Abstract
Background
Acid secretion is intimately associated with most upper gastrointestinal diseases. Helicobacter pylori infection is a major environmental factor modifying acid secretion.
Aim
To study the association between the pattern of H pylori gastritis and gastric secretory function in a large number of subjects without specific upper gastrointestinal disease.
Methods and materials
Maximal acid output (MAO) was measured in 255 patients with dyspepsia showing normal endoscopy. Activity and severity of gastritis, atrophy and H pylori infection were assessed in body and antral biopsies. The correlations of histological parameters as well as age, sex, height, weight, smoking, serum gastrin, pepsinogen I and II, and their ratio with MAO were determined. Multiple linear regression was used to show the best possible predictors of MAO.
Results
Negative relationships: Body atrophy and body‐combined (active and chronic) inflammatory scores showed a potent inverse correlation with MAO (correlation coefficients (CC) 0.59 and 0.50, respectively). Body:antral chronic gastritis ratio and body:antral combined inflammation ratio (both with CC = 0.49) and age (CC = 0.44) were also inversely correlated with MAO. Intestinal metaplasia at both antral and body sites had negative relationships with acid output with CC = 0.23 and 0.20, respectively. Positive relationships: Serum pepsinogen I, body H pylori density:combined inflammation ratio and pepsinogen I:II ratio with CC of 0.38, 0.38 and 0.30, respectively, correlated with MAO. The H pylori density: combined inflammation of both antrum and body positively correlated with MAO (CC = 0.29 and 0.38, respectively). Male sex and patient height also positively correlated with acid output. Modelling showed that body combined inflammatory score, body atrophy, age and serum pepsinogen I are independent predictors of acid output (R2 = 0.62).
Conclusion
Combination of body gastritis, body atrophy, age and serum pepsinogen I can be used as predictors of acid‐secretory state in populations infected with H pylori.
A close association is found between the level of gastric acid secretion and the type of disease affecting the upper gastrointestinal tract. Duodenal ulceration occurs in patients with high acid secretion,1,2,3,4,5 gastro‐oesophageal reflux disease and its complications in patients with normal6 or high levels of acid secretion,7,8,9 gastric ulceration in patients with moderately reduced secretion10 and gastric cancer in patients with profoundly reduced or absent acid secretion.11,12,13,14
The level of acid secretion is thought to have a role in the aetiology of these common upper gastrointestinal diseases. Increased gastric acid secretion and duodenal acid load predispose to duodenal ulceration as shown by the Zollinger–Ellison syndrome.15,16 Absence of gastric acid secretion is thought to predispose to gastric cancer by allowing colonisation of the stomach by carcinogen‐synthesising bacteria and also by the reflex hypergastrinaemia stimulating cell proliferation.17,18,19 The role of acid in the aetiology of gastro‐oesophageal reflux disease is shown by the therapeutic efficacy of acid‐inhibitory drugs.
There has been a marked change in the pattern of upper gastrointestinal disease in the Western world over the past century. The incidence of gastric cancer has progressively fallen,20,21,22 that of duodenal ulceration has risen and then fallen, and the incidence of reflux disease and its malignant complications has increased.22,23 These changes in incidence of disease are thought to be partly due to a rise in gastric acid secretion. There are also marked geographical variations in incidence of upper gastrointestinal disease. Reflux disease and its complications are more common in the Western world versus the Eastern world and the opposite trend is seen for gastric cancer. Again, these variations are believed to be related partly to regional variations in levels of gastric acid secretion.
A major factor known to influence gastric acid secretion is Helicobacter pylori infection, which colonises the gastric mucosa of >50% of the world's populations. The effect of the infection on acid secretion is related to the pattern of gastritis, which it induces in the stomach. Previous studies24,25,26 have examined acid secretion and H pylori gastritis in specific diseases—for example, duodenal ulceration, gastric ulceration and gastric cancer. In patients with duodenal ulcer having antral‐predominant gastritis, eradicating H pylori lowers serum gastrin, and basal and gastrin‐releasing peptides stimulate acid.27 In patients with low acid secretion due to body‐predominant atrophic gastritis, eradicating H pylori increases acid secretion.28 In our study, we examined the relationship between the pattern of H pylori gastritis and gastric acid secretion in a large number of patients without specific upper gastrointestinal disease. For various reasons acid secretion is now rarely measured, and we have therefore also assessed the ability to predict the level of acid secretion from gastric histology and serological markers.
Aim
To assess the value of gastric histology, serological markers and other characteristics as predictors of gastric acid secretion in patients infected with H pylori.
Methods and materials
Study population
The study included 255 patients with H pylori‐positive dyspepsia, showing normal upper gastrointestinal endoscopy (ie, no evidence of mucosal ulcerations, erosions or neoplastic changes), who had undergone acid secretory studies in the Gastrointestinal Unit, Gartnavel General Hospital, Glasgow, UK over the past 10 years. Patients who had taken proton pump inhibitor treatment in the past year or H2 blocker treatment within the previous 3 weeks were not included. Their H pylori infection was confirmed by positive results in at least one of two methods, 14C urea breath test and gastric histology. In all, 123 (48.2%) patients were men and 132 (51.8%) were women. Mean age was 43.4 (SD 13.2) years and range of 18–84 years. Median (interquartile range (IQR)) height, weight and body mass index (BMI (weight (kg)/height2 (m2)) of the patients were 167 (16) cm, 70 (19) kg and 25 (5) kg/m2, respectively.
Histological assessment
Endoscopic biopsies of both the antrum and body region of the stomach were available in 175 patients. The processed biopsy specimens were stained with haematoxylin–eosin and H pylori‐specific Cresyl fast violet stain. All slides were assessed by a single pathologist for H pylori density as well as type and severity of gastritis. The histological criteria applied for evaluation of gastritis were based on the updated Sydney classification of gastritis as follows29:
Gastritis: presence of inflammatory cells of any type in the lamina propria.
Grade of inflammation: severity of infiltration of lymphocytes and plasma cells.
Activity of inflammation: Presence of neutrophils in the inflammatory infiltrate. The sum of the grade and activity was expressed as combined inflammatory score (CIS) for each participant with maximum possible score of 6.30
Mucosal atrophy: defined as the separation of the mucosal glands and a decrease in the thickness of mucosa, which is greater in severity than that seen in the inflammation of the lamina propria, and usually associated with an increase in the stromal matrix.
Intestinal metaplasia: presence of goblet cells with or without other cellular elements of intestinal mucosal and glandular epithelium.
Acid output measurement
After a 12 h fast, an orogastric tube (Anderson, New York, New York, USA) was swallowed and its position in the dependent part of the stomach was checked using the water recovery test.31 Intermittent suction was then applied using an intermittent suction unit (Omeda, Columbia, Maryland, USA) that applies suction for 20 s in each 32‐s cycle. An intravenous infusion of Pentagastrin (Peptavlon; ICI, Cheshire, UK) was started at a rate of 0.6 μg/kg/h to stimulate maximal acid secretion. Gastric juice was collected for 1 h in 15‐min aliquots.
The volume of each 15‐minute gastric juice collection was recorded and its hydrogen ion concentration was measured by titration with 0.1 mol/l sodium hydroxide to pH 7 using an autotitrator (Radiometer ETS 822; Copenhagen, Denmark). The acid output per 15‐minute period was then calculated by multiplying the volume by the hydrogen ion concentration. Maximal acid output (MAO) in response to pentagastrin was calculated by taking the mean of two highest consecutive 15‐min collections and expressed as mmol/h.
Serological assay
Blood was drawn from each patient during the visit to the endoscopy unit. Separated serum samples were stored at −70°C until analyses. Serum pepsinogen I and II were assayed with ELISA using monoclonal antibodies to pepsinogen I and II (BIOHIT Diagnostics, Biohit, Devon, UK). Serum gastrin‐17 was measured using an ELISA kit from the same supplier. All procedures were carried out according to the manufacture's instructions and results of pepsinogen I and II reported in μg/l and pmol/l for gastrin‐17. The pepsinogen I:II ratio was calculated and reported in fraction. H pylori cytotoxin‐associated gene A (CagA) antibodies were determined using a polyclonal enzyme immunoassay method, as previously described.32
Statistical analysis
Except for MAO values, all other variables had a relatively non‐symmetric distribution; hence a non‐parametric test (Spearman's rho) was used for bivariate correlations. All results were presented with a two‐sided significance level. A partial correlation analysis with zero orders was used to estimate possible confounders. To test the differences in mean MAO in different levels of gastritis and atrophy, we used one‐way analysis of variance (ANOVA) and the retrospective analysis Dunnett test if appropriate. Mann–Whitney U test was used for comparing the age of male and female patients.
Stepwise linear regression was carried out to determine the histological and serological predictors of MAO. Body atrophy score, antral atrophy score, serum pepsinogen I, serum pepsinogen I:II ratio, serum gastrin, age, H pylori density of antrum and body, or H pylori in one of two sites and body CIS were selected as independent variables and MAO as the dependent variable. Main criteria for probability of F to enter and F to remove of independent variables were ⩽0.05 and ⩾0.10, respectively. In addition to the standardised coefficients, colinearity statistics were presented for each variable. For most statistical analysis, we used SPSS V.12.0.
Results
Acid output and H pylori‐related gastritis
Density of H pylori colonisation in the antral mucosa was positively associated with acid output with a correlation coefficient (CC) of 0.31 (p<0.01), but H pylori density of the body did not correlate significantly with the acid secretion (CC = −0.11, p = 0.19). Recalculation of this relationship using a mean score of H pylori density at any site showed a positive correlation with acid output (CC = 0.17, p<0.05).
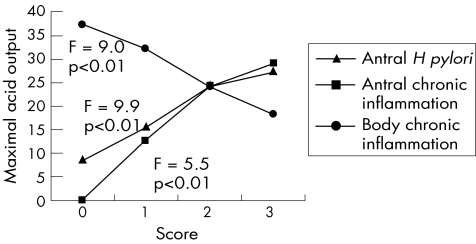
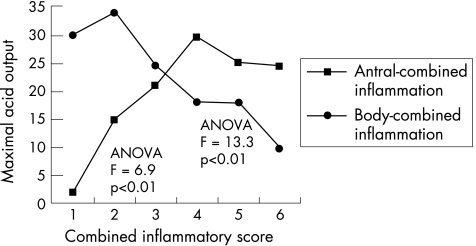
Chronic inflammation of the antrum was positively associated (CC = 0.27, p<0.01) with acid output, although there was a more potent negative association between chronic inflammation of the body mucosa and acid output (CC = −0.41, p<0.01; fig 1). Active inflammation in the antrum did not show any significant relationship with acid output, but in the body it showed a negative correlation (CC = −0.28, p<0.01) with acid output. CISs (ie, active and chronic) of the antrum showed a positive correlation with acid output reaching a peak of 29.3 mmol/h at histological score 4. However, from score 4 to 6 we found a slight decrease in acid output with the lowest value of 24.2 mmol/h at score 6. CIS of the body after a peak of 33.7 mmol/h at score 2 showed a persistent downward trend with minimal acid output of 9.5 at score 6. Statistical tests with ANOVA showed a significant difference between mean acid output among patients with different CISs (F = 6.9, p<0.5). Also, a significant inverse correlation of body combined inflammation with acid output was apparent (CC = −0.50, p<0.01; fig 2).
Figure 1 Relationships of Helicobacter pylori (H pylori) infection, antral and body chronic inflammation with maximal acid output.
Figure 2 Relationship of antral and body combined inflammation with maximal acid output.
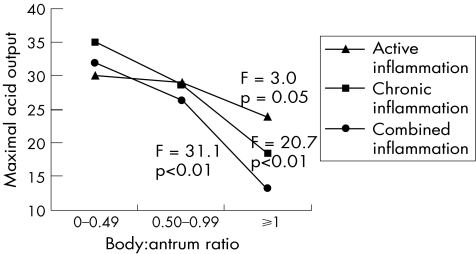
Two other indices, body:antral chronic inflammation ratio and body:antral combined inflammation ratio were tested for their relationship with acid output. Both of them showed a significant inverse correlation with acid output, with CC = −0.49 (p<0.01). As shown in fig 3, the body:antrum ratio of active inflammation had a similar inverse correlation with acid output but with a relatively weak significance (CC = −0.17, p = 0.07 and F = 3.0, p = 0.05).
Figure 3 Relationship of body:antrum ratios of active, chronic and combined inflammation with maximal acid output.
The density of H pylori per each score of combined inflammation was also evaluated regarding relationship with acid output. Those indices at both antral and body sites were positively correlated with acid output with CC values of 0.29 (p<0.01) and 0.38 (p<0.01), respectively.
Acid output and gastric atrophy
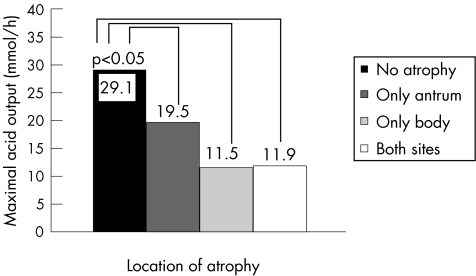
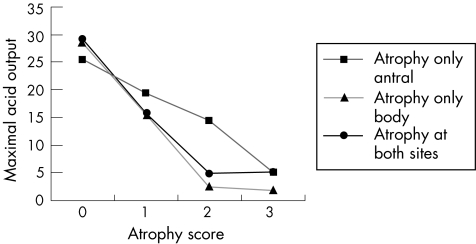
Antral‐confined atrophy was detected in 19 (12.6%) patients and atrophy limited to the body in 27 (17.9%) patients. Only 11 (7.3%) patients had multifocal atrophy. Mean (SD) acid output in patients without atrophy was 29.1 (12.2) mmol/h. In patients with antral atrophy only, acid output was 19.5 (11.1), in patients with body atrophy only and multifocal atrophy, acid output was reduced at 11.5 (10.1) and 11.9 (7.2) mmol/h, respectively. Using one‐way ANOVA, there were significant differences between groups regarding acid output (F = 22.0, p<0.01). The Dunnett t test showed that acid output in each of the groups with atrophy was significantly different compared with the non‐atrophic group (all p<0.05; fig 4).
Figure 4 Mean values of maximal acid output in patients with atrophy in different locations.
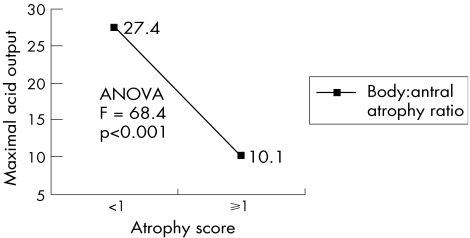
As seen in fig 5, both antral and body atrophy scores had a significant inverse correlation with acid output. The CC for antral atrophy was −0.30 (p<0.01) and for body atrophy it was −0.59 (p<0.01). The ratio of body:antral atrophy also showed a prominent reverse relationship with acid output, with ratio <1 for an output of 27.4 mmol/h and ratio⩾1 for an output of 10.1 mmol/h (fig 6). Zero‐order partial correlation showed that the relationship between body atrophy and acid output could be exaggerated with age and body‐combined inflammation. After controlling for age, CC dropped from −0.54 to −0.48 (11% decrease). Controlling body‐combined inflammation also decreased the CC of body atrophy score and acid output from −0.54 to −0.40 (26% decrease). By controlling both variables, CC dropped from −0.54 to −0.47 (31%).
Figure 5 Relationship of antral and body atrophy with maximal acid output.
Figure 6 Relationship of body:antral atrophy ratio with maximal acid output. ANOVA, analysis of variance.
Intestinal metaplasia at both antral and body sites had a negative relationship with acid output with CC = 0.23 (p<0.01) and CC = 0.20 (p<0.05), respectively.
Acid output and pepsinogen I, pepsinogen I:II ratio and gastrin‐17
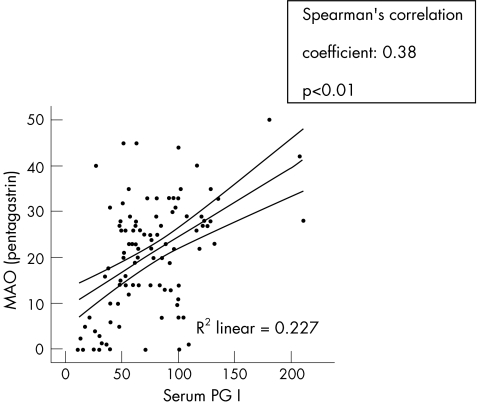
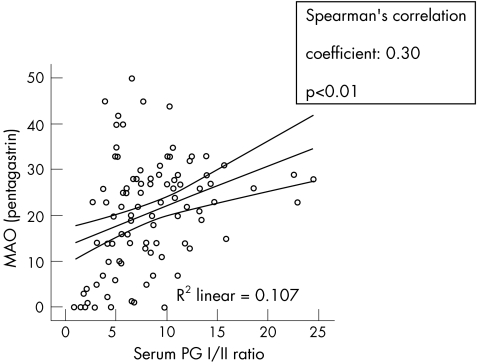
The median serum pepsinogen I and II concentrations of all participants were 73.5 μg/l (IQR 48.5) and 9.6 μg/l (IQR 8.0), respectively. The calculated median pepsinogen I:II ratio was 7.6 (IQR 5.5). Serum pepsinogen I was positively correlated with acid output, with CC = 0.38 (p<0.01). No relationship was found between acid output and serum pepsinogen II, but the ratio of pepsinogen I:II had a positive CC = 0.30 (p<0.01) with acid output (figs 7, 8).
Figure 7 Relationship of serum pepsinogen I (PG I) with maximal acid output (MAO).
Figure 8 Relationship of serum pepsinogen I:II ratio (PG I:II) with maximal acid output (MAO).
Median serum gastrin‐17 of the enrolled patients was 2.2 pmol/l. There was no significant correlation between acid output and serum gastrin‐17.
CagA serology; relationship with acid output and gastric histology
In 177 patients who had sufficient serum for anti‐CagA serology, mean (SD) acid output was similar in patients with positive versus negative CagA serology with 28.3 (11.2) and 26.7 (12.3) mmol/h, respectively. However, there was a correlation between anti‐CagA antibody titre and inflammatory histological parameters with respect to antral active inflammation (CC = 0.35, p<0.01), body active inflammation (CC = 0.33, p<0.01) and body chronic inflammation (CC = 0.24, p<0.05). No significant relationship was found between anti‐CagA antibody and atrophic or metaplastic changes in gastric mucosa.
Other factors associated with acid output
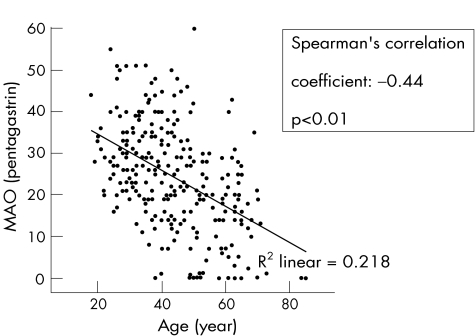
Age had an inverse relationship with acid output (CC = −0.44, p<0.01; fig 9). Calculation of the relationship between acid output and age in men and women separately showed a slightly greater inverse relationship among men than among women (−0.41 v −0.38).
Figure 9 Relationship of age with maximal acid output (MAO).
Regarding sex, men had mean (SD) acid output of 28.5 (13.2) mmol/h and women 21.4 (10.4) mmol/h. The difference with the independent t test was significant (p<0.01). Women were slightly older than men (median age 44 v 40 years, Mann–Whitney U test, p<0.05). Two other variables, body CIS and body atrophy were evaluated in both sexes, and we found no significant difference between men and women.
Smokers who smoked ⩾3 cigarettes per day had mean (SD) acid output of 29.9 (9.1) mmol/h compared with non‐smokers who had mean (SD) acid output of 26.5 (12.6) mmol/h. The difference between the two groups was at borderline significance (p = 0.05). The major histological changes such as antral‐combined inflammation, body‐combined inflammation and body atrophy were not significantly different between smoker and non‐smoker groups, but serum level of pepsinogen I was significantly higher in smokers than non‐smokers (125.4 (IQR 58.5) v (95.3 (IQR 69) p<0.05).
The patient's height showed a positive significant correlation with acid output, with CC = 0.43 and p<0.01. Weight also had a positive but less potent relationship (CC = 0.26, p<0.01) with acid output, but BMI did not show any significant relationship (CC = 0.02, p = 0.84). Sex subgroups were not different regarding the relationship of acid output and these variables.
Best predictors of acid output
To determine the best predictors of acid output, all variables, which had an obvious and marked linear relationship with acid, were selected. Body atrophy score, antral atrophy score, serum pepsinogen I, serum pepsinogen I:II ratio, serum gastrin, age, H pylori density of antrum and body, H pylori in one of the two sites and body CIS were entered as independent variables and acid output as dependent variable. The stepwise model produced four models. In the final model, according to standardised coefficients, body‐combined inflammation (β = −0.34), body atrophy (β = −0.30), serum pepsinogen I (β = 0.26) and age (β = −0.26) were the most independent predictors of acid output. All other variables were excluded from the model according to enter and remove criteria. The R and R2 in the final model were 0.79 and 0.62, respectively (table 1).
Table 1 Summary of stepwise linear regression analysis for predictors of maximal acid output.
| Predictors | Unstandardised coefficients | Standardised coefficients | p Value | Colinearity statistics | ||
|---|---|---|---|---|---|---|
| B | SE | β | Tolerance* | VIF† | ||
| Intercept (constant) | 42.38 | 7.00 | 0.00 | |||
| Body combined Inflammation | −2.56 | 0.79 | −0.34 | 0.00 | 67 | 1.50 |
| Body atrophy | −5.44 | 1.94 | −0.30 | 0.01 | 63 | 1.60 |
| Age | −0.33 | 0.11 | −0.26 | 0.00 | 97 | 1.04 |
| Serum pepsinogen I | 0.04 | 0.01 | 0.26 | 0.01 | 86 | 1.16 |
Dependent variable: maximal acid output.
R = 0.79, R2 = 0.62, SE of the estimate = 8.43
*Tolerance is the percentage of the variance in a given predictor that cannot be explained by other predictors.
†A variance inflation factor (VIF) >2 is usually considered problematic regarding colinearity with other predictors.
Discussion
H pylori infection produces a wide variety of patterns of gastritis.33,34,35,36,37,38,39,40,41,42 The pattern of gastritis varies in the extent to which it affects the antrum versus body region of the stomach and in the extent to which it produces acute inflammation, chronic inflammation, intestinal metaplasia and atrophy. Our study indicates that these different patterns of gastritis are associated with different levels of gastric acid secretion.
The antrum and body region of the stomach both have an important role in regulating gastric acid secretion. The antral mucosa contains the G cells, which release gastrin. This hormone is released in response to ingestion of protein‐containing food and it stimulates the oxyntic mucosa to secrete acid.43 The hormone acts on the gastrin receptors on the enterochromaffin‐like (ECL) cells of the oxyntic mucosa causing them to release histamine, which acts in a paracrine fashion, binding to the H2 receptors on the parietal cells and thereby stimulating them to secrete acid.44,45,46 Gastrin also exerts a trophic influence on the ECL cells and parietal cells of the oxyntic mucosa.47,48
H pylori gastritis influences the function of the antral mucosa, stimulating an increased release of gastrin by the G cells.27,49,50,51,52 It is unclear whether this is due to the influence of inflammatory cytokines53,54,55 or the ammonia produced by the urease activity of the bacterium, raising antral surface pH and thus blocking the physiological inhibition of gastrin release by luminal acid.56H pylori antral gastritis will thus tend to increase gastric acid secretion by increasing gastrin release. If the infection produces atrophy of the antral mucosa, the rise in gastrin will be moderated due to loss of G cells.57,58,28 As gastrin is released by ingestion of food, the main influence of the antrum is in meal‐stimulating acid secretion, which is technically difficult to measure. We assessed acid secretion in response to stimulation by exogenous gastrin, which acts directly on the oxyntic mucosa. Our study will therefore not have detected changes in meal‐stimulated acid secretion due to disturbances in antral function.
Our study shows the strong correlation between H pylori body gastritis and reduced gastric acid secretion. Both inflammation and atrophy of the body mucosa were strongly associated with reduced gastric acid secretion. The effect of the atrophy is clearly explained by loss of acid‐secreting parietal cells. The inflammation of the body mucosa is thought to produce functional inhibition of acid secretion. We, and others, have shown that eradicating H pylori in patients with low acid secretion produces recovery of secretion, which is associated with resolution of the body gastritis without any change in atrophy.28,59 The mechanism by which H pylori‐induced inflammation impairs the function of the oxyntic mucosa is not clear. However, the infection stimulates production of interleukin 1β, which is a powerful inhibitor of acid secretion, blocking the release of histamine from the ECL cells and also inhibiting the function of the parietal cells.60,61 Our study clearly shows that atrophy and inflammation are both independently associated with low acid secretion.
The serum concentrations of pepsinogens are indicators of gastric atrophy and consequently of gastric secretory function. Pepsinogen I originates from gastric fundic glands,62 whereas pepsinogen II is secreted by glands of the body and antral region of the stomach as well as the proximal duodenum.63 We found pepsinogen I to be the most useful predictor of the disturbance of acid secretion in our patients infected with H pylori. This is consistent with our histological studies confirming that atrophy of the body mucosa was the most powerful predictor of low acid secretion, as pepsinogen I is a strong predictor of body atrophy.
We also examined the influence of a variety of demographic characteristics of the patients on acid secretion. Our data showed that smokers who smoked ⩾3 cigarettes per day had greater acid secretion than non‐smokers. We found no significant relationship between cigarette smoking and major histological factors, but our data showed a higher level of pepsinogen I among smokers. Chronic smoking is associated with higher gastric acid secretion in subjects with and without ulcer.64,65,66,67 In addition, there is a significant positive association between the number of cigarettes smoked and the magnitude of acid secretion.68 Nicotine administrated intravenously stimulates acid secretion in humans.69 Chronic cigarette smoking stimulates the vagus and activates the parietal cells to enhance acid output.68 Increased serum pepsinogen I in smokers was another finding of our study, which could be related to their higher acid secretion with a parallel increase in chief cell secretory capacity.65
Our data indicate a marked decline in acid secretion with increasing age in male and female patients infected with H pylori. The median acid output in 20‐year‐olds was 35 mmol/h, which fell to 15 mmol/h in 70‐year‐olds. This negative association between acid secretion and age in patients infected with H pylori has been previously observed.70 By contrast, acid output has been reported to remain steady or increase with age in patients infected with H pylori.71 The lower acid secretion in the older patients infected with H pylori can be explained partly (but not completely) by more severe body gastritis and higher prevalence of atrophy.70,72,73 It is unclear to what extent the higher prevalence of low acid secretion and that of body gastritis and atrophy in older patients is due to increasing age or a cohort effect, where those born at an earlier time have increased predisposition to develop these abnormalities. In some patients with severe body atrophy, autoimmune gastritis may be an aetiological factor for hypochlorhydria in advanced age. This may result as a consequence of the interaction between H pylori infection and host response to the organism's antigens, and to gastric autoantigens including gastric H+K+ATPase.74
Our study showed that male patients have greater acid output than females. This finding is consistent with previous work,75,76 but its biological explanation has not been clearly defined. In women, smaller gastric surface area and corresponding smaller parietal cell mass or lower parietal cell reactivity or both may be responsible.76,77,78 The patient's height positively correlated with acid output, and this effect was similar in both sexes, suggesting that body surface may explain the greater acid secretion in men. Iijima et al70 reported that in subjects infected with H pylori, there was a more marked fall in acid output with increasing age in men versus women. This is consistent with our finding.
Owing to the number and variety of factors affecting acid secretion and the potential for interaction between them, we carried out stepwise linear regression analysis. This indicated four independent factors, (1) body inflammation, (2) body atrophy, (3) serum pepsinogen I and (4) age, as powerful predictors of acid secretion. A combination of these factors had a strong relationship with acid output and could be used for prediction of acid output in individual patients.
It should be emphasised that this study shows the association between the patterns of gastritis and gastric acid secretion but does not in itself indicate the nature of the association. Previous studies28,79,80 have shown that eradication of H pylori infection causes recovery of gastric acid secretion in patients with body‐predominant atrophic gastritis, thereby providing evidence that this histological pattern of gastritis can inhibit acid secretion. However, it is important to recognise also that acid secretion may affect the pattern of gastritis. This is shown by the fact that inhibition of acid secretion by proton pump inhibitors causes the pattern of gastritis to change from an antral‐predominant gastritis to a body‐predominant gastritis and may also cause acceleration of the atrophic process.81
Irrespective of the complex nature of the association between the pattern of gastritis and acid secretion, our study provides data allowing the prediction of acid secretion based on gastric histology, serological markers and patient characteristics. This may be useful in estimating levels of acid secretion in different populations as well as in individual patients.
Abbreviations
ANOVA - analysis of variance
BMI - body mass index
CagA - cytotoxin‐associated gene A
CIS - combined inflammatory score
ECL - enterochromaffin‐like
IQR - interquartile range
MAO - maximal acid output
Footnotes
Competing interests: None declared.
This study was approved by the Ethics Committee of the Western Infirmary, Glasgow, UK.
References
- 1.Hunt J N, Kay A W. The nature of gastric hypersecretion of acid in patients with duodenal ulcer. BMJ 19544902(Suppl)1444–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baron J H. The relationship between basal and maximum acid output in normal subjects and patients with duodenal ulcer. Clin Sci 196324357–370. [PubMed] [Google Scholar]
- 3.Koster K H. Gastric acid secretion in patients with duodenal ulcer. Scand J Gastroenterol 19661199–206. [PubMed] [Google Scholar]
- 4.Broome A, Bergstrom H, Olbe L. Maximal acid response to histamine in duodenal ulcer patients subjected to resection of the antrum and duodenal bulb followed by vagotomy. Gastroenterology 196752952–958. [PubMed] [Google Scholar]
- 5.Zhu H, Pace F, Sangaletti O.et al Gastric acid secretion and pattern of gastroesophageal reflux in patients with esophagitis and concomitant duodenal ulcer. A multivariate analysis of pathogenetic factors. Scand J Gastroenterol 199328387–392. [DOI] [PubMed] [Google Scholar]
- 6.Jahadi M R, Chandler J P. Detecting gastroesophageal reflux by pH recording and acid reflux test. Am Surg 197238281–284. [PubMed] [Google Scholar]
- 7.Csendes A, Larrain A, Uribe P. Gastric acid secretion in patients with a symptomatic gastroesophageal reflux and patients with esophageal strictures. Ann Surg 1974179119–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collen M J, Lewis J H, Benjamin S B. Gastric acid hypersecretion in refractory gastroesophageal reflux disease. Gastroenterology 199098654–661. [DOI] [PubMed] [Google Scholar]
- 9.Davenport H W. Is the apparent hyposecretion of acid by patients with gastric ulcer a consequence of a broken barrier to diffusion of hydrogen ions into the gastric mucosa? Gut 19656513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlborg L, Dahlgren S, Nordgren B. Gastric secretion of hydrochloric acid and sialic acid in patients with peptic ulcer and gastric cancer during intravenous infusion of histamine. Scand J Gastroenterol 19705427–431. [PubMed] [Google Scholar]
- 11.Kobayashi S, Kizu M, Kasugai T. Gastric acid secretion in relation to gross type of gastric cancer. Am J Gastroenterol 197360366–371. [PubMed] [Google Scholar]
- 12.Ogoshi K, Kondoh Y, Tajima T.et al Histologic type and gastric acid secretion in gastric cancer. Tumour Biol 199415263–268. [DOI] [PubMed] [Google Scholar]
- 13.Miyaji M, Ogoshi K, Tajima T.et al Association between serum gastrin levels, gastric acid secretion and age in early gastric cancer. Tumour Biol 199718311–320. [DOI] [PubMed] [Google Scholar]
- 14.Konturek S J, Starzynska T, Konturek P C.et al Helicobacter pylori and CagA status, serum gastrin, interleukin‐8 and gastric acid secretion in gastric cancer. Scand J Gastroenterol 200237891–898. [DOI] [PubMed] [Google Scholar]
- 15.Kaye M D, Rhodes J, Beck P. Gastric secretion in duodenal ulcer, with particular reference to the diagnosis of Zollinger‐Ellison syndrome. Gastroenterology 197058476–481. [PubMed] [Google Scholar]
- 16.Annibale B, De Magistris L, Corleto V.et al Zollinger‐Ellison syndrome and antral G‐cell hyperfunction in patients with resistant duodenal ulcer disease. Aliment Pharmacol Ther 1994887–93. [DOI] [PubMed] [Google Scholar]
- 17.Itoh T, Tatsuta M, Tamura H.et al Studies on serum gastrin of the patients with gastric cancer. Am J Gastroenterol 19776856–63. [PubMed] [Google Scholar]
- 18.Konturek P C, Hartwich A, Zuchowicz M.et al Helicobacter pylori, gastrin and cyclooxygenases in gastric cancer. J Physiol Pharmacol 200051(4 Pt)737–749. [PubMed] [Google Scholar]
- 19.Takaishi S, Cui G, Frederick D M.et al Synergistic inhibitory effects of gastrin and histamine receptor antagonists on Helicobacter‐induced gastric cancer. Gastroenterology 20051281965–1983. [DOI] [PubMed] [Google Scholar]
- 20.Locke GR I I I, Talley N J, Carpenter H A.et al Changes in the site‐ and histology‐specific incidence of gastric cancer during a 50‐year period. Gastroenterology 19951091750–1756. [DOI] [PubMed] [Google Scholar]
- 21.Kaneko S, Yoshimura T. Time trend analysis of gastric cancer incidence in Japan by histological types, 1975–1989. Br J Cancer 200184400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parkin D M, Bray F, Ferlay J.et al Global cancer statistics, 2002. CA Cancer J Clin 20055574–108. [DOI] [PubMed] [Google Scholar]
- 23.Sharma P, Sampliner R E. The rising incidence of esophageal adenocarcinoma. Adv Intern Med 200146137–153. [PubMed] [Google Scholar]
- 24.Rademaker J W, Hunt R H. Helicobacter pylori and gastric acid secretion: the ulcer link? Scand J Gastroenterol Suppl 199118771–77. [PubMed] [Google Scholar]
- 25.McColl K E, Fullarton G M, Chittajalu R.et al Plasma gastrin, daytime intragastric pH, and nocturnal acid output before and at 1 and 7 months after eradication of Helicobacter pylori in duodenal ulcer subjects. Scand J Gastroenterol 199126339–346. [DOI] [PubMed] [Google Scholar]
- 26.DeCross A J, Marshall B J. The role of Helicobacter pylori in acid‐peptic disease. Am J Med Sci 1993306381–392. [DOI] [PubMed] [Google Scholar]
- 27.El‐Omar E, Penman I, Dorrian C A.et al Eradicating Helicobacter pylori infection lowers gastrin mediated acid secretion by two thirds in patients with duodenal ulcer. Gut 1993341060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El‐Omar E M, Oien K, El‐Nujumi A.et al Helicobacter pylori infection and chronic gastric hyposecretion. Gastroenterology 199711315–24. [DOI] [PubMed] [Google Scholar]
- 29.Dixon M F, Genta R M, Yardley J H.et al Classification and grading of gastritis. The updated Sydney system. International workshop on the histopathology of gastritis, Houston 1994. Am J Surg Pathol 1996201161–1181. [DOI] [PubMed] [Google Scholar]
- 30.El–Omer E, Koien K, Muraay L.et al Increased prevalence of precancerous changes in relatives of gastric cancer patients: critical role of H. pylori. Gastroenterology 200011822–30. [DOI] [PubMed] [Google Scholar]
- 31.Hassan H A, Hobsley H. Positioning of subjects and of nasogastric tube during a gastric secretion study. BMJ 19701458–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Penelope M W, Cratbree J E, Forman Gastric cancer, cytotoxin‐associated gene A positive Helicobacter pylori, and serum pepsinogen: an international study. Gastroenterology 1999116269–276. [DOI] [PubMed] [Google Scholar]
- 33.Drumm B, Sherman P, Cutz E.et al Association of Campylobacter pylori on the gastric mucosa with antral gastritis in children. N Engl J Med 19873161557–1561. [DOI] [PubMed] [Google Scholar]
- 34.Blaser M J. Gastric Campylobacter‐like organisms, gastritis, and peptic ulcer disease. Gastroenterology 198793371–383. [DOI] [PubMed] [Google Scholar]
- 35.Le Bodic M F, Barre P, Freland C.et al Campylobacter pylori and gastric mucosa: histological and bacteriological study and preliminary results of an epidemiological survey in the area of Nantes. Gastroenterol Clin Biol 198711543–549. [PubMed] [Google Scholar]
- 36.Rokkas T, Pursey C, Uzoechina E.et al Campylobacter pylori and non‐ulcer dyspepsia. Am J Gastroenterol 1987821149–1152. [PubMed] [Google Scholar]
- 37.Sipponen P, Varis K, Celderberg A.et al Campylobacter pylori is associated with chronic gastritis but not with active peptic ulcer disease. APMIS 19889684–88. [DOI] [PubMed] [Google Scholar]
- 38.Michaletz P A, Graham D Y. Gastritis. Bringing this enigma into sharper focus. Postgrad Med 19888398–100, 1036. [DOI] [PubMed] [Google Scholar]
- 39.Doodley C P, McKenna D, Humphreys H.et al Histological gastritis in duodenal ulcer: relationship to Campylobacter pylori and effect of ulcer therapy. Am J Gastroenterol 198883278–282. [PubMed] [Google Scholar]
- 40.Barthel J S, Westblom T U, Havey A D.et al Gastritis and Campylobacter pylori in healthy, asymptomatic volunteers. Arch Intern Med 19881481149–1151. [PubMed] [Google Scholar]
- 41.Mahony M J, Wyatt J I, Littlewood J M. Campylobacter pylori gastritis. Arch Dis Child 198863654–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siurala M, Sipponen P, Kekki M. Campylobacter pylori in a sample of Finnish population: relations to morphology and functions of the gastric mucosa. Gut 198829909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simonsen M. Gastrin. Lancet 1965191420–421. [PubMed] [Google Scholar]
- 44.Blair AJ I I I, Feldman M, Barnett C.et al Detailed comparison of basal and food‐stimulated gastric acid secretion rates and serum gastrin concentrations in duodenal ulcer patients and normal subjects. J Clin Invest 198779582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waldum H L, Sandvik A K, Brenna E.et al Gastrin‐histamine sequence in the regulation of gastric acid secretion. Gut 199132698–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eysselein V E, Kovacs T O, Kleibeuker J H.et al Regulation of gastric acid secretion by gastrin in duodenal ulcer patients and healthy subjects. Gastroenterology 1992102(4 Pt)1142–1148. [PubMed] [Google Scholar]
- 47.Hansen O H, Pedersen T, Larsen J K.et al Effect of gastrin on gastric mucosal cell proliferation in man. Gut 197617536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barrowman J A. The tropic action of gastro‐intestinal hormones. Digestion 19751292–104. [DOI] [PubMed] [Google Scholar]
- 49.Tarnasky P R, Kovacs T O, Sytnik B.et al Asymptomatic H. pylori infection impairs pH inhibition of gastrin and acid secretion during second hour of peptone meal stimulation. Dig Dis Sci 1993381681–1687. [DOI] [PubMed] [Google Scholar]
- 50.Lehmann F S, Schiller N, Cover T.et al H. pylori stimulates gastrin release from canine antral cells in primary culture. Am J Physiol 1998274(6 Pt)G992–G996. [DOI] [PubMed] [Google Scholar]
- 51.McColl K E, Gillen D, El‐Omar E. The role of gastrin in ulcer pathogenesis. Baillieres Best Pract Res Clin Gastroenterol 20001413–26. [DOI] [PubMed] [Google Scholar]
- 52.Ohkusa T, Miwa H, Nomura T.et al Improvement in serum pepsinogens and gastrin in long‐term monitoring after eradication of Helicobacter pylori: comparison with H. pylori‐negative patients. Aliment Pharmacol Ther 200420(Suppl 1)25–32. [DOI] [PubMed] [Google Scholar]
- 53.Beales I, Blaser M J, Srinivasan S.et al Effect of Helicobacter pylori products and recombinant cytokines on gastrin release from cultured canine G cells. Gastroenterology 1997113465–471. [DOI] [PubMed] [Google Scholar]
- 54.Russo F, Jirillo E, Clemente C.et al Circulating cytokines and gastrin levels in asymptomatic subjects infected by Helicobacter pylori. Immunopharmacol Immunotoxicol 20012313–24. [DOI] [PubMed] [Google Scholar]
- 55.Zavros Y, Merchant J L. Modulating the cytokine response to treat Helicobacter gastritis. Biochem Pharmacol 200569365–371. [DOI] [PubMed] [Google Scholar]
- 56.El Nujumi A M, Dorrian C A, Chittajallu R S.et al Effect of inhibition of Helicobacter pylori urease activity by acetohydroxamic acid on serum gastrin in duodenal ulcer subjects. Gut 199132866–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruiz B, Correa P, Fontham E T.et al Antral atrophy, Helicobacter pylori colonization, and gastric pH. Am J Clin Pathol 199610596–101. [DOI] [PubMed] [Google Scholar]
- 58.Ricci C, Vakil N, Rugge M.et al Serological markers for gastric atrophy in asymptomatic patients infected with Helicobacter pylori. Am J Gastroenterol 2004991910–1915. [DOI] [PubMed] [Google Scholar]
- 59.Gillen D, Wirz A A, McColl K E. Helicobacter pylori eradication releases prolonged increased acid secretion following omeprazole treatment. Gastroenterology 2004126980–988. [DOI] [PubMed] [Google Scholar]
- 60.Uehara A, Okumura T, Sekiya C.et al Interleukin‐1 inhibits the secretion of gastric acid in rats: possible involvement of prostaglandin. Biochem Biophys Res Commun 19891621578–1584. [DOI] [PubMed] [Google Scholar]
- 61.Saperas E, Yang H, Tache Y.et al Interleukin‐1 beta acts at hypothalamic sites to inhibit gastric acid secretion in rats. Am J Physiol 1992263(3 Pt)G414–G418. [DOI] [PubMed] [Google Scholar]
- 62.Samloff I M. Cellular localization of group I pepsinogen in human gastric mucosa by immunoflourescence. Gastroenterology 197161185–188. [PubMed] [Google Scholar]
- 63.Samloff I M, Liebman W M. Cellular localization of the group II pepsinogens in human stomach and duodenum by immunoflourescence. Gastroenterology 19736536–42. [PubMed] [Google Scholar]
- 64.Kaufmann D, Wilder‐Smith C H, Kempf M.et al Cigarette smoking, gastric acidity and peptic ulceration. What are the relationships? Dig Dis Sci 1990351482–1487. [DOI] [PubMed] [Google Scholar]
- 65.Parente F, Lazzaroni M, Sangaletti O.et al Cigarette smoking, gastric acid secretion, and serum pepsinogen I concentrations in duodenal ulcer patients. Gut 1985261327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lanas A, Hirschowitz B I. Influence of smoking on basal and on vagally and maximally stimulated gastric acid and pepsin secretion. Scand J Gastroenterol 199227208–212. [DOI] [PubMed] [Google Scholar]
- 67.Whitfield P F, Hobsley M. Comparison of maximal gastric secretion in smokers and non‐smokers with and without duodenal ulcer. Gut 198728557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ligny G, Van Ccauter J, Henry J P. The effect of cigarette smoking on the cicatrization of duodenal ulcers in patients treated with cimetidine. The role of acid hypersecretion. Rev Med Brux 198910233–238. [PubMed] [Google Scholar]
- 69.Mertz D P, Thongbhoubesra T. Effect of nicotine on the production of gastric acid (translation). Med Klin 197671147–155. [PubMed] [Google Scholar]
- 70.Iijima K, Ohara S, Koike T.et al Gastric acid secretion of normal Japanese subjects in relation to Helicobacter pylori infection, aging, and gender. Scand J Gastroenterol 200439709–716. [DOI] [PubMed] [Google Scholar]
- 71.Harouma K, Kamada T, Kawaguchi H.et al Effect of age and Helicobacter pylori infection on gastric acid secretion. J Gastroenterol Hepatol 200015277–283. [DOI] [PubMed] [Google Scholar]
- 72.Katelaris P H, Seow F, Lin B P.et al Effect of age, Helicobacter pylori infection, and gastritis with atrophy on serum gastrin and gastric acid secretion in healthy men. Gut 1993341032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feldman M, Cryer B, McArthur K E.et al Effects of aging and gastritis on gastric acid and pepsin secretion in humans: a prospective study. Gastroenterology 19961101043–1052. [DOI] [PubMed] [Google Scholar]
- 74.Uibo R. Contribution of epidemiological studies to gastritis immunology. Int Rev Immunol 20052431–54. [DOI] [PubMed] [Google Scholar]
- 75.Collen M J, Abdulian J D, Chen Y K. Age does not affect basal gastric acid secretion in normal subjects or in patients with acid‐peptic disease. Am J Gastroenterol 199489712–716. [PubMed] [Google Scholar]
- 76.Kekki M, Samloff I M, Ihamaki T.et al Age‐ and sex‐related behaviour of gastric acid secretion at the population level. Scand J Gastroenterol 198217737–743. [DOI] [PubMed] [Google Scholar]
- 77.Kekki M, Sipponen P, Siurala M. Age behaviour of gastric acid secretion in males and females with a normal antral and body mucosa. Scand J Gastroenterol 1983181009–1016. [DOI] [PubMed] [Google Scholar]
- 78.Grahnquist L, Ruuska T, Finkel Y. Early development of human gastric H,K‐adenosine triphosphatase. J Pediatr Gastroenterol Nutr 200030533–537. [DOI] [PubMed] [Google Scholar]
- 79.Haruma K, Mihara M, Okamoto E.et al Eradication of Helicobacter pylori increases gastric acidity in patients with atrophic gastritis of the corpus‐evaluation of 24‐h pH monitoring. Aliment Pharmacol Ther 199913155–162. [DOI] [PubMed] [Google Scholar]
- 80.Sipponen P, Kekki M, Seppala K.et al The relationships between chronic gastritis and gastric acid secretion. Aliment Pharmacol Ther 199610(Suppl 1)103–118. [DOI] [PubMed] [Google Scholar]
- 81.Kuipers E J, Uyterlinde A M, Pena A S.et al Increase of Helicobacter pylori‐associated corpus gastritis during acid suppressive therapy: implications for long‐term safety. Am J Gastroenterol 1995901401–1406. [PubMed] [Google Scholar]