Abstract
Background
Mouse mammary tumour virus (MMTV) has a proven role in breast carcinogenesis in wild mice and genetically susceptible in‐bred mice. MMTV‐like env gene sequences, which indicate the presence of a replication‐competent MMTV‐like virus, have been identified in some human breast cancers, but rarely in normal breast tissues. However, no evidence for a causal role of an MMTV‐like virus in human breast cancer has emerged, although there are precedents for associations between specific histological characteristics of human cancers and the presence of oncogenic viruses.
Aim
To investigate the possibility of an association between breast cancer and MMTV‐like viruses.
Methods
Histological characteristics of invasive ductal human breast cancer specimens were compared with archival MMTV‐associated mammary tumours from C3H experimental mice. The presence of MMTV‐like env DNA sequences in the human breast cancer specimens was determined by polymerase chain reaction and confirmed by Southern hybridisation.
Results
MMTV‐like env gene sequences were identified in 22 of 59 (37.3%) human breast cancer specimens. Seventeen of 43 (39.5%) invasive ductal carcinoma breast cancer specimens and 4 of 16 (25%) ductal carcinoma in situ specimens had some histological characteristics, which were similar to MMTV‐associated mouse mammary tumours. However, these similarities were not associated with the presence or absence of MMTV‐like gene sequences in the human breast tumour specimens. A significant (p = 0.05) correlation was found between the grade of the human breast cancer and similarity to the mouse mammary tumours. The lower the grade, the greater the similarity.
Conclusion
Some human breast cancer specimens, in which MMTV‐like env DNA sequences have been identified, were shown to have histological characteristics (morphology) similar to MMTV‐associated mouse mammary tumours. These observations are compatible with, but not conclusive of, an association between the presence of MMTV‐like env DNA sequences and some human breast cancers.
A viral aetiology for human breast cancer has long been postulated. This postulation is based on the proven role of mouse mammary tumour virus (MMTV) as the causal agent of mammary tumours in field and experimental mice. A virus with 95% sequence homology to MMTV, but only 57% to human endogenous retrovirus, has been identified in human breast tumours.1,2,3 This virus has been commonly referred to as an MMTV‐like virus.4
Substantial, but not conclusive, evidence suggests that this MMTV‐like virus may be associated with the aetiology of human breast cancer. This evidence has been reviewed by Holland and Pogo.4 The key evidence for a possible role of MMTV‐like viruses in breast cancer is the identification of a unique 660‐base pair segment of the MMTV envelope gene in more than one third of breast tumours in Western women, but rarely in normal breast tissues from normal women.1,5,6 There is evidence to suggest that this virus is exogenous and possibly transmitted through mother's milk or other environmental sources.7 However, recently developed evidence is suggestive of both an exogenous and an endogenous MMTV‐like virus having a role in human breast cancer aetiology as has been proved in rodents.3
Additional supportive evidence for a role of MMTV‐like virus in breast cancer aetiology has recently been published. This evidence includes the identification of MMTV‐like sequences in circulating lymphocytes and in intestinal lymphoid tissues (Peyer's patches) in patients with breast cancer, the location of MMTV‐like sequences in breast acini of breast cancers but not in adjacent non‐malignant epithelial cells and the identification of an MMTV‐like superantigen in human breast cancer, which is required in the mouse for transmission of MMTVs to lymphocytes.8,9,10 There is evidence that normal human cells can be infected by MMTV.11 Malignant transformation of normal human breast epithelial cells by exposure to MMTV has been shown.12 This transformation may be induced by signalling proteins encoded by the env gene of MMTV.12 A possible additional mechanism for transformation by infection of human breast cells with MMTV has been recently identified by Theodorou et al13 who identified an oncogene (Fgf10), which is activated by MMTV insertional mutagenesis in mouse mammary tumours and overexpressed in a subset of human breast carcinomas.
It is extremely difficult to establish conclusive evidence that a specific virus has a causal role for a specific cancer. The main reason for this difficulty is that infections with viruses may be common, but cancer associated with the same virus may be rare and in addition such viruses may only be oncogenic in combination with one or more cofactors, including the presence of other viruses. For example, Epstein–Barr virus may be the causal factor for Burkitt's lymphoma in the presence of malaria and for nasopharyngeal cancer in the presence of salted fish consumed by genetically susceptible populations. A further example is the development of Kaposi's sarcoma, which may require the presence of both HIV and (Kaposi's) herpes virus‐8. For these reasons, a series of evidentiary criteria for proof of viral oncogenesis have been developed.14 These evidentiary criteria include epidemiological and biological plausibility and the identification of specific viral genetic material in association with specific cancers, evidence of experimental malignant transformation of normal cells after exposure to the virus and associations between the presence of a specific virus and specific histology (morphology). With respect to MMTV‐like virus and human breast cancer, these and other evidentiary criteria have been met, with two important exceptions. These exceptions are (1) evidence of prior infection with the virus before the development of cancer and (2) associations between the presence of the virus in cancer cells and typical histological (morphological) characteristics. This study deals with the latter.
In this study, we hypothesised that the presence of MMTV‐like viral envelope gene sequences in human breast cancer specimens may be associated with histological characteristics typical of MMTV‐associated mouse mammary tumours. We have partly confirmed this hypothesis. However, this is not evidence of causation. This is because many different factors, including different viruses, may result in the same or similar histology. However, the reverse does not apply. If a virus is carcinogenic, the resulting cancer should be associated with a typical or specific histology. If this is not so, the virus is unlikely to be carcinogenic for a specific organ, cell or tissue. In addition, we have not shown a specific association between the presence of MMTV sequences and specific histological characteristics in breast cancer specimens.
Methods
Human specimens
Fifty nine unselected, formalin‐fixed, paraffin‐wax‐mounted invasive ductal carcinoma (IDC) and ductal carcinoma in situ (DCIS) breast cancer specimens were obtained from pathology service archives in Sydney, Australia. These specimens were used for the “blinded” study outlined later, which aimed to determine if MMTV‐like positive human breast tumour specimens had histological characteristics similar to MMTV‐associated mouse mammary tumours.
Mouse specimens
Forty four formalin‐fixed, paraffin‐wax‐mounted archival specimens of MMTV‐associated mammary tumours from C3H experimental mice were obtained from the Jackson Laboratory, Maine, USA.
Polymerase chain reaction
The PCR analyses were conducted at the Pogo Laboratories, Mount Sinai Hospital, New York, USA. Results from PCR analyses (using the same procedures) on 14 of these specimens were available from previous Sydney‐based studies. These two sets of data were compared to determine the reliability of the analyses.
Details of the PCR procedures are as follows. Genomic DNA from paraffin‐wax‐embedded cancer tissue was isolated according to the Qiagen DNeasy protocol. Tissues were treated with 1200 µl xylene and centrifuged for 6 min at full speed three times consecutively. The tissues were then treated with 1200 µl absolute ethanol and centrifuged for 5 min at full speed three times consecutively. The tissues were lysed overnight at 55°C in a shaking water bath and then treated with 4 µl RNase A (100 mg/ml). The tissues were eluted with 100 µl acetate buffer. The quality and concentration of genomic DNA was determined by spectrophotometry and amplification of the β‐globin gene (primers PC‐04, and GH‐20, Invitrogen Co, Grand Island, New York, USA). Samples that elicited poor results after initial β‐globin amplification were subjected to sodium acetate:ethanol precipitation and tested for the β‐globin gene a second time.
PCR was conducted on at least two separate occasions with primers designated 2N and 3N, as published by Wang et al.1 The sequences (5′–3′) of primers and probes are 2N—CCTACATCTGCCTGTGTTAC; 3N—ATCTGTGGCATACCTAAAGG; and 3L—TACAGGTAGCAGCACGTATG.
Southern hybridisation
Southern hybridisation was carried out according to the previously published protocol, using 3L as the probe.1 This single 250‐base pair sequence was used for amplification because the samples used in this study were fixed in formalin and embedded in paraffin wax. Larger sequences are difficult to amplify from DNA extracted from fixed specimens. The use of a single primer can be justified because of the many previous publications based on this primer and the consistent presence of this primer in breast cancer specimens in which a wide series of large env sequences have been identified and confirmed by full sequencing.2,4
To further overcome doubts about the reliability of data based on PCR for MMTV‐like sequences, we reviewed the data and 10 specimens from a previous study in which in situ PCR analyses were used to identify MMTV‐like sequences in additional breast cancer specimens.9
Histology
The term “histological characteristics” has been used in this article to describe “tissue morphology” because use of the latter term varies between scientific disciplines.
The histological appearance of human breast cancers is well known. By contrast, the histological appearance of MMTV‐associated mammary tumours in mice is not well known. Historically, MMTV‐associated mammary tumours in mice have been classified as Dunn types A, B and C. Approximately 90% of all such tumours in mice are of types A or B.15 Type A tumours consist of small round cells of regular size and shape with loose connective tissue, surrounding a small lumen. Type B tumours are of more solid appearance with occasional glands and cysts. All breast tumours from the C3H mice showed the typical morphology of Dunn type A or B. MMTV is integrated into the genome of mammary tumours in all such mice.16
Both the human and mouse specimens were stained with haematoxylin and eosin in the same laboratory (Saint Vincent's Hospital, Sydney) by standard methods.
Statistical considerations
Cohen's κ measurement was used for the assessment of the consistency of the results of PCR analyses from the two separate laboratories.
The relationship between the identification of MMTV‐like env gene sequences in human breast cancer specimens and histological characteristics similar to MMTV‐associated mouse mammary tumours was investigated using the Pearson χ2 test for independence to determine if two categorical variables (presence of MMTV‐like sequences and similarity to mouse tumour histological characteristics) are related. The same method was used to investigate the relationship between the grade of human breast cancer specimens and histological characteristics similar to MMTV‐associated mouse mammary tumours.
To simplify the statistical analyses, where both IDC and DCIS were present in the same specimen, the specimen was classified according to the dominant type.
The SPSS statistical package was used for all analyses.
Results
Histology and PCR analysis
The histological characteristics of the human breast cancers and the MMTV‐associated mouse mammary tumours were compared by WD, an experienced histopathologist. Human breast cancers are heterogeneous, with the histological characteristics often varying in different parts of the tumour. This is in contrast with MMTV‐associated mouse mammary tumours, which are homogeneous, and the histological patterns are usually similar throughout the tumour. In the Discussion, we suggest that this difference may be because of the involvement of multiple viruses in human breast cancer as compared with only MMTV in mouse mammary cancer.
These histological assessments were conducted with the identity of the specimens and the PCR assessments blinded. The histological assessments and PCR analyses were conducted in separate laboratories. Neither the histopathologist nor the laboratory scientists working in the separate laboratories were aware of the other's outcomes. The correlations of the two sets of data were conducted independently by JSL. The human specimens were classified for tumour type and grade according to standard criteria (modified Bloom and Richardson Criteria). The MMTV‐associated mouse tumours were assessed as types A and B. The human breast cancers were classified as “similar” or “not similar” to the mouse type A and B tumours if there was any area of the tumour which showed similarity.
The results of the PCR analyses conducted on the same 14 specimens to identify MMTV‐like env sequences in the two separate laboratories were the same for 12 of the specimens (κ 0.7; p = 0.005). Accordingly, the results of the PCR analyses can be considered to be significantly but not perfectly reliable. An image of a PCR gel positive for MMTV‐like sequences extracted from one of these 14 specimens has previously been published.6
Of the 59 unselected breast cancer specimens, 43 were classified as IDC and 16 were classified as DCIS.
MMTV‐like env gene sequences were identified in 22 of 59 (37.3%) human breast cancer specimens (table 1).
Table 1 Histological similarity of MMTV‐like positive human breast cancer specimens to MMTV‐associated mouse mammary tumours.
| Grade | Histology similar to mouse | Histology not similar to mouse | |
|---|---|---|---|
| Human IDC breast cancer specimens | |||
| Positive MMTV sequences, n = 19 | 1 | 3 | 2 |
| 2 | 5 | 4 | |
| 3 | 2 | 3 | |
| Negative MMTV sequences, n = 24 | 1 | 3 | 1 |
| 2 | 3 | 6 | |
| 3 | 1 | 10 |
| Human DCIS breast cancer specimens | |||
|---|---|---|---|
| Positive MMTV sequences, n = 3 | 1 | ||
| 2 | 2 | ||
| 3 | 1 | ||
| Negative MMTV sequences, n = 13 | 1 | 2 | |
| 2 | 1 | 3 | |
| 3 | 2 | 5 |
In all, 17 of 43 (39.5%) IDC breast cancer specimens had some histological characteristics which were similar to MMTV‐associated mouse mammary tumours. However, these similarities were not significantly correlated (χ2 = 1.56; p = 0.21) with the presence or absence of MMTV‐like gene sequences in specific human breast tumour specimens. Some of the human breast cancer specimens had histological characteristics similar to Dunn type A mouse mammary tumours (collections of round cells with little cytoplasm and a central alveoli or acini), others were similar to Dunn type B (collections of round cells with little cytoplasm without a central alveoli or acini), others were similar to mixed Dunn types A and B.
Four of 16 (25.0%) DCIS specimens had some histological characteristics, which were similar to MMTV‐associated mouse mammary tumours. The number of DCIS specimens is too small to justify a formal statistical analysis. However, the trends are the same for DCIS and IDC—namely, no obvious correlation between the presence of MMTV‐like sequences and similarity to MMTV associated mouse mammary tumours.
The similarity of human IDC breast tumour specimens (including both MMTV‐like sequence positive and negative breast tumours) with MMTV‐associated mouse mammary tumours is significantly (χ2 = 5.85; p = 0.05) associated with the grade of the human cancer (table 1). The lower the grade of the human tumour, the greater the similarity to mouse mammary tumours. There was no difference with respect to similarity of Dunn type A or type B mouse mammary tumours and human breast tumours.
To reduce the possibility of contamination, which can lead to false PCR results when standard methods are used, previously published data based on in situ PCR and reassessment of the histological characteristics of 10 breast cancer tumours were included in this study.9 In all, 4 of 10 human specimens with both IDC and DCIS elements from the previous investigation in which MMTV‐like gene sequences had been identified by both standard and in situ PCR had histological characteristics similar to MMTV‐associated mouse mammary tumours. These images have previously been published.9
Histological characteristics
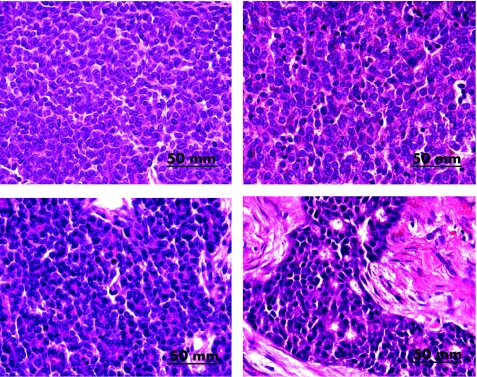
Figure 1 shows the histological characteristics of two selected mouse mammary tumours and human breast cancers. The selection of these images is to show the similarities between the mouse and human cancer specimens. As seen in fig 1, the histological characteristics of some mouse mammary tumour and human breast cancer specimens are virtually identical. There are masses of densely packed round cells with little cytoplasm and little intercellular connective tissue in both the specimens. The human breast cancer cells have approximately 10–15% greater diameter than the mouse mammary tumour cells. However, it must be emphasised that few (3/59 specimens) of the whole human breast cancer specimens are identical to the mouse mammary tumours. Other specimens that were classified as similar to mouse specimens had patches of malignant tissue which were similar to mouse mammary tumours.
Figure 1 Histological characteristics of MMTV‐associated mouse mammary tumours (magnification ×600) compared with human IDC specimens. Left (top and bottom): mouse mammary tumour specimens; right (top and bottom): human IDC specimens. The upper left mouse mammary tumour specimen is a type B tumour. The histological characteristics of this mouse mammary tumour are virtually identical to the human breast cancer specimen (upper right). Masses of densely packed round cells with little cytoplasm and little intercellular connective tissue are seen in both the mouse and human specimens. The mouse mammary tumour specimen (bottom left) is a mixed type A and type B tumour. The histological characteristics of this mouse mammary tumour and the (lower right) human breast cancer specimen are similar except that the human breast cancer specimen has collections of densely packed round cancer cells. Sheets of connective tissue separate these collections of cancer cells. The mouse mammary tumour specimen (lower left) has masses of densely packed round cells with little surrounding connective tissue. The mouse tumour is homogeneous, with similar cancer cells throughout the specimen. The human tumour is heterogeneous, with similar cancer cells confined to one area of the specimen.
These observations reflect the heterogeneous pattern of human breast cancer specimens, which is in contrast with the homogeneous pattern of most MMTV‐associated mouse mammary tumours.
Discussion
We have shown that 35.6% of an unselected series of human breast cancer specimens have some histological characteristics similar to Dunn type A or type B MMTV‐associated mouse mammary tumour specimens. However, such similarity for the whole human breast cancer specimen is unusual. Most human specimens, which we classified as being similar to mouse specimens, had regions of similarity, but were not similar throughout the whole specimen. The similarity of the human breast cancer specimens was shown to be largely dependent on the grade of the cancer; the lower the grade of cancer, the greater the similarity of human and mouse specimens.
MMTV‐like env gene sequences were identified in 22 of 59 (37.3%) human breast cancer specimens (table 1). However, there were no associations between the presence of MMTV‐like sequences and similarity of human breast cancer to MMTV‐associated mouse mammary tumours.
Reliability of these observations
The assessment of histological characteristics has obvious subjective elements. However, we believe that the findings have validity because of the blinded nature of the assessments. In addition, others can readily observe the histological characteristics presented in this paper in any series of human breast cancer specimens taken from patients in Western countries (MMTV‐associated breast cancer seems to be rarely present in Chinese and Vietnamese patients).6,17
The findings based on PCR are also considered to be reliable. The primers used in the PCR analyses were identical to those previously used to successfully identify MMTV‐like env gene sequences in four independent laboratories.1,5,6,18
The data based on PCR analyses conducted in the two different laboratories on the same 14 human breast cancer specimens in this study were consistent, but not perfect. There seems to be two reasons for this: (1) the probable low MMTV‐like viral load in breast cancers and (2) the use of formalin‐fixed specimens in PCR analyses.
In addition, the similarity between human breast cancer specimens and MMTV‐associated mouse mammary specimens was observed in several of the additional breast cancer specimens in which MMTV‐like env gene sequences had been identified by both in situ PCR and standard PCR. In situ PCR has advantages over standard PCR because the risk of contamination is low and the location of the viral DNA can be visualised.
General discussion
More than 20 years ago, Wellings19 observed that some human breast cancer specimens had histological characteristics similar to MMTV‐associated mouse mammary tumours. However, there is a belief that “spontaneous”—that is, MMTV‐associated mouse mammary—tumours do not resemble most human breast cancers.15 This belief seems to be based on the observations made by Hamilton20 in 1974, and the conventional wisdom that the most common form of human breast cancer is scirrhous carcinoma. The term scirrhous carcinoma was used historically to describe IDCS which were characterised by streams of elongated malignant cells surrounded by dense connective tissues which in turn lead to the rock (scirrhous) character of the tumour. These past observations and beliefs obviously differ from our current observations.
There is a plausible explanation for these different observations. Owing to mammographic screening, the diagnosis of human breast cancer is being made at an increasingly early stage of neoplastic progression. Therefore, many of the breast cancer specimens that we have assessed have some of the histological characteristics of hyperplasia. These characteristics are similar to those of Dunn types A and B in MMTV‐associated mammary tumours in mice. In addition, the MMTV‐associated mammary tumours in mice, which have been used for comparison with human breast cancers, are probably also in the early stages of neoplastic progression. This is because experimental mice are commonly killed soon after the detection of mammary tumours, without being kept alive until their natural deaths.
The presence of MMTV‐like env gene sequences in 37.3% of the human breast tumours in this study is compatible with our previous observations of an increasing prevalence of MMTV‐like sequences in the progression from normal breast pathology to breast cancer.21
There are precedents for a correlation between the presence of an oncogenic virus and the development of specific histological characteristics in an associated cancer. The classic example is the association between human papilloma virus (HPV), cervical intraepithelial neoplasia and human cervical cancer.22 With respect to cervical intraepithelial neoplasia, there is also an inverse association between grade and expression of virus morphology (koilocytosis), and retention of tissue architecture. This is the same phenomenon as we observed in this current investigation. However, we have not shown a valid association between the presence of MMTV‐like env gene sequences in human breast tumours and characteristic histological patterns. We have no evidence to explain this lack of association and can only speculate about the reasons. Perhaps an MMTV‐like virus “hits and runs”. Perhaps an MMTV‐like virus initially acts in concert with other viruses to cause breast cancer, and then leaves no trace after it has initiated oncogenic change. The identification of a range of oncogenic viruses in human breast cancer offers some credence to this speculation, particularly with respect to HPV, whose presence on some human breast tumours is associated with its typical histological characteristics.23,24,25
It should be noted that differing influences may result in the same or similar abnormal phenotypes of cells. For example, drugs, carcinogenic toxins and radiation plus spontaneous mutations may cause aplastic anaemia, but the cell phenotype is the same for all causes. Although the phenotype or histological characteristics of MMTV‐associated mammary tumours in experimental mice seems to be a consistent phenomenon, it is possible that additional viruses such as the Epstein–Barr virus and HPV may also be associated with human breast cancers and could account for the heterogeneous histological pattern of human breast cancers.24,25
There is also the possibility that MMTV‐like viruses and other viruses may be attracted to and located in malignant tumours after their creation. This parasitic phenomenon seems to occur with Chlamydia infections of pre‐existing atheromatous plaques. In our view, such a parasitic phenomenon is unlikely with respect to MMTV‐like viruses and human breast cancer. This is because of the conclusive experimental evidence of the causal role of MMTV in experimental mice and the evidence that MMTV is associated with malignant transformation of normal human breast epithelial cells.4,12
If MMTV‐like viruses have a role in breast carcinogenesis, such a role is likely to be complex. These complexities include the potential influence of cofactors such as sex and growth hormones, interaction between MMTV‐like viruses and other viruses, and the possibility that an MMTV‐like virus exists in both exogenous and endogenous forms, which may combine to cause carcinogenesis.
This is the first study to investigate the possible associations between the presence of MMTV‐like sequences and breast cancer morphology. Additional studies with greater numbers of fresh tissue specimens are needed to confirm these initial observations. This is because PCR analyses on fresh tissues potentially offer greater sensitivity and reliability than analyses of fixed tissues.
We speculate that the heterogeneous morphology of human breast cancer may be due to the influence of several different oncogenic viruses, including MMTV‐like virus, Epstein–Barr virus and HPV.
Conclusions
Some human breast cancers in which MMTV‐like env DNA sequences have been identified show histological characteristics (morphology) similar to MMTV‐associated mouse mammary tumours. These observations are compatible with, but not conclusive of, an association between the presence of MMTV‐like env DNA sequences and some human breast cancers.
Acknowledgements
We thank Dr J Hirsh of the Henderson Research Centre, Ontario, Canada, for advice and guidance; Dr R Cardiff of the University of California, USA, for the donation of the initial archival mouse specimens; Dr I Mikaelian of the Jackson Laboratory, Maine, USA, for the donation of the additional mouse specimens; Dr B Pogo, Mount Sinai School of Medicine, New York, USA, for support and encouragement; and E Carpenter for conducting the PCR analyses in her laboratory
Abbreviations
DCIS - ductal carcinoma in situ
HPV - human papilloma virus
IDC - invasive ductal carcinoma
MMTV - mouse mammary tumour virus
Footnotes
Funding: This investigation was partially supported by the US Department of Defense breast cancer research program and the Cooper Medical Research Foundation.
Competing interests: None.
Ethical approval: The Australian Institutional Ethics Authorities approved this investigation.
The images were prepared by the Illustrations and Imaging services at the University of New South Wales, Sydney.
References
- 1.Wang Y, Holland J F, Bleiweiss I J.et al Detection of mammary tumor virus env gene‐like sequences in human breast cancer. Cancer Res 1995555173–5179. [PubMed] [Google Scholar]
- 2.Liu B, Wang Y, Melana S M.et al Identification of a proviral structure in human breast cancer. Cancer Res 2001611754–1759. [PubMed] [Google Scholar]
- 3.Szabo S, Haislip A M, Traina‐Dorge V.et al Human, rhesus monkey and feline sequences highly similar to mouse mammary tumor virus sequences. Microsc Res Tech 200568209–221. [DOI] [PubMed] [Google Scholar]
- 4.Holland J F, Pogo B G T. Mouse mammary tumor virus‐like viral infection and human breast cancer. Clin Cancer Res 2004105647–5649. [DOI] [PubMed] [Google Scholar]
- 5.Etkind P, Du J, Khan A.et al Mouse mammary tumor virus‐like env gene sequences in human breast tumors and a lymphoma of a breast cancer patient. Clin Cancer Res 200061273. [PubMed] [Google Scholar]
- 6.Ford C E, Tran D, Deng Y M.et al Mouse mammary tumour like virus prevalence in breast tumours of Australian and Vietnamese women. Clin Cancer Res 200391118–1120. [PubMed] [Google Scholar]
- 7.Wang Y, Pelisson I, Melana S M.et al MMTV‐like env gene sequences in human cancer. Arch Virol 2001146171–180. [DOI] [PubMed] [Google Scholar]
- 8.Lushnikova A A, Kryukova I N, Rotin D L.et al Detection of the env MMTV‐homologous sequences in mammary carcinoma patient intestine lymphoid tissue. Dokl Biol Sci 2004399423–426. [DOI] [PubMed] [Google Scholar]
- 9.Ford C E, Faedo M, Rawlinson W D. Mouse mammary tumor virus‐like transcripts and DNA are found in affected cells of human breast cancer. Clin Cancer Res 2004107284–7289. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Jiang J ‐ D, Xu D.et al A mouse mammary tumor virus‐like long terminal repeat superantigen in human breast cancer. Cancer Res 2004644105–4111. [DOI] [PubMed] [Google Scholar]
- 11.Indik S, Guensburg W H, Salmons B.et al Mouse mammary tumor virus infects human cells. Cancer Res 2005656651–6659. [DOI] [PubMed] [Google Scholar]
- 12.Katz E, Lareef M H, Rassa J C.et al MMTV encodes an ITAM responsible for transformation of mammary epithelial cells in three‐dimensional culture. J Emerg Med 2005201431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Theodorou V, Boer M, Weigelt B.et al Fgf10 is an oncogene activated by MMTV insertional mutagenesis in mouse mammary tumors and overexpressed in a subset of human breast carcinomas. Oncogene 2004236047–6055. [DOI] [PubMed] [Google Scholar]
- 14.Vonka V. Causality in medicine: the case of tumours and viruses. Philos Trans R Soc London 20003551831–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardiff R D, Wellings S R. The comparative pathology of human and mouse mammary glands. J Mammary Gland Biol Neoplasia 19994105–122. [DOI] [PubMed] [Google Scholar]
- 16.Vaage J, Smith G H, Asch B B.et al Mammary tumorogenesis and tumor morphology in four C3H sublines with and without exogenous mammary tumor virus. Cancer Res 1986462096–2100. [PubMed] [Google Scholar]
- 17.Day N K, Witkin S S, Sarkar N H.et al Antibodies reactive with murine mammary tumor virus in sera of patients with breast cancer: geographic and family studies. Proc Natl Acad Sci USA 1981782483–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine P H, Pogo B G ‐ T, Klouj A.et al Increasing evidence for a human breast carcinoma virus with geographic differences. Cancer 2004101721–726. [DOI] [PubMed] [Google Scholar]
- 19.Wellings S R. A hypothesis of the origin of human breast cancer from the terminal ductal lobular unit. Pathol Res Pract 1980166515–535. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton J M. Comparative aspects of mammary tumors. Adv Cancer Res 1974191–45. [DOI] [PubMed] [Google Scholar]
- 21.Ford C E, Faedo M, Crouch R.et al Progression from normal breast pathology to breast cancer is associated with increasing prevalence of mouse mammary tumor virus‐like sequences in men and women. Cancer Res 2004644755–4759. [DOI] [PubMed] [Google Scholar]
- 22.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 20022342–350. [DOI] [PubMed] [Google Scholar]
- 23.Lawson J S, Tran D D, Rawlinson W R. From Bittner to Barr—a viral, diet and hormone breast cancer aetiology hypothesis. Breast Cancer Res 2001381–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Villiers E ‐ M, Sanstrom R E, zur Hausen H.et al Presence of papilloma sequences in condylomatous lesions of the mamillae and in invasive carcinoma of the breast. Breast Cancer Res 20057R1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glaser S L, Hsu J L, Gulley M L. Epstein‐Barr virus and breast cancer: state of the evidence for viral carcinogenesis. Cancer Epidemiol Biomarkers Prev 200413688–697. [PubMed] [Google Scholar]