. All histological sections were reviewed by a pathologist (PHT) without knowledge of the patient's clinical course, and foci of CCLs on representative slides were selected and demarcated for morphometric assessment of nuclei. Sections with fixation or histotechnical artefacts, and incomplete sections, were excluded. CCLs in completely benign specimens, adjacent to foci of atypical hyperplasia and DCIS, and CCLs with nuclear atypia were marked for morphometric nuclear analysis.
The histological diagnoses of atypical ductal and lobular hyperplasia, and DCIS followed described criteria.10 CCLs with marked nuclear atypia on mammotome core biopsy, as judged by light microscopy, were advised for open excision biopsy, and managed akin to atypical ductal hyperplasia (ADH) discovered on core biopsy.
Subsequent open excision specimens were also fixed in formalin, macroscopically described, serially sliced, processed, embedded in paraffin wax and evaluated histologically in the usual manner.
Morphometric measurement
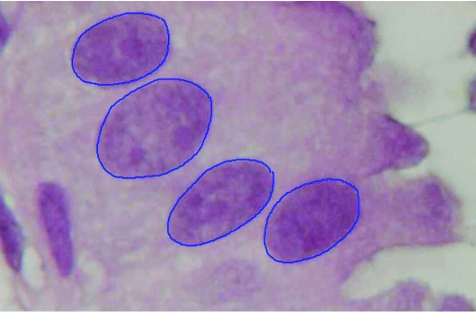
Nuclear morphometrical analysis of the representative areas containing CCLs was carried out by CNL in a blinded manner—that is, without prior knowledge of the histological assessment, using a computer‐assisted imaging system consisting of a light microscope (Leitz Aristoplan, Germany), a high‐resolution digital camera (Olympus DP50, Japan) and an image acquisition software (Viewfinder Lite V.1.0.135, California, USA). At least 10 images of each focus were captured digitally at 100× magnification and pixel size 1392×1040. These images were analysed using ImageJ 1.33u, and 60 nuclei per focus, totalling to an average of >120 nuclei per case, were randomly selected and the contours outlined using a digital pen and tablet (Wacom, USA). Only non‐overlapping nuclei with easily detectable boundaries were included (fig 1). The nuclear morphometric parameters examined were nuclear area and perimeter, circularity factor and feret's diameter. Circularity factor, a dimensionless shape descriptor, is defined by the formula (4π×area)/perimeter2, where the value of 1 corresponds to a perfect circle. Feret's diameter, also known as the maximum calliper length, is defined as the greatest linear distance between any two points on the perimeter.
Figure 1 Nuclei of columnar cell lesion with atypia outlined using a digital pen and graphic tablet (haematoxylin and eosin staining; original magnification 400×).
Statistical analysis
For comparison of the means of continuous variables between groups, two‐sided unpaired Student's t test was carried out when the variances of the groups were equal, as ascertained by the F test. An alternative Welch t test was carried out when the groups had different variances. All analyses were carried out using GraphPad Prism (V.4.00 for Windows). Values of p<0.05 were considered to be significant.
Results
Of the 40 mammotome core biopsy specimens studied histologically, 8 (20%) showed CCLs with nuclear atypia, whereas the remaining CCLs were without nuclear atypia (CNA), although 4 showed accompanying atypical lobular hyperplasia (ALH), 18 showed accompanying ADH and 1 showed accompanying DCIS. Mean age of the patients with CCLs with nuclear atypia and CNA was 54.1 (range 44–72) years and 48.9 (range 40–63) years, respectively.
Nuclear morphometry
Table 2 shows a comparison of the nuclear morphometric measurements from biopsy specimens that displayed CCLs with nuclear atypia with those that displayed CNA on core biopsy (including those accompanying atypical hyperplasia and DCIS). Mean nuclear area was significantly larger (1.15 fold) in specimens with CCLs showing nuclear atypia than in those that did not display atypia (p = 0.04). Correspondingly, mean nuclear perimeter of the CCLs with nuclear atypia group was larger (p = 0.03). Mean feret's diameter, a measure of maximum calliper length, was also found to be longer in the group having CCLs with nuclear atypia (p = 0.019), although no significant difference was established for circularity. No significant differences were observed for nuclear morphometric parameters in CCLs in completely benign biopsy specimens versus those accompanying atypical hyperplasia (both ductal and lobular) and DCIS, with the exception of circularity, where the nuclei of innocuous CCL in completely benign biopsy specimens were closer in shape to a circle than the atypical hyperplasia (both ductal and lobular) and DCIS (table 3).
Table 2 Comparison of mean nuclear morphometric features of columnar cell lesions (CCLs) with nuclear atypia (CA) and CCLs without nuclear atypia (CNA).
| Mean values of nuclear morphometric parameters | ||||
|---|---|---|---|---|
| Area (μm2) | Perimeter (μm) | Circularity | Feret's diameter (μm) | |
| CA (n = 8) | 35.31 (2.122) | 22.08 (0.6918) | 0.881 (0.016) | 8.576 (0.3067) |
| CNA (n = 32) | 30.65 (0.6329) | 20.40 (0.2239) | 0.908 (0.005) | 7.746 (0.1085) |
| p Value | 0.04 | 0.03 | NS | 0.019 |
NS, not significant.
Data are presented as mean (SEM).
Table 3 Comparison of mean nuclear morphometric features of columnar cell lesions (CCLs) in completely benign biopsy specimens (innocuous CCL) and CCLs associated with atypical hyperplasia and ductal carcinoma in situ.
| Mean values of nuclear morphometric parameters | ||||
|---|---|---|---|---|
| Area (μm2) | Perimeter (μm) | Circularity | Feret's diameter (μm) | |
| Innocuous CCLs (n = 8) | 30.05 (1.322) | 20.25 (0.4649) | 0.927 (0.005) | 7.471 (0.2610) |
| CCLs associated with AH/DCIS (n = 24) | 30.41 (0.7087) | 20.39 (0.2496) | 0.902 (0.006) | 7.832 (0.1156) |
| p Value | NS | NS | 0.005 | NS |
AH, atypical hyperplasia; CCL, columnar cell lesion; DCIS, ductal carcinoma in situ; NS, not significant.
Data are presented as mean (SEM).
Table 4 shows the nuclear morphometric findings of CCL in completely benign biopsy specimens (innocuous CCL) and CCLs with nuclear atypia, where nuclear perimeter and feret's diameter are considerably larger in CCLs with nuclear atypia than in innocuous CCL; whereas nuclei of the CCLs with nuclear atypia are less circular or round than the innocuous CCLs.
Table 4 Comparison of mean nuclear morphometric features of innocuous columnar cell lesions (CCLs) and CCLs with nuclear atypia.
| Mean values of nuclear morphometric parameters | ||||
|---|---|---|---|---|
| Area (μm2) | Perimeter (μm) | Circularity | Feret's diameter(μm) | |
| Innocuous CCL (n = 8) | 30.05 (1.322) | 20.25 (0.4649) | 0.927 (0.005) | 7.471 (0.2610) |
| CCL with nuclear atypia (n = 8) | 35.31 (2.122) | 22.08 (0.6918) | 0.881 (0.016) | 8.576 (0.3067) |
| p Value | 0.05 | 0.04 | 0.02 | 0.01 |
CCL, columnar cell lesion.
Data are presented as mean (SEM).
Open excision biopsy specimens
Table 5 summarises the open excision outcomes. A total of 24 women underwent open excision biopsy, after core biopsy diagnoses of atypical hyperplasia, CCL with nuclear atypia and DCIS.
Table 5 Outcomes of open excision follow‐up of 24 cases.
| Mammotome core biopsy diagnosis | Outcome of follow‐up excisions (n = 24) | |||
|---|---|---|---|---|
| DCIS | ADH | Benign | Lobular neoplasia | |
| ADH (%)* (n = 15) | 6 (40) | 8 (53.3) | 1 (6.7) | 0 |
| CA (%)* (n = 5) | 1 (20) | 3 (60) | 1 (20) | 0 |
| ALH (n = 3) | 1 | 1 | 0 | 1 |
| DCIS (n = 1) | 1 | 0 | 0 | 0 |
ADH, atypical ductal hyperplasia; ALH, atypical lobular hyperplasia; CA, CCL with nuclear atypia; CCL, columnar cell lesion; DCIS, ductal carcinoma in situ.
*Values expressed as percentage of occurrence of a particular outcome in that group.
In comparing the significance of CCL with nuclear atypia (n = 5) and ADH (n = 15) found on core biopsy in terms of their open excision outcomes, it was shown that a larger proportion (40% v 20%) of cases with ADH as opposed to those cases having CCLs with nuclear atypia were subsequently upstaged to DCIS. Conversely, a larger proportion of cases diagnosed with CCLs having nuclear atypia on core biopsy remained benign (20% v 6.7%) on open excision histology.
Discussion
Quantitative nuclear morphometry has been shown to be of prognostic value in invasive breast cancer and DCIS by several reports.11,12,13,14 In particular, nuclear size and size variation have been widely acknowledged as parameters for subjective and quantitative assessment of nuclear pleomorphism.12 In the context of CCLs, although certain nuclear features such as increased nuclear:cytoplasmic ratio, which results in the appearance of nuclear enlargement, nuclear hyperchromasia, irregular chromatin pattern and presence of nucleoli have been noted and are currently used in the histopathological diagnosis of atypia for CCLs,3,15 no published study to date has, however, reported on the nuclear morphometry of this group of lesions quantitatively.
Given the issue of diagnostic consistency of CCLs, as shown in a recent reproducibility study by Tan et al,7 and the emerging evidence that at least some CCLs with atypia may represent either a precursor of DCIS or the earliest morphological manifestation of DCIS,3 we examined the nuclear morphometry of this group of lesions and found that nuclear area, perimeter and feret's diameter were considerably larger in CCLs with atypia than in those without atypia, regardless of the presence of accompanying atypical hyperplasia or DCIS. This finding of larger nuclear area in CCLs with atypia than in those without atypia provides supporting evidence to the observation of increased nuclear:cytoplasmic ratio in CCLs with atypia. That nuclear perimeter and feret's diameter show correspondingly higher values in CCLs with atypia is expected under the premise of larger nuclear area and no marked difference in circularity. Although it is tempting to postulate that nuclear area and perimeter, as well as feret's diameter, can be potentially useful as adjunctive, quantitative criteria to aid in evaluation of CCLs, particularly to distinguish those with atypia from those without, the practical limitation to this lies in the establishment of validated cut‐off values for these parameters. The confirmation of nuclear enlargement in CCLs with atypia may also be reflective of the accumulation of abnormal genetic material, and may therefore lend weight to the consideration of these lesions being neoplastic in origin. These results also mirror what we previously found in a nuclear morphometry study of DCIS.14 When CCLs in completely benign biopsy specimens (innocuous CCL) were compared against CCLs associated with atypical hyperplasia or DCIS (excluding those with nuclear atypia), we found no marked differences in nuclear morphometric values other than circularity, whereby innocuous CCLs disclosed nuclear shapes closer to a circle. However, when CCLs with nuclear atypia were compared against innocuous CCLs, nuclear perimeter and feret's diameter were expectedly larger and nuclear shape less circular in the CCLs with nuclear atypia. Although the difference in nuclear area in both groups was of borderline significance (p = 0.05), the mean nuclear area was larger in those with nuclear atypia. These findings suggest that CCLs without nuclear atypia, regardless of whether they are identified in a completely benign background or accompanying atypical hyperplasia or DCIS, are similar in nuclear characteristics.
Take‐home messages
Columnar cell lesions (CCLs) of the breast encompass a spectrum of benign to atypical entities, with variably dilated terminal duct lobular units lined by columnar epithelial cells with apical snouts.
An issue of concern is the lack of consistency in the diagnosis of CCLs with cytologic atypia.
Nuclear morphometry reveals that nuclear area, perimeter and feret's diameter of CCLs with atypia were significantly greater that CCLs without atypia.
Open excision biopsies in 24 women with CCLs on core biopsy that were associated with atypical hyperplasia, nuclear atypia or DCIS showed upstaging to DCIS in 40% of biopsy diagnosed conventional atypical ductal hyperplasia versus 20% of CCLs with nuclear atypia.
Nuclear enlargement is the key parameter to rely upon to make a diagnosis of CCL with significant nuclear atypia.
Clinical follow‐up studies have established that ADH represents a generalised risk factor for subsequent development of breast cancer, which is about four to five times that of the reference population.8,16,17 Although CCLs with marked nuclear atypia, otherwise also known as flat epithelial atypia, are regarded as potentially neoplastic or as a possible precursor to invasive tubular carcinoma,1 there is still little data on its correlation with subsequent open excision biopsy when found as an isolated lesion on core biopsy. Although the diagnosis of ADH is made based on the extent of cytoarchitectural abnormality of affected duct spaces, CCLs with atypia are discerned on the degree of observed nuclear atypia in ducts lined by columnar cells. In this study, the excision biopsy outcomes of ADH and CCLs with atypia found on initial core biopsy specimens show that a higher proportion of ADH on core biopsy is associated with a more sinister open excision outcome than in those CCLs with atypia. The limitation to this preliminary conclusion is the small number of cases in each group with subsequent open excision follow‐up.
In conclusion, nuclear morphometry may be a useful adjunctive tool in confirming marked nuclear atypia in breast CCLs, provided appropriate validated cut‐offs can be established. Its utility is, however, hampered by the generally less than widespread availability of the software in diagnostic surgical pathology laboratories and the length of time required to trace outlines of a sufficient number of nuclei. Nevertheless, our study has affirmed that nuclear enlargement is perhaps the key histological parameter that can be relied on to make a diagnosis of CCL with marked nuclear atypia.
Abbreviations
ADH - atypical ductal hyperplasia
CCL - columnar cell lesion
CNA - CCL without nuclear atypia
DCIS - ductal carcinoma in situ
Footnotes
Competing interests: None declared.
References
- 1.Ho B C S, Tan P H. Flat epithelial atypia: concepts and controversies of an intraductal lesion of the breast. Pathology 200537105–111. [DOI] [PubMed] [Google Scholar]
- 2.Fraser J L, Raza S, Chorny K.et al Columnar alterations with prominent apical snouts and secretions: a spectrum of changes frequently present in breast biopsies performed for microcalcifications. Am J Surg Pathol 1998221521–1527. [DOI] [PubMed] [Google Scholar]
- 3.Schnitt S J, Vincent‐Salomon A. Columnar cell lesions of the breast. Adv Anat Pathol 200310113–124. [DOI] [PubMed] [Google Scholar]
- 4.Wellings S R, Jensen H M, Marcum R G. An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst 197555231–273. [PubMed] [Google Scholar]
- 5.Goldstein N S, O'Malley B A. Cancerization of small ectatic ducts of the breast by ductal carcinoma in situ cells with apocrine snouts. Am J Clin Pathol 1997107561–566. [DOI] [PubMed] [Google Scholar]
- 6.McLaren B K, Gobbi H, Schuyler P A.et al Immunohistochemical expression of estrogen receptor in enlarged lobular units with columnar alteration in benign breast biopsies. A nested case‐control study. Am J Surg Pathol 200529105–108. [DOI] [PubMed] [Google Scholar]
- 7.Tan P H, Ho B C S, Selvarajan S.et al Pathological diagnosis of columnar cell lesions of the breast: are there issues of reproducibility? J Clin Pathol 200558705–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs T W, Connolly J L, Schnitt S J. Non‐malignant lesions in breast core needle biopsies: to excise or not to excise? Am J Surg Pathol 2002261095–1110. [DOI] [PubMed] [Google Scholar]
- 9.Nasser S M. Columnar cell lesions: current classification and controversies. Semin Diag Pathol 20042118–24. [DOI] [PubMed] [Google Scholar]
- 10.NHS Breast Screening Programme Pathology reporting of breast disease. NHSBSP publication No 58. The Royal College of Pathologists. Sheffield: NHS cancer screening programmes, 2005
- 11.Kronqvist P, Kuopio T, Collan Y. Morphometric grading of invasive ductal breast cancer. I. Threshold for nuclear grade. Br J Cancer 199878800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baak J P, Van Dop K, Kurver P H.et al The value of morphometry to classic prognosticators in breast cancer. Cancer 198556374–382. [DOI] [PubMed] [Google Scholar]
- 13.Aaltomaa S, Lipponen P, Papinaho S.et al Nuclear morphometry and DNA flow cytometry as prognostic factors in female breast cancer. Eur J Surg 1992158135–141. [PubMed] [Google Scholar]
- 14.Tan P H, Goh B B, Chiang G.et al Correlation of nuclear morphometry with pathologic parameters in ductal carcinoma in situ of the breast. Mod Pathol 200114937–941. [DOI] [PubMed] [Google Scholar]
- 15.Rosen P P.Rosen's breast pathology. 2nd edn. Philadelphia: Lippincott Williams & Wilkins, 2002215
- 16.Dupont W D, Page D L. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med 1985312146–151. [DOI] [PubMed] [Google Scholar]
- 17.Dupont W D, Parl F F, Hartman W H.et al Breast cancer risk associated with proliferative breast disease and atypical hyperplasia. Cancer 1993711258–1265. [DOI] [PubMed] [Google Scholar]