Abstract
Aims
To clarify the role of β‐catenin in digestive endocrine carcinogenesis, a large and representative series of gastroenteropancreatic endocrine tumours was analysed in order to determine the incidence and pattern of β‐catenin changes and to analyse the clinical and histological characteristics of the tumours presenting immunohistochemically detectable changes in β‐catenin expression.
Methods
229 cases of gastroenteropancreatic endocrine tumours (stomach, 11; duodenum and ampulla, 29; jejunum and ileum, 51; appendix, 13; colon and rectum, 17; and pancreas, 108) were studied by immunohistochemistry to assess the pattern of distribution of β‐catenin (membranous, cytoplasmic or nuclear). DNA was analysed to detect mutations in exon 3 of the CTNNB1 gene.
Results
The distribution of immunoreactive β‐catenin protein was membranous in 164 cases, cytoplasmic in 58 cases and nuclear in seven cases. No mutation was detected in exon 3 of the CTNNB1 gene in any case. The seven cases with nuclear accumulation of β‐catenin were large tumours (mean size 44 (standard deviation (SD) 18.5) mm) with metastases, including liver metastases in five cases, high Ki‐67 index (mean 34% (SD 16.5%)) and cyclin D1 overexpression; p53 accumulation was detected in six cases. Five patients died of disease; the mean (SD) survival was 13.6 (4.8) months.
Conclusions
Immunohistochemically detectable nuclear accumulation of β‐catenin is infrequent in gastroenteropancreatic endocrine tumours and is usually not associated with mutations in CNNTB1 exon 3. Changes in β‐catenin expression are late events in digestive endocrine carcinogenesis, associated with tumour progression and dissemination.
β‐Catenin, one of the mediators of the Wnt signalling pathway, has a pivotal role in intracellular signalling and represents a key element in one of the most important pathways of epithelial carcinogenesis. Direct or indirect changes in the Wnt signalling pathway may result from mutations in the gene coding for β‐catenin itself or for its partners, such the APC gene product.1 Such genetic changes usually result in the cytoplasmic accumulation of free β‐catenin, eventually followed by its nuclear translocation. The existence of change can therefore be suspected by immunohistochemical demonstration of the nuclear localisation of β‐catenin. However, this sign is not specific: nuclear localisation of β‐catenin may be secondary to epigenetic mechanisms or to changes in the expression of some of its partners, such as cadherins, especially in invasive tumour cells.
Direct or indirect evidence supports the involvement of the Wnt signalling pathway in some subsets of endocrine tumours, such as pituitary adenomas.2 However, there is considerable controversy about the pattern and frequency of changes in β‐catenin, at both the protein and gene levels, in the endocrine tumours of the digestive tract and the pancreas.3 This may be because most studies have been conducted on limited series of cases, representing selected subsets of digestive endocrine tumours. We were therefore prompted to re‐evaluate the possible role of β‐catenin in digestive endocrine carcinogenesis. For this purpose, we designed a study of a large series of primary digestive endocrine tumours, representative of their various locations, with the following aims:
To determine the incidence and pattern of changes in β‐catenin according to the site of the endocrine tumour; and
To analyse the clinical, histological, phenotypic and genetic characteristics of the tumours showing immunohistochemically detectable changes in β‐catenin expression.
Materials and methods
Study group
We retrieved 229 cases of gastroenteropancreatic endocrine tumours from the files of the Department of Pathology, Hôpital Edouard Herriot, Lyon, France. All tumours were submitted for surgical or endoscopic resections between 1985 and 2004. From 1985 to 1997, only material fixed in Bouin's fluid was available. After 1997, at least one sample of tumour tissue was fixed in buffered formalin. In all cases, the endocrine nature of the tumour was verified by the immunodetection of chromogranin A and synaptophysin (table 1). Clinical information and follow‐up data were obtained from clinical charts. All available histological material was reviewed. Tumours were classified according to the World Health Organization criteria into the following groups: well‐differentiated endocrine tumours (either benign or of uncertain behaviour), well‐differentiated endocrine carcinomas, and poorly differentiated endocrine carcinomas.
Table 1 List of the antibodies used in the study .
| Antibodies | Clone | Source |
|---|---|---|
| Chromogranin A | LKZH10 | Boehringer (Mannheim, Germany) |
| Synaptophysin | SVP38 | Sigma (St Louis, Missouri, USA) |
| β‐Catenin | CAT‐5H10 | Zymed (San Francisco, California, USA) |
| 14 | Transduction Laboratories (Lexington, Kentucky, USA) | |
| Ki‐67 | MIB‐1 | Dako (Glostrup, Denmark) |
| Cyclin D1 | CYCD1 | Biocare Medical (Concord, California, USA) |
| p53 | DO7 | Dako |
Table 2 gives the main pathological characteristics of the patients included in the study group.
Table 2 Clinical, pathological and functional features of the 229 cases included in the study.
| Site | Number of cases | Age (years) median (SD) | Sex M/F | Tumour size (cm) median (SD) | Histological classification (WHO, 2000)* | Metastases (number of cases) | |||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | Total | Liver metastases | |||||
| Stomach | 11 | 58 (15) | 9/2 | 2.2 (1.6) | 7 | 3 | 1 | 3 | 1 |
| Duodenum | 22 | 57 (13.6) | 13/9 | 2 (2.3) | 18 | 5 | 0 | 5 | 1 |
| Ampulla | 7 | 56 (12.6) | 4/3 | 2.1 (0.9) | 2 | 5 | 0 | 5 | 1 |
| Jejunum and ileum | 51 | 64 (9.1) | 30/21 | 2.2 (1.4) | 0 | 49 | 2 | 45 | 24 |
| Appendix | 13 | 34 (20.5) | 6/7 | 0.8 (0.5) | 12 | 1 | 0 | 0 | 0 |
| Colon–rectum | 17 | 57 (12.8) | 10/7 | 2.8 (2.5) | 7 | 8 | 2 | 7 | 3 |
| Pancreas | 108 | 50 (14.1) | 48/60 | 3.5 (2.7) | 51 | 54 | 3 | 55 | 31 |
| Total | 229 | 54 (15.1) | 120/109 | 2.7 (2.3) | 97 | 125 | 8 | 120 | 61 |
F, female; M, male; WHO, World Health Organization.
*WHO classification: 1, well‐differentiated tumour; 2, well‐differentiated carcinoma; 3, poorly differentiated carcinoma.
Immunohistochemical studies
Table 1 lists the antibodies used. Two commercially available antibodies were used for β‐catenin immunodetection; they gave comparable results in tissue sections. An indirect streptavidin–biotin immunoperoxidase technique was applied to deparaffinised tissue sections. For β‐catenin, the following features were analysed:
Cellular localisation of immunostaining: membranous, nuclear or cytoplasmic;
Percentage;
Distribution of tumour cells with nuclear or cytoplasmic location of immunoreactive β‐catenin.
For evaluation of labelling for cyclin D1, Ki‐67 and p53, the number of positive tumour cells was evaluated as a percentage of 1000 consecutive cells.
Mutation analysis
Genomic DNA was extracted from 10‐µm‐thick sections of formalin‐fixed, paraffin‐wax‐embedded tissue, after tumour cell microdissection. Only tissue material fixed in buffered formalin, available in 148 cases, was used for mutation analysis. In three cases with nuclear immunoreactivity of β‐catenin, genomic DNA was also extracted from frozen tissue material to verify the sensitivity of the extraction technique. To screen for mutations in exon 3 of the CTNNB1 gene coding for β‐catenin, extracted genomic DNA was amplified by polymerase chain reaction. The following primers were designed to amplify a 152‐bp fragment located between bases 817 and 1044: sense, 5′‐ATGGAACCAGACAGAAAAG‐3′; antisense, 5′‐TACAGGACTTGGGAGGTATC‐3′ (MWG Biotech, Ebersberg, Germany). Polymerase chain reaction products were purified from agarose gels and sequenced with an ABI PRISM 310 sequencer (Applied Biosystems, Foster City, California, USA). For each case tested, DNA was sequenced from two independent samples with two different sets of primers. Positive controls included samples of buffered formalin‐fixed, paraffin‐wax‐embedded material from tumours known to harbour mutations of exon 3 of the CNNTB1 gene, and were processed under the same conditions, including 10 cases of pseudopapillary and solid tumour of the pancreas and four cases of hepatoblastoma.
Statistical analysis
For comparisons of the frequencies of clinical or pathological characteristics, the χ2 test with Yates' correction was used. The non‐parametric Student's t test was used for comparison of means; p<0.05 was considered significant.
Results
Patterns of β‐catenin expression
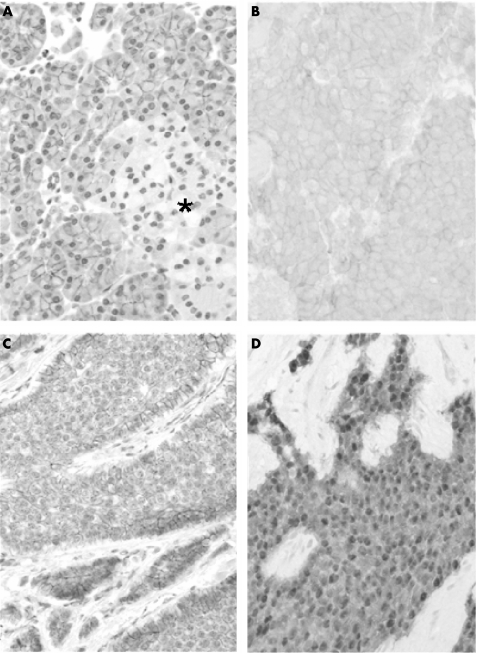
Immunoreactive β‐catenin was detected in all cases studied and presented three main patterns of subcellular distribution (fig 1).
Figure 1 β‐catenin expression in normal and neoplastic gastroenteropancreatic endocrine cells. In normal endocrine pancreatic cells (A), β‐catenin is expressed at low levels all over the islet of Langerhans (*) as compared with adjacent exocrine pancreatic cells. In endocrine tumours, β‐catenin usually retains a membranous distribution; the expression level is either homogeneous (B) or heterogeneous (C) within the tumour. The apparent up regulation of β‐catenin at the periphery of tumour nodules (C) can be seen. Nuclear accumulation (D) of immunoreactive β‐catenin is readily visible in this case. Immunoperoxidase 250×.
A strictly membranous pattern of expression (fig 1B,C), comparable to that observed in normal gastroenteropancreatic endocrine cells (fig 1A), was present in 164 cases. The apparent intensity of expression level ranged from faint to strong; it was often heterogeneous within the same tumour; the highest apparent levels of expression were usually observed at the periphery of tumour lobules or over tumour cells in contact with the connective stroma (fig 1C). A cytoplasmic pattern of expression was observed in 58 cases; it was usually associated with variable levels of membranous expression. Its apparent intensity level ranged from faint to moderate.
Nuclear accumulation of β‐catenin was observed in only seven tumours (fig 1D); the immunoreactive protein showed either a strictly nuclear distribution or a mixed nuclear and cytoplasmic distribution; the apparent expression level ranged from moderate to intense. Table 3 gives the site of these tumours.
Table 3 Topographic distribution of cases with nuclear expression of β‐catenin.
| Site | Total cases (n) | Cases with nuclear localisation of β‐catenin, n (%) |
|---|---|---|
| Stomach | 11 | 1 (9) |
| Duodenum | 22 | 0 |
| Ampulla | 7 | 1 (14.2) |
| Jejunum and ileum | 51 | 0 |
| Appendix | 13 | 0 |
| Colon | 9 | 2 (22.2) |
| Rectum | 8 | 2 (25) |
| Pancreas | 108 | 2 (1.8) |
In six cases, all cells showed a nuclear distribution of immunoreactive β‐catenin; in the remaining case, about 50% of tumour cells, with a heterogeneous distribution within the tumour, were reactive. We observed no example of tumour with nuclear accumulation of β‐catenin in cell foci located along the tumour margins.
In the seven cases with nuclear accumulation of β‐catenin, cyclin D1 was detectable in the nuclei of 10–80% of tumour cells (median (standard deviation (SD)) 30% (27.6%)). The Ki‐67 labelling index ranged from 15% to 70% (median 34% (SD 16.5%)). A nuclear accumulation of p53 was detected in six of the seven cases; the percentage of p53‐positive tumour cells ranged from 10% to 100% (median 39.2% (SD 33.6%)).
Mutation analysis
Under our technical conditions, no mutation in exon 3 of the CNNTB1 gene was detected in any case tested, after extraction from either paraffin‐wax‐embedded or frozen tissue. We verified that, under the same technical conditions, we were able to detect mutations in exon 3 of the CNNTB1 gene in our positive controls.
Correlations with clinical and pathological features
Table 4 summarises the main clinical and pathological features of the cases presenting with either membranous, cytoplasmic or nuclear distribution of immunoreactive β‐catenin.
Table 4 Correlations between β‐catenin status and clinicopathological characteristics.
| Distribution of immunoreactive β‐catenin | Age (years) median (SD) | Sex M/F | Tumour size (mm) median (SD) | Histological classification (WHO, 2000)* | Metastases, n (%) | |||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | Total | Liver metastases | ||||
| Membranous and/or cytoplasmic | 54 (15.3) | 116/106 | 27 (23.8) | 97 | 121 | 5 | 113 (50.9) | 55 (24.7) |
| Nuclear | 62 (9.4) | 4/3 | 44 (18.5) | 0 | 4 | 3 | 7 (100) | 5 (71.5) |
F, female; M, male; WHO, World Health Organization.
*WHO classification: 1, well‐differentiated tumour; 2, well‐differentiated carcinoma; 3, poorly differentiated carcinoma.
The seven cases presenting with a nuclear distribution of immunoreactive β‐catenin showed several distinctive features. All of them were classified, according to the World Health Organization criteria, as endocrine carcinomas, either well‐differentiated (n = 4) or poorly differentiated (n = 3). Their median (SD) size was large (44 (18.5) mm v 27 (23.8) mm in cases without nuclear β‐catenin accumulation; p = 0.066). All seven tumours were metastatic at the time of diagnosis; 5 (71.5%) of them had liver metastases. By contrast, 50.9% of the tumours with membranous or cytoplasmic expression of β‐catenin were metastatic at the time of diagnosis and 24.7% had liver metastases; the differences were significant (p = 0.03 and 0.02, respectively). Follow‐up data were available in six cases with nuclear accumulation of β‐catenin; 5 (71.5%) patients died of disease; their mean (SD) survival was 13.6 (4.8) months. Follow‐up data were available in 154 cases with either membranous or cytoplasmic expression of β‐catenin; only 21.2% died from disease (p<0.05) and their mean (SD) survival was 44 (32.8) months (p = 0.048).
Discussion
Our results, based on a large and representative series, show that immunohistochemically detectable nuclear accumulation of β‐catenin
is infrequent in gastroenteropancreatic endocrine tumours;
is usually not associated with detectable mutations in exon 3 of the CNNTB1 gene;
is observed in tumours with malignant and aggressive behaviour.
Conflicting results exist in the current literature about the incidence of changes in β‐catenin in digestive or pancreatic endocrine tumours, as detected by immunohistochemistry or mutation analysis. Two pioneer works suggested a high incidence of nuclear or cytoplasmic accumulation of β‐catenin in certain subsets of digestive endocrine tumours. In one series,4 nuclear accumulation of immunoreactive β‐catenin was reported in 8 of 22 (36%) cases of intestinal endocrine tumours and in 2 of 7 (29%) cases of pancreatic tumours; in a second series,5 including 72 cases of gastric, duodenal, appendiceal and rectal endocrine tumours, nuclear staining was observed in 35% of cases. No mutation in exon 3 of the CNNTB1 gene was detected in the first series,4 whereas in the second series,5 the incidence of mutations amounted to 37.5%, including cases with only cytoplasmic staining. However, these results have not been reproduced by more recent studies. At the protein level, no case of nuclear staining has been reported in several recent studies of endocrine tumours originating from the various segments of the digestive tract.6,7,8,9,10 At the gene level, two studies of digestive11 and pancreatic12 endocrine tumours failed to detect any mutation in exon 3 of the CNNTB1 gene. However, most of these studies have been conducted in short series, and bias sampling could not be excluded.
To avoid such problems, we studied a large series of 229 cases of well‐characterised endocrine tumours representative of the various segments of the digestive tract and the pancreas. As in previous studies,4,5 our results confirm that nuclear accumulation of β‐catenin can be detected in some cases of gastroenteropancreatic endocrine tumours, but with a much lower incidence than previously reported: <5% instead of 30–35%.4,5 Our results are in line with those reported in other studies of endocrine tumours of the same embryological origin, such as the lung.13
In line with most previous studies, our mutation analysis study could not detect any mutation in exon 3 of CNNTB1 gene, even in cases with nuclear accumulation of the corresponding protein. It is unlikely that a lack of sensitivity of our technique could account for these negative results, as, under the same technical conditions, we were able to detect mutations in several positive controls; for the same reason, it is also unlikely that the different primers used in our study and in previous ones5 may have led to different results. However, we cannot exclude the existence of mutations in other regions of the CNNTB1 gene, which are not usually investigated in most mutation analysis studies, as exon 3 has proved to be a mutational hot spot and encodes a domain essential for the function of the corresponding protein. However, extended analyses of the CNNTB1 gene would be necessary before considering and exploring other genetic mechanisms to explain the nuclear accumulation of β‐catenin observed in some gastroenteropancreatic endocrine tumours.
In addition, we could not detect any nuclear accumulation of β‐catenin along the invasive margins of endocrine tumours; this is in sharp contrast with results on digestive adenocarcinomas, especially from the colon, in which this pattern is frequent; this suggests that the role of β‐catenin in tumour invasion is different in endocrine and exocrine tumours.
Our data suggest that in gastroenteropancreatic endocrine tumours, the nuclear accumulation of the β‐catenin, even in the absence of gene mutations, may have functional and clinical consequences. In our series, as in previous ones,4,5 nuclear accumulation of β‐catenin protein was associated with an overexpression of cyclin D1 and a high proliferative activity. Moreover, in our series, all tumours presenting with a nuclear accumulation of β‐catenin were large lesions, classified as endocrine carcinomas, because of signs of local invasion or metastatic dissemination, and showing features usually associated with aggressive endocrine tumours, such as the frequent nuclear expression of p53. These observations suggest that changes in the Wnt pathway, confirmed by changes in β‐catenin status, may be late events in digestive endocrine carcinogenesis, associated with tumour progression and dissemination.
In our study, as in previous ones,4,5 tumours with nuclear accumulation of β‐catenin were found in any segment of the digestive tract as well as in the pancreas; in our series, they were more frequent in the hindgut, but this remains to be confirmed in larger series. In any case, available data suggest that in contrast with other molecular mechanisms involved in digestive endocrine carcinogenesis, such as MEN1 mutations14 or chromosome X allelic losses,15 changes in the Wnt pathway are not site dependent.
Conclusion
Our study, based on a large and representative series of gastroenteropancreatic endocrine tumours, confirms that changes in the Wnt pathway are possible but infrequent events during digestive endocrine carcinogenesis, and suggests that they are associated with tumour progression and dissemination.
Take‐home messages
Nuclear accumulation of β‐catenin is infrequent in gastroenteropancreatic endocrine tumours.
It is usually not associated with mutations in exon 3 of the CTNNB1 gene.
Changes in β‐catenin expression are likely to be late events in digestive endocrine carcinogenesis, associated with tumour progression and dissemination.
Acknowledgements
We thank Dominique Bourchany, Evelyne Walch and Sylvie Baldassini for their expert technical assistance. We also thank Jean‐Jacques Madjar, MD, PhD (DTAMB gene sequencing platform, Université Claude Bernard Lyon 1, La Doua, Villeurbanne, France) for help.
Footnotes
Competing interests: None declared.
References
- 1.Debruyne P, Vermeulen S, Mareel M. The role of the E‐cadherin/catenin complex in gastrointestinal cancer. Acta Gastroenterol Belg 199962393–402. [PubMed] [Google Scholar]
- 2.Semba S, Han S Y, Ikeda H.et al Frequent nuclear accumulation of β‐catenin in pituitary adenoma. Cancer 20019142–48. [DOI] [PubMed] [Google Scholar]
- 3.Semba S, Yamakawa M, Sasano H. The cadherin‐catenin superfamily in endocrine tumors. Endocr Pathol 2001121–13. [DOI] [PubMed] [Google Scholar]
- 4.Semba S, Kusumi R, Moriya T.et al Nuclear accumulation of β‐catenin in human endocrine tumors: association with Ki‐67 (MIB‐1) proliferative activity. Endocr Pathol 200011243–250. [DOI] [PubMed] [Google Scholar]
- 5.Fujimori M, Ikeda S, Shimizu Y.et al Accumulation of β‐catenin protein and mutations in exon 3 of β‐catenin gene in gastrointestinal carcinoid tumor. Cancer Res 2001616656–6659. [PubMed] [Google Scholar]
- 6.Kuroda N, Mizushima S, Guo L.et al Goblet cell carcinoid of the appendix: investigation of the expression of β‐catenin and E‐cadherin. Pathol Int 200151283–287. [DOI] [PubMed] [Google Scholar]
- 7.Barshack I, Goldberg I, Chowers Y.et al Different β‐catenin immunoexpression in carcinoid tumours of the appendix in comparison to other gastrointestinal carcinoid tumors. Pathol Res Pract 2002198531–536. [DOI] [PubMed] [Google Scholar]
- 8.Li C C, Hirowaka M, Qian Z R.et al Expression of E‐cadherin, β‐catenin, and Ki‐67 in goblet cell carcinoids of the appendix: an immunohistochemical study with clinical correlation. Endocr Pathol 20021347–58. [DOI] [PubMed] [Google Scholar]
- 9.Li C C, Xu B, Hirokawa M.et al Alterations of E‐cadherin, α‐catenin and β‐catenin expression in neuroendocrine tumours of the gastrointestinal tract. Virchows Arch 2002440145–154. [DOI] [PubMed] [Google Scholar]
- 10.Zhang P J, Furth E E, Cai X.et al The role of β‐catenin, TGF β3, NGF2, FGF2, IGFR2, and BMP4 in the pathogenesis of mesenteric sclerosis and angiopathy in midgut carcinoids. Hum Pathol 200435670–674. [DOI] [PubMed] [Google Scholar]
- 11.Stancu M, Wu T T, Wallace C.et al Genetic alterations in goblet cell carcinoids of the vermiform appendix and comparison with gastrointestinal carcinoid tumors. Mod Pathol 2003161189–1198. [DOI] [PubMed] [Google Scholar]
- 12.Gerdes B, Ramaswamy A, Simon B.et al Analysis of β‐catenin gene mutations in pancreatic tumors. Digestion 199960544–548. [DOI] [PubMed] [Google Scholar]
- 13.Pelosi G, Scarpa A, Puppa G.et al Alteration of the E‐cadherin/β‐catenin cell adhesion system is common in pulmonary neuroendocrine tumors and is an independent predictor of lymph node metastasis in atypical carcinoids. Cancer 20051031154–1164. [DOI] [PubMed] [Google Scholar]
- 14.Forsberg L, Zablewska B, Piehl F.et al Molecular and genetic mechanisms of tumorigenesis in multiple endocrine neoplasia type‐1. Int J Mol Med 2001151653–1664. [DOI] [PubMed] [Google Scholar]
- 15.Pizzi S, D'Adda T, Azzoni C.et al Malignancy‐associated allelic losses on the X‐chromosome in foregut but not in midgut endocrine tumours. J Pathol 2002196401–407. [DOI] [PubMed] [Google Scholar]