Abstract
Sinus histiocytosis with massive lymphadenopathy (SHML), also designated as Rosai–Dorfman disease (RDD), is a rare benign reactive lymphoproliferative disorder. It is defined by a characteristic histopathology with sinus histiocytosis and haemophagocytosis known as emperipolesis. In histiocytes S100 is strongly expressed, whereas CD1a staining typically is negative. The disease mainly manifests at a single lymph node; however, multilocular and extranodal affection can occur. Causative infectious agents, and virus infections in particular, have repeatedly been suspected, although until now the origin of the disease has been unclear. Four cases of RDD (two nodal sites and two extranodal upper respiratory tract sites) were analysed for parvovirus B19 (B19) infection by immunohistochemistry to detect B19 capsid proteins VP1/VP2. In all the four cases, huge numbers of B19‐positive cells were partly detected. The positive cells were identified either as lymphocytes or, in one extranodal case, also as respiratory epithelial cells. This is the first report of B19 infection in RDD tissue, indicating that B19 may be associated with the pathogenesis of SHML.
Sinus histiocytosis with massive lymphadenopathy (SHML) was first described in 1969 by Rosai and Dorfman,1 giving rise to the term Rosai–Dorfman disease (RDD). RDD is a benign lymphoproliferative disorder that typically occurs as self‐limited, mainly unilateral, painless lymphadenopathy.2 In the initial stage, preceding RDD symptoms, fever and pharyngitis may be observed.2 Most often, in as many as 90% of patients, the lymph nodes of the cervical region are primarily affected. Multilocular or extranodal manifestations of SHML (ENSHML), however, affecting various organs—for example, the skin, soft tissue, upper or lower respiratory tract, bone, brain and liver—can be observed in 43% of patients.2,3 RDD is defined by a characteristic histology showing massive, although non‐malignant, accumulation of histiocytes and lymphophagocytosis and erythrophagocytosis, which is known as emperipolesis in lymph node sinuses and affected extranodal tissues.1,2 By immunotyping in RDD histiocytes, especially strong expression of S100 protein and expression of various pan‐macrophage antigens such as CD68 is observed, whereas CD1a staining, which is found in Langerhans cell histiocytosis, is typically negative.4,5
Additional clinical findings in patients with RDD comprise haematological and immunological abnormalities, such as normocytic or microcytic anaemia or autoimmune haemolytic anaemia.2,6 Furthermore, affection of joints with polyarthralgia or rheumatoid arthritis, glomerulonephritis, diabetes mellitus, asthma and, rarely, serological autoimmune findings such as rheumatoid factor and antinuclear antibodies are reported as associated features in RDD.3
The nature and origin of RDD causing lymphoproliferation, and its peculiar histopathology have long been discussed.3 Infectious agents, and virus infections in particular, have repeatedly been suggested to have a primary role in the development of RDD.3 Although there has been some evidence of association of Epstein–Barr virus (EBV) and human herpes virus 6, the pathogenic mechanism in RDD is still unclear.7,8,9
Parvovirus B19 (B19) is the pathogen in erythema infectiosum.10 It primarily affects red blood progenitor cells through the erythrocyte P antigen receptor, causing haemolytic anaemia.11,12 In immunocompromised people or patients with haematological disorders, B19 can cause severe aplastic crisis.13 In prenatal infection, particularly in the second trimester fetus, severe anaemia with fatal fetal hydrops may occur.14 Accompanying arthralgia and prolonged arthropathy are known features of B19 infection.15,16 More recently, B19 has been shown to be associated with a variety of inflammatory disorders characterised or accompanied by lymphoproliferation or autoimmunological phenomena, such as rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis; vasculitides, such as giant‐cell arthritis, autoimmune haemolytic anaemia, interstitial lung disease with hepatitis and myositis; and adult and neonatal cardiomyopathy.16,17,18 In a recent study, we detected B19 capsid proteins in the lymphocytes of about 90% of synovial tissues analysed in patients with rheumatoid arthritis.19 To investigate a potential role of parvovirus B19 in RDD, we analysed four cases of RDD for the presence of B19 virus infection by immunohistochemistry, detecting B19 VP1/VP2 capsid proteins. Two patients had SHML; in the other two cases, ENSHML sites in the upper respiratory tract were analysed.
Case selection and histories
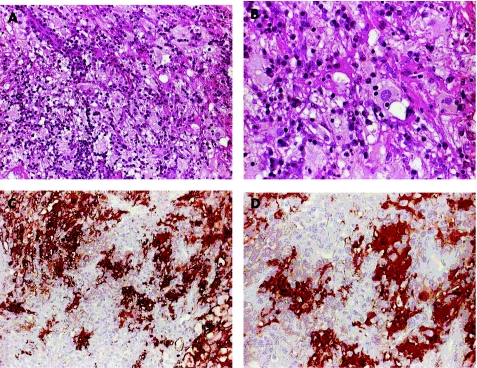
Four cases of RDD were analysed (table 1). The diagnosis of RDD or SHML was based on the characteristic histopathological findings of mainly histiocytosis with emperipolesis, (fig 1A,B) combined with immunophenotypical detection of S100 protein and negative CD1a antigen expression (S100+/CD1a−; fig 1C,D). Two patients (cases 1 and 2) had only nodal affection of cervical or thoracic wall lymph nodes, respectively. The other two patients (cases 3 and 4) showed extranodal manifestation of RDD in the upper respiratory tract, with documented association of cervical lymph nodes (cases 3 and 4) and other organ sites (case 3); in the last two cases only biopsy material from extranodal manifestation sites was investigated for B19 infection.
Table 1 Synopsis of data and clinical findings in the analysed patients with RDD.
| Case no | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Age (in years) at diagnosis | 43 | 67 | 63 | 43 |
| Sex (M/F) | F | F | M | M |
| Nodal manifestation sites | Thoracic wall | Cervical | Cervical, upper arm, axillar | Cervical |
| Extranodal manifestation sites | ND | ND | URT, kidney, skin | URT |
| Preceding symptoms | Laryngitis | URT infection | NR | NR |
| Duration | 1–2 months | 3 months | >7 years | >2 years |
| Associated anamnestical information | Chronic monoarthropathy | Diabetes mellitus type 2 | Polymyalgia rheumatica | NR |
| Associated laboratory findings | — | ANA antibodies+ | Anaemia, hyper‐ gammaglobulinaemia. | — |
| Infection serology or direct virus analyses by IHC or ISH | NA | CMV−, HIV−, EBV−, IgG+/IgM−, borellia−, toxo−, plasma−, IgG+/IgM− | IHC: CMV−, HPV−, EBV−, HSV1/2−; ISH: EBV−,CMV− | NA |
| Tissue with positive B19 detection (IHC) | Lymph node | Lymph node | URT (nasal cavity mucosa) | URT (sinus mucosa) |
ANA, anti‐nuclear antibodies; CMV, cytomegalovirus; EBV, Epstein–Barr virus; F, female; HPV, human papilloma virus; HSV1/2, human simplex virus type 1 and type 2; Ig, immunoglobulin; IHC, immunohistochemistry; ISH, in situ hybridisation; M, male; NA, not analysed; ND, not detected; NR, not reported; RDD, Rosai–Dorfman disease; URT, upper respiratory tract; +, positive; −, negative.
Figure 1 Histomorphology and S100 immunolabelling of Rosai–Dorfman disease (RDD) in affected nasal mucosa (case 3). (A,B) Haematoxylin and eosin staining showing lymphoplasmocytic infiltration and intermingling of huge amounts of large pale histiocytes with lymphopagocytosis (A, ×20); emperipolesis can be observed in several histiocytes showing internalised lymphocytes in the cytoplasm (B, ×40). (C,D) Peroxidase‐mediated S100 staining (dimethylaminoazobenzene, brown) showing strong S100 expression in RDD histiocytes (C, ×20; D, ×40).
Case 1
A 43‐year‐old woman was admitted to the gynaecology department owing to a newly developed palpable node at the lateral border of the right mammary gland, at the thoracic wall. Sonography disclosed a hypoechoic node suspicious for a lymph node. Anamnestically, laryngitis accompanied by high fever >39°C shortly before the development of the node was reported. The node was surgically removed. Characteristic histopathological and immunophenotypical findings led to the diagnosis of RDD. After surgery, there was no recurrence of any specific symptoms; however, in a follow‐up investigation several years later, chronic unilateral osteoarthritis of the right shoulder joint persisting over about the same period was mentioned. Serological analyses for infectious diseases have not, unfortunately, been carried out.
Case 2
After an upper respiratory infection, a 67‐year‐old woman presented with bilateral, cervical lymphadenopathy persisting for about 3 months with varying lymph node size and intermittent pain. Anamnestically, diabetes mellitus type 2 and arterial hypertony were reported.
Clinical evaluation and ultrasonography showed (bilateral, but predominantly right‐sided) several enlarged oval hypoechoic lymph nodes with a size up to a maximum of 2×1.3 cm in the upper cervical angle. Additionally, in both parotid glands hypoechoic areas were detected, suspicious for enlarged lymph nodes; one larger hypoechoic structure of 1.2×2.5 cm was detected in the left parotid gland suspicious for a benign parotid tumour. The largest cervical lymph node was surgically removed. The histopathological and immunophenotypical analysis (S100+; CD1a−) showed RDD. Serological analyses were negative for cytomegalovirus, HIV and Borrelia burgdorferi; for Toxoplasma gondii as well as for EBV (EBV‐viral capsid antigen and Epstein–Barr nuclear antigen (test))‐positive immunoglobulin (Ig)G but negative IgM were detected, hinting at an infection the patient had earlier. A positive titre of anti‐nuclear IgA of 1:40 was detected.
Case 3
A 63‐year‐old man presented to the otorhinolaryngeal department with an extremely enlarged cervical lymph node, a submucosal tumorous lesion of the right main nasal cavity and an enlarged lymph node of 1 cm diameter on the right proximal arm. Ten months earlier, rheumatoid factor negative arthralgia had led to the diagnosis of polymyalgia rheumatica, which disappeared after corticosteroid treatment. After diagnostic removal of the lymph nodes, a diagnosis of RDD was established from the characteristic histopathological and immunophenotypical findings. The consecutively excised nasal tumour tissue similarly showed an unusually severe diffuse infiltration of the mucosal tissue by large histiocytes with foamy cytoplasm and mainly small mature lymphocytes and emperipolesis (fig 1A,B). The B lymphocyte infiltrates were immunohistochemically polyclonal, as high amounts of plasma cells with κ or λ chains were observed. Positive S100 and negative CD1a immunostaining, as in the nodal sites, indicated an extranodal manifestation of RDD or ENSHML (fig 1C,D). Direct virus analyses of the primary affected lymph node tissues yielding virus proteins (immunohistochemistry) of human papilloma virus, cytomegalovirus, EBV and human simplex virus type 1 and 2 or virus DNA (EBV, cytomegalovirus; DNA‐in situ hybridisation) did not identify any local virus infection. Extended analyses by differential phenotyping (25F9, MRP8, MRP14, 27E10, RM3/1, MAC387, lysocyme, Ki‐M1P, Ki‐M4P, CD14, CD68, CD30, human leucocyte antigen‐DR, CD1a, Lag) and RNA‐in situ hybridisation (HMSE‐1, c‐fms, M‐CSF, RelB) in affected tissues of this patient provided evidence for the monocyte or macrophage, but not for the dendritic cell origin of the SHML histiocytes and cytokine relationship of the disorder.20 Additional clinical findings in the patient consisted of anaemia, polyclonal hypergammaglobulinaemia, slight leucocytosis with relative lymphopenia, and increased erythrocyte sedimentation rate and C reactive protein. Over a course of 7 years after the primary diagnosis, repeated diagnostic biopsies of partly newly developed lesions showed further RDD association of bilateral mucosa from the main nasal cavity, both kidneys, axillar and cervical lymph nodes, and skin with multiple indurated purple red lesions of the upper back, thorax and left arm. An initial corticoid treatment reduced the tumorous renal masses, and the patient was kept, under permanent low‐dose corticoid drugs, at a steady state of disease.
Case 4
A 43‐year‐old man had chronic sinusitis with hyperplastic changes of the left‐sided nasal cavity, maxillary and ethmoidal sinuses, with obstruction of the nasal cavity.
Biopsy material from polyps of the left maxillar sinus, the left lower nasal concha and the middle nasal tract showed dense subepithelial infiltration with partly follicular‐arranged lymphocytes and plasma cells, intermingling with huge masses of enlarged histiocytes with partly detectable lymphophagocytosis. Immunostaining was positive for S100 and negative for CD1a, leading to a diagnosis of extranodal RDD or ENSHML.
Clinical investigations showed hypothyreosis. A sonographically detected hypoechoic, scintigraphic negative thyroid node was bioptically analysed for RDD; no signs of RDD participation of the thyroid gland were detected.
Fifteen months later, unilateral cervical lymphadenopathy led to the surgical removal of five small and two enlarged cervical lymph nodes together twice up to 3×1.5×1 cm in size; both enlarged lymph nodes showed diffuse lymphocytic hyperplasia and moderate sinus histiocytosis compatible with the previous diagnosis of RDD, whereas the small lymph nodes did not show any pathological changes. Owing to ongoing problems of the upper respiratory tract after a further 12 months, more than 2 years after primarily establishing the diagnosis of RDD or ENSHML, mucosal tissue was surgically removed from the left maxillary and left ethmoidal sinuses. Histological examination showed scarified maxillar and ethmoidal sinus mucosa with massive plasma cellular infiltration (S100+; CD1a−). Serological analyses have, unfortunately, not been carried out in this patient.
Methods
B19 immunohistochemistry
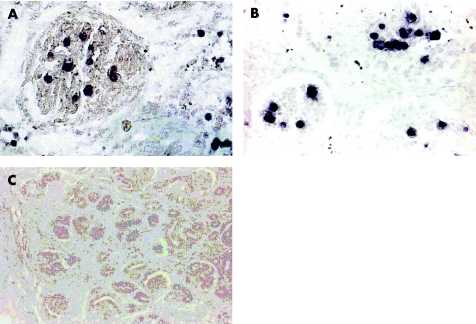
For parvovirus B19 detection, a monoclonal mouse antibody reactive to parvovirus B19 VP1 and VP2 capsid proteins was used (kindly provided by Dade Behring, Marburg, Germany). VP1 (structural protein 1) and VP2 (structural protein 2) genes overlap, whereas the VP2 open reading frame is totally included in the VP1 open reading frame.21,22 The antibody that was used recognises VP1 and VP2 proteins. In addition, immunohistochemical analysis was carried out using the commercially available monoclonal mouse anti‐B19 VP1/VP2 antibody R92F6 (Novocastra, Newcastle upon Tyne, UK). Kidney tissue of a B19‐infected hydropic fetus, analysed earlier by B19‐DNA‐in situ hybridisation (fig 2A), was used as positive control (fig 2B); by antibody staining, B19‐positive cells were specifically detected to the same extent and with the same histological distribution as that by in situ hybridisation, intravasal or in areas of fetal haematopoiesis.23 Immunohistochemical investigation of B19‐negative fetal kidney control tissue did not show positive staining of cells (fig 2C). B19 specificity of the antibody had been proved by immunoabsorption assay.19 Immunohistochemical analysis was carried out on routinely processed formalin‐fixed and paraffin‐wax‐embedded tissue sections. After dewaxing in xylene and rehydration in a descending alcohol series, tissue sections were subjected to proteolytic treatment in 0.1% pronase (Protease type XXIV, Sigma‐Aldrich, St Louis, Missouri, USA) in phosphate‐buffered saline (2–6 min, room temperature), followed by application of parvovirus B19 VP1/VP2 antibody (1:100 in 1% bovine serum albumin/phosphate‐buffered saline) and incubation for 1 h at room temperature. Finally, the primary antibody was detected by alkaline phosphatase‐mediated nitroblue tetrazolium staining, using secondary antibody steps according to the alkaline phosphatase‐anti‐alkaline phosphatase protocol. In positive cases, control experiments omitting the primary VP1/VP2 antibody showed negative staining results (fig 3D). Additional immunohistochemistry experiments, which were extended to all RDD sections, used the B19 VP1/VP2 antibody R92F6 (Novocastra) diluted 1:30 in 1% bovine serum albumin or phosphate‐buffered saline, following the above protocol or with alternative microwave pretreatment in 0.01 M citric acid, pH 6.0.
Figure 2 Control experiments in B19‐positive fetal kidney tissue from a fetal hydrops case detecting B19‐DNA and B19‐VP1/VP2 protein by DNA‐in situ hybridisation using a B19‐DNA probe analysed previously (A, ×40) and B19‐VP1/VP2 immunohistochemical analysis (B, ×40) using alkaline phosphatase‐mediated nitroblue tetrazolium or 5‐bromo‐4‐chloro‐3‐indoyl phosphate staining (purple black). Detection of DNA and protein show a similar amount and similar types of positive cells by either method. Negative B19VP1/VP2 staining in a B19‐negative fetal kidney tissue (C, ×20) shows that the B19 antibody does not non‐specifically recognise cells in the kidney.
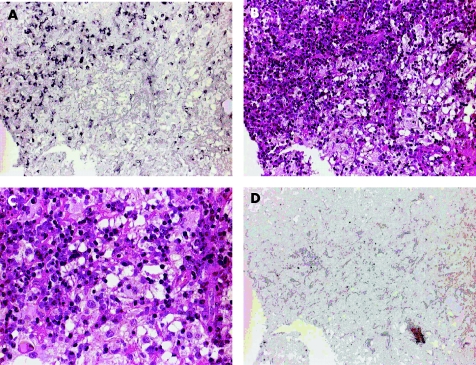
Figure 3 Localisation and morphological identification of B19‐positive cells comparing B19 immunohistochemistry (IHC) and respective haematoxylin and eosin (H&E) staining of Rosai–Dorfman disease (RDD)‐affected nasal mucosa tissue (case 3). (A) B19 VP1/VP2 IHC using alkaline phosphatase‐mediated nitroblue tetrazolium staining (purple black) showing an accumulation of multiple B19‐positive cells with lymphocyte morphology (×20). (B) H&E staining of the same tissue area as shown in (A), localising B19 detection in an area of dense lymphoplasmocytic infiltration and predominantly in a B19‐negative area with neighbouring histiocytic cells (×20). (C) H&E‐stained tissue at higher magnification (×40) showing lymphophagocytosis of histiocytes at the border to the lymphoplasmocytic infiltration. (D) IHC negative control of the respective RDD tissue omitting the B19 VP1/VP2 antibody, showing no specific staining of cells (×20).
Immunophenotyping and cell‐type characterisation
Tissue sections were analysed for expression of S100 protein and CD1a antigen by immunohistochemistry, using a polyclonal rabbit anti‐S100 antibody (DAKO, Hamburg, Germany; dilution 1:2000) and a monoclonal mouse anti‐CD1a antibody (Immunotech, Westbrook, Maine, USA), respectively. S100 and CD1a labelling was carried out by peroxidase‐mediated 3‐amino 9‐ethylcarbazole and dimethylaminoazobenzene staining, using the automated Nexus program (Ventana, Medical Systems, Tucson, Arizona, USA; S100) and a multistep detection, using a biotinylated secondary antibody, avidin or biotin peroxidase complex and tyramide signal amplification (CD1a), respectively.
Characterisation of B19‐positive cell types was carried out using a B cell‐specific mouse monoclonal CD20 antibody (DAKO; dilution1:30) and a T cell‐specific mouse monoclonal CD3 antibody (Novocastra; dilution 1:100). For immuno double staining after the earlier mentioned VP1 and VP2 detection, CD20 or CD3 labelling was carried out by peroxidase‐mediated 3‐amino 9‐ethylcarbazole staining using the automated Nexus program (Ventana).
Results
B19 immunohistochemical analysis was carried out in the excised lymph nodes of cases 1 and 2 with RDD, in the tumorous nasal tissue of case 3 and in primary biopsy material (left middle nasal tract, left lower concha and left maxilla) as well in recent biopsy material obtained 2 years later (left maxilla and left ethmoid) from the upper respiratory tract of case 4.
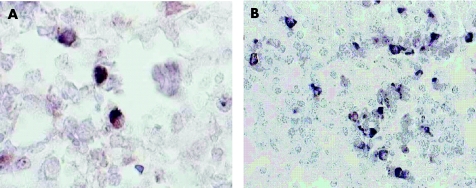
By B19 immunohistochemistry in both RDD lymph nodes (cases 1 and 2) and in all analysed extranodal RDD tissue specimens (cases 3 and 4) partly high amounts of B19 capsid‐positive cells were detected (fig 4). Interestingly, B19‐positive cells were identified mainly as lymphocytic cells (fig 3). By immuno double staining for T cell and B cell markers in both nodal cases (cases 1 and 2), B19‐positive cells were assigned to CD20‐positive B lymphocytes and CD3‐positive T lymphocytes (fig 5).
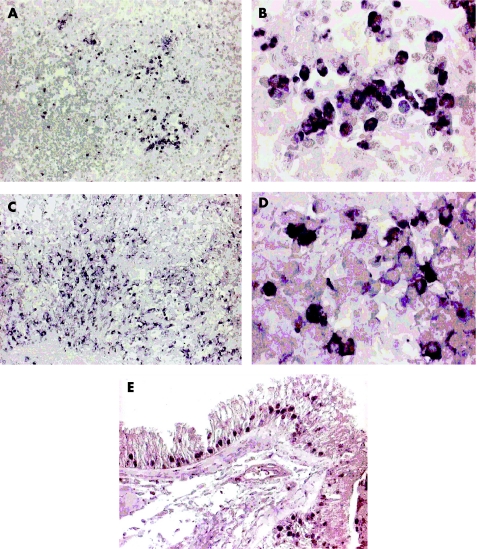
Figure 4 Detection of parvovirus B19 VP1 and VP2 capsid proteins in Rosai–Dorfman disease (RDD) tissues by immunohistochemistry using alkaline phosphatase‐mediated nitroblue tetrazolium staining (purple black) with or without methyl green counterstain. (A, B) Several B19‐positive cells in RDD lymph node tissue (case 2), partly accumulated and partly loosely scattered (A, ×20); at higher magnification (B, ×100) nuclear or cytoplasmic B19 staining can be observed, with positive cells showing lymphocyte morphology. (C, D) Multiple B19‐positive cells with lymphocyte morphology spread in the RDD‐affected nasal mucosa of case 3 (C, ×20; D, ×100). (E) Multiple B19‐positive respiratory epithelial cells detected in the affected sinus tissue of case 4 (×40).
Figure 5 Co‐immunostaining of B19 VP1/VP2 protein (nitroblue tetrazolium, purple black) and CD20 antigen (3‐amino 9‐ethylcarbazole, brown; A, ×100) or CD3 antigen (dimethylaminoazobenzene, brown; B, ×40) in the Rosai–Dorfman disease‐affected lymph node of case 1. Double labelling of B19‐positive cells with CD20 and CD3 antibodies shows the B lymphocyte and T lymphocyte origin of the virus‐infected cells.
B19‐positive histiocytes could not be detected in any case. However, accumulation of B19‐positive lymphocytic cells often occurred next to or intermingling with areas of dense histiocytosis in which emperipolesis was observed (fig 3). Clear evidence of phagocytosis of B19‐positive lymphocytes by the RDD histiocytes, however, could not be seen owing to methodical limitations (haematoxylin and eosin counterstain interfering with nitroblue tetrazolium staining).
In tissue samples from case 4, showing affection of the upper respiratory tract and virus positive lymphocytic cells in respiratory epithelial cells, regional staining for B19 VP1 and VP2 was observed (fig 4E).
Positive staining results were obtained in sections of the same tissue samples and in the same areas, respectively, using either the non‐commercial B19 antibody (Dade‐Behring) or the commercially available B19‐R92F antibody (Novocastra); it should be mentioned, however, that stronger staining intensity in general was obtained with the first.
Discussion
Infections, and virus infections in particular, have long been suspected of having a primary role in RDD.3 Human herpes virus‐6 infection has been reported in a small set of cases with RDD.7,8 However, conclusive evidence on the cause of RDD is still unavailable. B19 has more recently been implicated in inflammatory or autoimmune diseases.16,18 We analysed tissue sections of two patients with nodal RDD or SHML and two patients with ENSHML for the presence of B19 capsid proteins VP1 and VP2. We showed remarkable amounts of B19‐infected cells in the two nodal and the two extranodal RDD manifestations. This is the first report of B19 infection in RDD tissue indicating that B19 may be associated with the pathogenesis of RDD.
Several features of RDD, such as arthropathy and arthritis, autoimmune haemolytic anaemia and the association with autoimmune phenomena such as findings of rheumatoid factor are well‐known symptoms related to B19 infection, and are thus compatible with a virus‐induced origin of RDD.2,3,16,18
Interestingly, histiocytes, the characteristic and pathognomonic cells in SHML, did not show B19 infection. B19 capsid protein‐positive cells histomorphologically appeared as lymphocytic cells; B cellular and T cellular origin of infected cells was seen in both RDD lymph node tissues by immuno double labelling for CD20 and CD3.
This may indicate that primarily virus‐infected lymphocytes give rise to a secondary histiocytic reaction that eventually causes the typical histomorphology of RDD.
The observation of B19‐positive lymphocytes is unexpected, as B19 infection is known to occur mainly in the red blood cell lineage, using the erythrocyte P antigen (glycosphingolipid globoside Gb4) as a receptor.11 However, B19 infection has also been seen in several other cell types such as the T lymphocytes and B lymphocytes.24 Gb4 was identified as a major neutral glycosphingolipid in 11 tissues, particularly those of mesodermal origin.25 Furthermore, B19 capsid binding to several tissue‐specific glycosphingolipids was seen in various tissues, including granulocyte, kidney, liver and bowel tissue.25 The finding of B19 capsid proteins in lymphocytic cells thus further indicates that B19 infection is not restricted to the erythroid lineage and that different cellular receptors may also be used for B19 infection of other cell types.25 Likewise, besides anaemia, thrombopenia and lymphocytopenia have been reported as clinical findings associated with B19 infection, suggesting that these cell types may somehow be affected by B19.26 To rule out antibody‐specific staining failures, two different monoclonal B19 VP1/VP2 antibodies were used, showing qualitatively identical results; a cross reactivity with, for example, activated lymphocytes of the antibodies, however, may still be possible.
In synovial tissues of patients with rheumatoid arthritis, which like RDD often show massive lymphoplasmocytic infiltration, we showed B19 infection of lymphocytes in a recent study.19 Also, B19 infection was shown in a few other inflammatory synovial affections exhibiting a similar rheumatoid arthritis‐like histomorphology with lymphoplasmocytic proliferation.19 These observations hinted at a specific reactive histomorphological phenotype associated with B19 infection of specific cell types. It has to be mentioned, however, that huge masses of histiocytes are not observed in rheumatoid arthritis. The report of B19‐associated haemophagocytosis of histiocytes in the bone marrow of a patient with Evans syndrome is of interest in this regard.27 This report also links B19 infection to the distinctive histomorphological phenomenon of histiocytosis and emperipolesis. Even more remarkable is the nature of the underlying disease associated with B19 in this case. Evans syndrome is a haematological disorder defined by autoimmune destruction of at least two haematological cell types. Recently, it has been suggested that a subset of patients with Evans syndrome may have autoimmune lymphoproliferative syndrome (ALPS), a rare disorder of disrupted lymphocyte homeostasis showing lymphoproliferation and autoimmune features.28 In most patients with ALPS, defective Fas‐mediated apoptosis, caused by defects in the Fas gene and Fas ligand gene, is reported.28,29 Recently, cases of RDD or SHML associated with ALPS were described.30 Also, Fas gene mutations were detected in a small subset of patients with SHML without ALPS.3 A primary genetic background as in ALPS, however, is not documented in RDD. Summarising these data, it has been hypothesised, that in most cases RDD or SHML may be an acquired disorder of deregulation of apoptotic signalling pathways.3
We detected B19 infection in high numbers of cells in nodal and extranodal RDD tissues in a small set of cases. This finding indicates that B19 may have an important role in the development of RDD or SHML, thus adding another disease to the spectrum of B19‐related conditions. The possible mechanisms by which B19 may contribute to the specific pathological reactions, however, especially when infecting non‐permissive cell types, are still little understood.
Further studies on B19 cellular interactions using larger numbers of cases with RDD or SHML are required to elucidate the role of B19 in RDD or SHML.
Abbreviations
ALPS - autoimmune lymphoproliferative syndrome
EBV - Epstein–Barr virus
ENSHML - extranodular manifestation of SHML
RDD - Rosai–Dorfman disease
SHML - sinus histiocytosis with massive lymphadenopathy
Footnotes
Competing interests: None.
References
- 1.Rosai J, Dorfman R T. Sinus histiocytosis with massive lymphadenopathy; a newly recognized benign clinicopathological entity. Arch Pathol 19698763–70. [PubMed] [Google Scholar]
- 2.Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai‐Dorfman disease): review of the entity. Semin Diagn Pathol 1990719–73. [PubMed] [Google Scholar]
- 3.McClain K L, Natkunam Y, Swerdlow S H. Atypical cellular disorders. Hematology (Am Soc Hematol Educ Program) 2004238–296. [DOI] [PubMed]
- 4.Eisen R N, Buckley P J, Rosai J. Immunophenotypic characterization of sinus histiocytosis with massive lymphadenopathy (Rosai‐Dorfman disease). Semin Diagn Pathol 1990774–82. [PubMed] [Google Scholar]
- 5.Paulli M, Rosso R, Kindl S.et al Immunophenotyic characterization of the cell infiltrate in five cases of sinus histiocytosis with massive lymphadenopathy (Rosai‐Dorfman disease). Hum Pathol 199223647–654. [DOI] [PubMed] [Google Scholar]
- 6.Grabczynska S A, Toh C T, Francis N.et al Rosai‐Dorfman disease complicated by autoimmune haemolytic anaemia: case report and review of a multisystem disease with cutaneous infiltrates. Br J Dermatol 2001145323–326. [DOI] [PubMed] [Google Scholar]
- 7.Levine P H, Jahan N, Murari P.et al Detection of human herpesvirus 6 in tissues involved by sinus histiocytosis with massive lymphadenopathy (Rosai‐Dorfman disease). J Infect Dis 1992166291–295. [DOI] [PubMed] [Google Scholar]
- 8.Luppi M, Barozzi P, Garber R.et al Expression of human herpesvirus‐6 antigens in benign and malignant lymphoproliferative diseases. Am J Pathol 1998153815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsang W Y, Yip T T, Chan J K. The Rosai‐Dorfman disease histiocytes are not infected by Epstein‐Barr virus. Histopathology 19942588–90. [DOI] [PubMed] [Google Scholar]
- 10.Anderson M J, Lewis E, Kidds I M.et al An outbreak of erythema infectiosum associated with human parvovirus infection. J Hyg (Lond) 19849385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown K E, Anderson S M, Young N S. Erythrocyte P antigen: cellular receptor for B19 parvovirus. Science 1993262114–117. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava A, Lu L. Replication of B19 parvovirus in highly enriched hematopoietic progenitor cells from normal bone marrow. J Virol 1988623059–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young N. Hematologic and hematopoietic consequences of B19 parvovirus infection. Semin Hematol 198825159–172. [PubMed] [Google Scholar]
- 14.Tolfvenstam T, Papadogiannakis N, Norbeck O.et al Frequency of human parvovirus B19 infection in intrauterine fetal death. Lancet 20013571494–1497. [DOI] [PubMed] [Google Scholar]
- 15.White D G, Woolf A D, Mortimer P P.et al Human parvovirus arthropathy. Lancet 19851419–421. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell L A. Parvovirus B19 Nonstructural (NS1) protein as a transactivator of interleukin‐6 synthesis: common pathway in inflammatory sequelae of human parvovirus infections? J Med Virol 200267267–274. [DOI] [PubMed] [Google Scholar]
- 17.de la Rubia J, Moscardo F, Arriaga F.et al Acute parvovirus B19 infection as a cause of autoimmune hemolytic anemia. Haematologica 200085995–997. [PubMed] [Google Scholar]
- 18.Lehmann H W, von Landenberg P, Modrow S. Parvovirus B19 infection and autoimmune disease. Autoimmun Rev 20032218–223. [DOI] [PubMed] [Google Scholar]
- 19.Mehraein Y, Lennerz C, Ehlhardt S.et al Detection of parvovirus B19 capsid proteins in lymphocytic cells in synovial tissue of autoimmune chronic arthritis. Mod Pathol 200316811–817. [DOI] [PubMed] [Google Scholar]
- 20.Middel P, Hemmerlein B, Fayyazi A.et al Sinus histiocytosis with massive lymphadenopathy: evidence for its relationship to macrophages and for a cytokine‐related disorder. Histopathology 199935525–533. [DOI] [PubMed] [Google Scholar]
- 21.Shade R O, Blundell M C, Cotmore S F.et al Nucleotide sequence and genome organization of human parvovirus B19 isolated from the serum of a child during aplastic crisis. J Virol 198658921–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cotmore S F, McKie V C, Anderson L J.et al Identification of the major structural and nonstructural proteins encoded by human parvovirus B19 and mapping of their genes by procaryotic expression of isolated genomic fragments. J Virol 198660548–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehraein Y, Rehder H, Draeger H G.et al Die Diagnostik fetaler Virusinfektionen durch in‐situ‐Hybridisierung. Geburtshilfe Frauenheilkd 199151984–989. [DOI] [PubMed] [Google Scholar]
- 24.Kerr J R. Pathogenesis of human parvovirus B19 in rheumatic disease. Ann Rheum Dis 200059672–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooling L L, Koerner T A, Naides S J. Multiple glycosphingolipids determine the tissue tropism of parvovirus B19. J Infect Dis 19951721198–1205. [DOI] [PubMed] [Google Scholar]
- 26.Lehmann H W, von Landenberg P, Modrow S. Parvovirus B19 infection and autoimmune disease. Autoimmun Rev 20032218–223. [DOI] [PubMed] [Google Scholar]
- 27.Uike N, Miyamura T, Obama K.et al Parvovirus B19‐associated haemophagocytosis in Evans‐Syndrome: aplastic crisis accompanied by severe thrombocytopenia. Br J Haematol 199384530–532. [DOI] [PubMed] [Google Scholar]
- 28.Teachy D T, Manno C S, Axsom K M.et al Unmasking Evans syndrome: T‐cell phenotype and apoptotic response reveal autoimmune lymphoproliferative syndrome (ALPS). Blood 20051052443–2448. [DOI] [PubMed] [Google Scholar]
- 29.Rieux‐Laucat F, Le Deist F, Fischer A. Autoimmune lymphoproliferative syndromes: genetic defects of apoptosis pathways. Cell Death Differ 200310124–133. [DOI] [PubMed] [Google Scholar]
- 30.Maric I, Pittaluga S, Dale J.et al Sinus histiocytosis with massive lymphadenopathy in patients with autoimmune lymphoproliferative syndrome. Mod Pathol 200417258A. [DOI] [PubMed] [Google Scholar]