Abstract
The sellar region is the site of a large number of pathological entities arising from the pituitary and adjacent anatomical structures, including brain, blood vessels, nerves and meninges. The surgical pathology of this area requires the accurate identification of neoplastic lesions, including pituitary adenoma and carcinoma, craniopharyngioma, neurological neoplasms, germ cell tumours, haematological malignancies and metastases, as well as non‐neoplastic lesions such as cysts, hyperplasias and inflammatory disorders. This review provides a practical approach to the diagnosis of pituitary specimens that are sent to the pathologist at the time of surgery. The initial examination requires routine haematoxylin and eosin staining to establish whether the lesion is a primary adenohypophysial proliferation or one of the many other pathologies that occurs in this area. The most common lesions resected surgically are pituitary adenomas. These are evaluated with several special stains and immunohistochemical markers that are now available to accurately classify these pathologies. The complex subclassification of pituitary adenomas is now recognised to reflect specific clinical features and genetic changes that predict targeted treatments for patients with pituitary disorders.
The sellar region is the site of different pathological entities arising from the pituitary and adjacent anatomical structures including brain, blood vessels, nerves and meninges. The surgical pathology of this area requires the accurate identification of neoplastic lesions, including pituitary adenoma and carcinoma, craniopharyngioma, neurological neoplasms, germ cell tumours and haematological malignancies, as well as non‐neoplastic lesions such as cysts, hyperplasia and inflammatory lesions.1,2 Box 1 lists the various pathologies that occur in the sella turcica.
Among all these entities, primary pituitary adenomas are the most common. A recent meta‐analysis showed that the estimated prevalence of pituitary adenomas was 14.4% in postmortem studies and 22.2% in radiological studies, with an overall estimated prevalence of 16.9%.3 These figures contravene the conventional view of pituitary tumours as rare; pituitary adenomas are indeed common in the general population. Although not often lethal, these lesions have a major effect on quality of life and are being recognised with increasing frequency. Moreover, the management of these lesions has seen major changes with the development of new pharmacotherapeutic agents, surgical approaches and radiotherapeutic techniques.
It therefore behoves the surgical pathologist to have a rational approach to the diagnosis of pituitary specimens. Owing to the variety of pathologies in the sellar region and the diverse modalities of therapeutic intervention, the pathologist has an important role in the diagnosis and management of the patient with pituitary pathology.
The initial approach
The handling of tissue obtained at pituitary surgery is important to ensure accurate and complete diagnosis. Adequate tissue fixed in formalin for histology and immunohistochemistry is paramount. In rare cases, there may be a need for ultrastructural analysis. As this situation is not often predicted clinically, it is recommended that a small piece of tissue be routinely fixed for electron microscopy and retained in the event that it is needed. Currently there is no need for special handling of tissue for other diagnostic techniques.
Most pituitary specimens are small and the tissue may be compromised by requests for intraoperative consultation. Freeze artefacts resulting from quick section handling can preclude further evaluation. In some centres, pathologists elect to use smear technology for intraoperative consultation to prevent this artefact; this method uses less tissue but requires experience for interpretation. Sometimes, the only diagnostic tissue is in the material used for the intraoperative procedure. We therefore recommend that this procedure be limited for use only when the clinical situation is unusual or when the surgeon encounters unexpected findings.
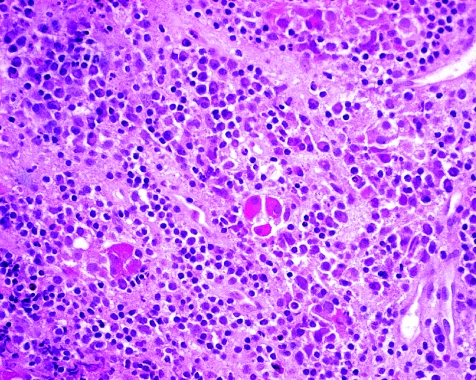
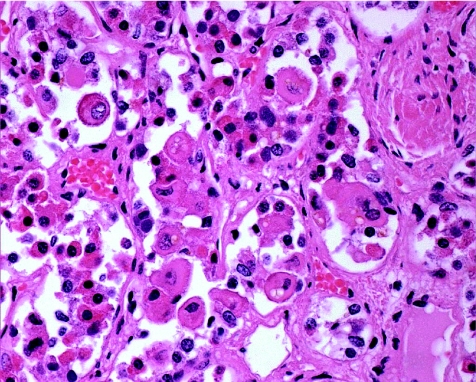
The initial evaluation of a pituitary specimen is the examination of slides stained with haematoxylin and eosin. This routine stain allows pathologists to histopathologically evaluate and determine whether the lesion is composed of adenohypophysial cells and therefore likely to be hyperplasia, adenoma or, very rarely, carcinoma. Other lesions will be identified or at least considered in this evaluation. Cysts such as Rathke's cleft cyst, arachnoid cysts and dermoid cysts will be recognised on the basis of clinical and radiological findings preoperatively and confirmed with the identification of the appropriate cyst lining.4 Hypophysitis of any type5 can be readily recognised with this conventional stain (fig 1). In addition, this analysis can also diagnose other types of tumours that arise in the sella. In some cases, the distinction between these unusual lesions and pituitary adenoma can be difficult and will require ancillary testing using various immunohistochemical stains as appropriate.
Figure 1 Lymphocytic hypophysitis. The adenohypophysis is infiltrated by chromic inflammatory cells, including lymphocytes and plasma cells. The few residual acidophils and basophils of the parenchyma are prominent.
Box 1 Classification of pituitary pathology*
-
Neoplastic
-
Benign†
Pituitary adenoma
Craniopharyngioma
Gangliocytoma/ganglioglioma
Granular cell tumour
Meningioma
Schwannoma
Chordoma
Vascular and mesenchyma tumours
-
Malignant
Pituitary carcinoma
Gliomas
Germ cell tumour
Lymphoma/leukaemia/Langerhans' cell histiocytosis
Vascular and mesenchymal tumours
Metastases
Miscellaneous (salivary gland lesions, melanoma, etc)
-
Non‐neoplastic
Hyperplasia
-
Inflammatory lesions
Infectious
Immune
-
Cysts
Rathke's cleft cysts
Arachnoid
Dermoid/epidermoid
Aneurysms
Meningoencephalocele
Hamartoma
Brown tumour of bone
*Surgical pathology only, not including developmental and metabolic lesions for which biopsy samples are not taken.
†Although classified as benign, many of these lesions are locally invasive and cause major morbidity and mortality.
This short review does not permit a discussion of the various neuropathological entities that can involve the sella. These include gliomas, meningiomas, schwannomas and chordomas. Unique tumours of the hypothalamus are the hypothalamic neuronal gangliocytomas and gangliogliomas, which are rare but can give rise to clinical features of hormone excess that can mimic pituitary adenoma.6 In the neurohypophysis, granular cell tumours are rarely diagnosed in a surgical pathology specimen but they have a characteristic histological appearance that rarely requires ancillary studies.1
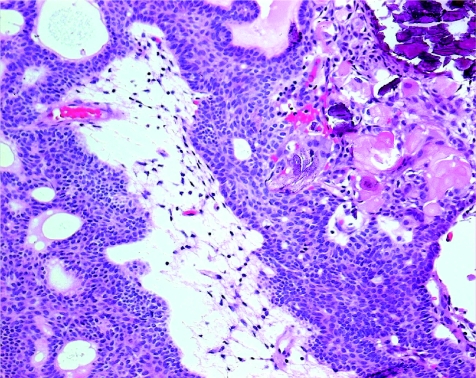
Craniopharyngioma is a unique tumour of the sellar region that is derived from the remnants of Rathke's pouch. These lesions have a characteristic morphology that requires only routine haematoxylin and eosin staining for identification and classification (fig 2). They are composed of cords or islands of epithelial cells with squamoid differentiation in a loose fibrous stroma with intervening cysts. The epithelial islands have palisade cells at the periphery.1 They are subclassified as adamantinomatous, which harbour mutations of the β‐catenin gene as a specific molecular pathogenetic mechanism,7,8 and papillary.
Figure 2 Craniopharyngioma. The squamous epithelium of this lesion lies in a loose fibrous stroma with prominent keratinisation and calcification (top right).
Germ cell tumours of the sella resemble those lesions in other sites of the body.9 Haematological malignancies of the sella are usually systemic diseases but occasionally arise as primary plasmacytomas or lymphomas.10,11 Most patients present with headache, visual impairment with cranial nerve dysfunction, panhypopituitarism and systemic changes that raise the possibility of a distinct disease entity.12
Metastatic malignancies are common in the pituitary,13,14 usually at a late stage of disseminated malignancy when the clinical diagnosis is known. Occasionally, however, these lesions are detected early, and particularly in patients with no known history of a primary malignancy, they can be clinically challenging. The lesions that most often give rise to pituitary metastasis are carcinomas of the breast, lung and prostate. The metastatic tumour is usually associated with neurohypophysis and extrasellar structures, giving rise to a clinical presentation of diabetes insipidus and nerve palsies that is not consistent with pituitary adenoma.15,16 The common lesions are readily diagnosed on routine histopathology, but a metastatic well‐differentiated neuroendocrine carcinoma (fig 3) can be a diagnostic dilemma.15,17 The expression of specific markers, such as thyroid transcription factor 1, cdx‐2 or peptides of gut or lung origin, may be required to distinguish these lesions from a primary pituitary neoplasm.
Figure 3 Metastatic neuroendocrine carcinoma. This metastasis from a primary endocrine carcinoma of the lung mimics a pituitary adenoma in architecture and cytology. Both lesions can be positive for synaptophysin and chromogranin; other markers are therefore required to establish the diagnosis. Strong nuclear positivity for thyroid transcription factor 1 (not shown) confirmed the diagnosis.
Primary adenohypophysial cell proliferations
Once a pituitary lesion is determined to be composed of epithelial cells with neuroendocrine differentiation and thought to be of primary pituitary origin, the classification of the lesion will require several steps: the lesion must be identified as hyperplasia or neoplasia; the cell population responsible for the proliferation must be established; and, in the case of a neoplasm, the behaviour, prognosis and potential treatment of choice must be determined.
Adenoma or hyperplasia?
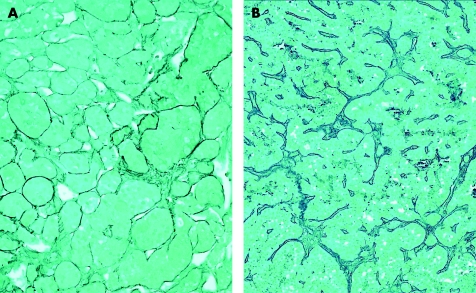
Hyperplasia is defined as a cell proliferation induced by a known stimulus and is a controlled process that stops when the stimulus is removed. Pituitary hyperplasia can be physiological, as when lactotrophs proliferate during pregnancy, or pathological, as when induced by excess hypophysiotropic hormones. Examples of pathological hyperplasia include cases of primary target organ failure, such as primary hypothyroidism,18,19 or hormone excess produced by neoplasms, such as hypothalamic gangliocytomas, or ectopic sources of growth hormone‐releasing hormone or corticotropin‐releasing hormone, such as bronchial, gastroenteropancreatic, adrenal or prostatic endocrine tumours.20 Hyperplasia can be clinically indistinguishable from adenoma. Radiological imaging can sometimes identify differences: in hyperplasia the proliferation is diffuse and there is no normal rim that is enhanced with gadolinium.21 However, this subtle difference is often overlooked and, unfortunately, it falls to the pathologist to make the diagnosis. In this regard, the reticulin stain is a useful tool. Normal adenohypophysis is formed by small acini of pituitary cells surrounded by an intact reticulin network. In hyperplasia, the acinar architecture is maintained and the reticulin network is preserved, but the acini are increased in size (fig 4A). In contrast, pituitary adenomas are characterised by complete disruption of the reticulin fibre network (fig 4B). Immunohistochemical stains are required to determine the hyperplastic cell population, and this technique will identify the admixed normal cells that stain for all of the normal adenohypophysial hormones.1
Figure 4 Reticulin staining in hyperplasia and neoplasia. (A) The lesion has an intact reticulin pattern but focally dilated acini; this is an example of thyrotroph hyperplasia. (B) In contrast, the lesion has total breakdown of acinar architecture, consistent with adenoma.
Classification of pituitary adenomas
Clinically, pituitary adenomas are classified as hormonally active or functioning adenomas and non‐functioning adenomas that often present with visual impairment and hypopituitarism, especially in men. About two thirds of clinically diagnosed lesions are functioning lesions associated with excess hormone production. These are further subclassified according to histological morphology.
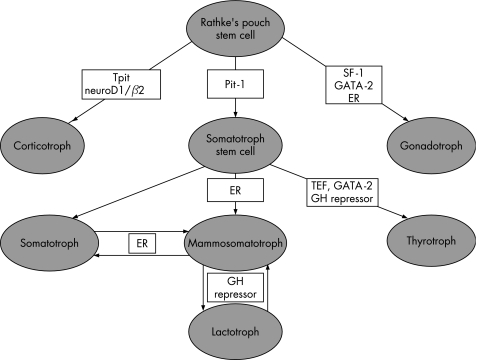
The pituitary is composed of at least six distinct cell types and each cell is responsible for the production and secretion of one or more specific hormones. The recent advances in molecular biology have clarified the cytodifferentiation of adenohypophysial cells.22,23 During development, an organised, complex process of cell differentiation is orchestrated by specific transcription factors (fig 5). These factors also have a role in determining the cytodifferentiation and hormone production of pituitary adenomas, and knowledge of their significance can help in classifying adenomas.24,25,26,27,28
Figure 5 Pathways of cell differentiation in adenohypophysis. The three main pathways of cell differentiation are determined by transcription factors that can serve as diagnostic markers. ER, oestrogen receptor; GH, growth hormone; SF‐1, steroidogenic factor 1; TEF, thyrotroph embryonic factor.
Adenohypophysial cells arise from Rathke's pouch stem cells. Corticotrophs are the first cells to differentiate in the human fetal pituitary. This process is determined by the novel Tpit transcription factor29 that mediates its action in concert with Ptx1 and neuroD1,30,31 which were previously identified as corticotroph upstream transcription element‐binding proteins. The second line of differentiation temporally in the human gland is through Pit‐1. This protein has restricted expression in the pituitary and activates the growth hormone, prolactin (PRL) and β‐thyrotropin (β‐TSH) genes.32,33,34,35,36,37 Pit‐1 initially determines growth hormone expression and the somatotroph phenotype. Expression of oestrogen receptor allows the expression of PRL and growth hormone in a bihormonal population of mammosomatotrophs.38 The development of mature lactotrophs depends on the presence of a putative growth hormone repressor that is yet unidentified. Some of the Pit‐1‐expressing cells further express thyrotroph embryonic factor39 and develop into thyrotrophs in the presence of a growth hormone repressor and GATA‐2.40 In physiological states, somatotrophs, mammosomatotrophs and lactotrophs transdifferentiate in a reversible fashion.41 In animal models, somatotrophs can also transdifferentiate into thyrotrophs in severe hypothyroidism and this too is thought to be reversible.42 These changes indicate the fluidity of this population of four cell types that are all dependent on Pit‐1. The third line of cytodifferentiation is that of the gonadotrophs which are determined by steroidogenic factor‐1 and GATA‐2 in the presence of oestrogen receptor.28,40
The various cell types give rise to tumours that can be clinically functioning or silent, and some tumour types have morphological variants that are thought to reflect differing pathogenetic mechanisms or responses to treatment. Table 1 defines these entities and summarises the clinical–pathological classification of pituitary adenomas.1,2 A more refined approach can subclassify adenomas on the basis of the pattern of immunoreactivity and, occasionally, ultrastructural features, which reflect the various pathogenetic mechanisms underlying these lesions.
Table 1 Clinicopathological classification of pituitary adenomas.
| Clinically functioning adenomas | Clinically silent adenomas |
|---|---|
| Adenomas causing GH excess | |
| Somatotroph adenomas | Silent somatotroph adenomas |
| Mammosomatotroph adenomas | |
| Adenomas causing hyperprolactinaemia | |
| Lactotroph adenomas | Silent lactotroph adenomas |
| Lactotroph adenomas with GH reactivity | |
| Adenomas causing TSH excess | |
| Thyrotroph adenomas | Silent thyrotroph adenomas |
| Adenomas causing ACTH excess | |
| Corticotroph adenomas | Silent corticotroph adenomas |
| Adenomas causing gonadotropin excess | |
| Gonadotroph adenomas | Silent gonadotroph adenomas |
| Plurihormonal adenomas | Hormone‐negative adenomas |
ACTH, adrenocorticotrophic hormone; GH, growth hormone; TSH, thyrotropin.
The following discussion, which includes a detailed morphological subclassification (table 2), provides a brief overview of the approach to the diagnosis of these lesions
Table 2 Morphological classification of pituitary adenomas.
| Tumour | Transcription factor | Hormones | Cam 5.2 keratin staining |
|---|---|---|---|
| Adenomas containing GH | Pit‐1 | ||
| Somatotroph adenomas | GH | ||
| Densely granulated | α‐subunit | Perinuclear | |
| Sparsely granulated | Fibrous bodies | ||
| Mammosomatotroph adenomas | GH, PRL, α‐subunit | ||
| Mixed somatotroph–lactotroph adenomas | |||
| Plurihormonal GH‐producing adenomas | GH, PRL α‐subunit, β‐TSH | ||
| Adenomas containing PRL | Pit‐1 | ||
| Lactotroph adenomas | PRL | ||
| Sparsely granulated | PRL (Golgi pattern) | ||
| Densely granulated | PRL (diffuse) | ||
| Acidophil stem cell adenomas | PRL, GH | Fibrous bodies | |
| Adenomas containing TSH | Pit‐1, TEF GATA‐2 | ||
| Thyrotroph adenomas | α‐subunit, β‐TSH | ||
| Adenomas containing ACTH | Tpit | ||
| Densely granulated | ACTH | ||
| Sparsely granulated | ACTH | ||
| Crookes' cell adenoma | ACTH | Dense bands | |
| Adenomas containing gonadotropins | SF‐1, ER, GATA‐2 | ||
| Gonadotroph adenomas | α‐subunit, β‐FSH, β‐LH | ||
| Plurihormonal adenomas | Multiple | ||
| Silent subtype 3 | Multiple | ||
| Unusual | Multiple | ||
| Hormone‐negative adenomas | None | ||
| Null‐cell adenomas | None |
ACTH, adrenocorticotrophic hormone; ER, oestrogen receptor; FSH, follicle‐stimulating hormone; GATA‐2, GH, growth hormone; PRL, prolactin; SF‐1, steroidogenic factor‐1; TEF, thyrotroph embryonic factor.
When the clinical history indicates hormone excess
In a patient with known Cushing's disease, the differential diagnosis is pituitary adenoma versus corticotroph hyperplasia. Pituitary adenoma is far more common, but the distinction is important and requires the use of reticulin staining and adrenocorticotrophic hormone (ACTH) immunohistochemistry as detailed above. In addition, the pathologist has the added tool of Crookes' hyaline change, a morphological marker of feedback suppression that indicates the status of normal corticotrophs (fig 6). In the absence of this change, the patient has either a misdiagnosis or corticotroph hyperplasia. Pseudo‐Cushing's syndrome is a significant medical pitfall that can occasionally result in unnecessary pituitary surgery. When there is no Crookes' hyalinisation of corticotrophs, a careful evaluation of reticulin and corticotroph distribution is required, which can be very difficult.43,44
Figure 6 Crooke's hyaline change. Non‐tumorous corticotrophs in the pituitary of a patient with raised glucocorticoids show accumulation of hyaline material as a concentric whorl in the cytoplasm; the hyaline can trap large lysosomes known as “enigmatic bodies” which are also found in corticotrophs. This change is characteristic of non‐tumorous corticotrophs in patients with functioning pituitary corticotroph adenomas; pituitary adenomas rarely show diffuse hyalinisation, forming the “Crooke's cell adenoma” (not shown).
The presence of Crooke's hyalinisation confirms that the patient has raised circulating glucocorticoids, but the differential diagnosis includes pituitary adenoma, ectopic ACTH secretion, primary adrenal pathology or iatrogenic administration of glucocorticoids. The clinical history, radiological imaging and elaborate biochemical evaluation including inferior petrosal sinus sampling may be required to distinguish these disorders.
The identification of an ACTH‐immunoreactive adenoma can be easy when the lesion is large. However, these adenomas are often small, about 1–2 mm in size. They may require multiple sections through the specimen to be identified. Generally, the larger lesions tend to be obvious adenomas but not obviously basophilic adenomas, as they are usually sparsely granulated, chromophobic adenomas. Periodic acid‐Schiff positivity and weak ACTH reactivity makes the diagnosis evident, but these stains can also be equivocal in this setting. The addition of Tpit is helpful, but as yet no commercially antisera or antibodies are available for this marker.
The classic microadenomas associated with the most florid examples of Cushing's disease are densely granulated adenomas composed of strongly basophilic cells. These adenomas exhibit strong periodic acid‐Schiff positivity. Immunohistochemically, they express ACTH and generally have strong reactivity with the Cam 5.2 antibody to keratins 7 and 8.
In some patients, there is no evidence of an adenoma but the non‐tumorous pituitary shows extensive Crooke's hyaline change. The outcome of surgery alone will indicate the true nature of this disorder; as very small microadenomas can be lost during surgery, perhaps suctioned during aspiration of blood in the operative field, an operative success can be assumed if biochemical abnormality is achieved along with regression of clinical signs and symptoms. The pathologist can only issue a report that indicates the presence of Crooke's hyaline change, consistent with Cushing's syndrome. In contrast, a surgical failure will require more careful clinical evaluation of the patient to exclude an ectopic source of ACTH or ACTH‐like peptide, primary adrenal disease or a missed pituitary lesion.
Crooke's cell adenoma is a rare variant of corticotroph adenoma. In this unusual lesion, the adenomatous cells exhibit the features of suppressed corticotrophs. These tumours are often associated with atypical clinical histories, and the diagnosis may be unclear, or there may be a history of cyclical Cushing's syndrome. The morphology of these tumours can be quite atypical as well, with prominent nuclear pleomorphism and large cells that can resemble gangliocytoma or metastatic carcinoma. This rare entity is defined by periodic acid‐Schiff positivity and immunoreactivity for ACTH, as well as the dense ring of keratin that fills the tumour cell cytoplasm, and is identified with Cam 5.2 staining.
When the patient has acromegaly or gigantism, the diagnosis of a growth hormone‐secreting adenoma is almost certain. However, these patients rarely have somatotroph hyperplasia owing to ectopic production of growth hormone‐releasing hormone by endocrine tumours as described above. The reticulin is a critical stain to exclude this possibility and ensure appropriate management for these patients.
Growth hormone‐secreting adenomas may be bihormonal mammosomatotroph adenomas or plurihormonal adenomas of the Pit‐1 family that make TSH. Monohormonal growth hormone‐producing adenomas can be densely or sparsely granulated. The distinctions can affect medical treatment in the event of surgery,45,46 therefore accurate classification is important. Indeed, it seems that the most important distinction is between densely and sparsely granulated types, as the pathophysiology of these lesions will determine their response to the current available treatments: somatostatin analogues versus growth hormone antagonists and possibly dopamine agonists.
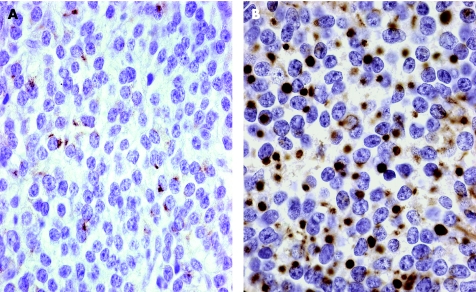
All of these adenomas stain for Pit‐1 and to variable degrees for the growth hormone. Mammosomatotrophs stain for PRL as well, and the unusual plurihormonal adenomas contain β‐TSH. All of the densely granulated variants are acidophilic and also contain an α‐subunit. The sparsely granulated somatotroph adenoma is the most difficult to diagnose, as it is often either negative or only weakly positive for growth hormone. The most critical immunostain in this setting is the Cam 5.2 keratin stain. It identifies perinuclear keratin in all of the densely granulated adenomas, including mammosomatotrophs and plurihormonal lesions (fig 7A). In contrast, it clearly decorates fibrous bodies in the sparsely granulated adenomas (fig 7B). These fibrous bodies can be identified without the keratin stain, as the tumour cells often have bilobed or concave, pleomorphic nuclei that are distorted by pale homogeneous eosinophilic globules.
Figure 7 Keratin patterns in somatotroph adenomas. (A) Densely granulated somatotroph and mammosomatotroph adenomas show a perinuclear pattern of keratin identified with the Cam 5.2 antibody. (B) Sparsely granulated somatotroph adenomas have a unique pattern of staining that identifies globular “fibrous bodies”.
The patient presenting with hyperprolactinaemia is usually given medical treatment. The patients who come for surgery either have failed medical treatment or major adverse effects induced by all of the dopaminergic agonists now available. As most lactotroph adenomas respond well to these drugs with hormone normalisation and tumour shrinkage, it is important for the pathologist to exclude the many other causes of hyperprolactinaemia, including hypophysitis and all of the various neoplasms identified in the initial part of this article.
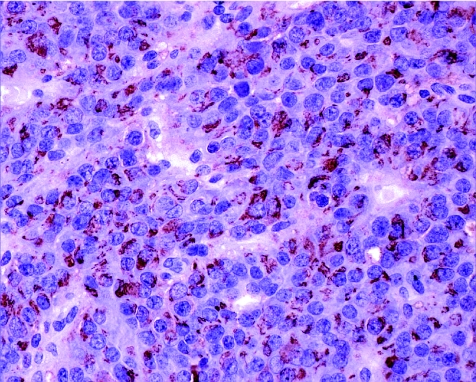
Lactotroph adenomas are subclassified into sparsely granulated and densely granulated variants. Sparsely granulated adenomas are usually highly responsive to dopamine agonists and therefore usually show major changes due to the previous treatment. Only untreated adenomas of this type show the usually chromophobic morphology with abundant cytoplasm and characteristic juxtanuclear prolactin immunoreactivity (fig 8). More commonly, the lesions are composed of small cells in a fibrous stroma, resembling inflammation, plasmacytoma or lymphoma. The diagnosis is confirmed by the identification of strong nuclear positivity for Pit‐1; usually they have at least focal prolactin positivity.
Figure 8 Prolactin staining in sparsely granulated lactotroph adenoma. This tumour represents the vast majority of lactotroph adenomas. It shows a highly specific staining pattern for prolactin that is localised to the Golgi complex but is not stored in cytoplasmic secretory granules.
The rare densely granulated lactotroph adenomas are composed of acidophilic to chromophobic cells with strong and diffuse cytoplasmic positivity for prolactin. Another unusual pituitary adenoma causing hyperprolactinaemia is the so‐called “acidophil stem‐cell adenoma”, an oncocytic lesion characterised by Pit‐1 nuclear staining, variable prolactin and growth hormone reactivity, and fibrous bodies identified with the Cam 5.2 immunostain.
The presentation of a patient with TSH excess requires the exclusion of thyrotroph hyperplasia. The rare thyrotroph adenomas are usually highly infiltrative macroadenomas with stromal fibrosis and marked nuclear atypia. Immunohistochemically, thyrotroph adenomas express α‐subunit and β‐TSH.
Clinically non‐functioning adenomas
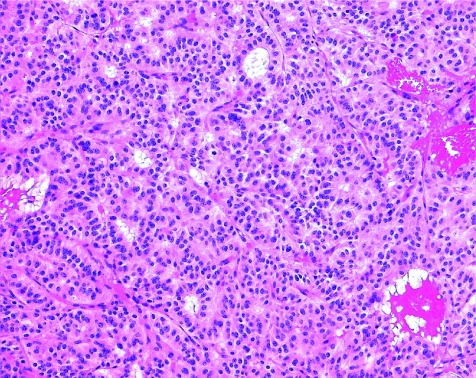
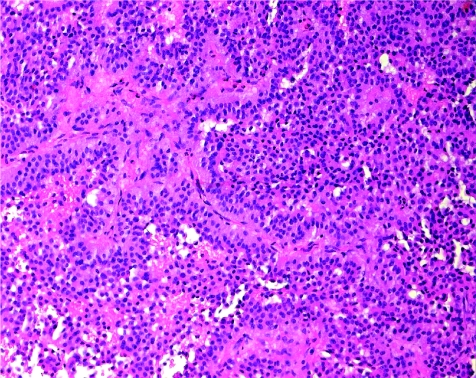
The diagnosis of a clinically non‐functioning adenoma requires appropriate classification for prognostication. The vast majority of these lesions are actually gonadotroph adenomas; these very rarely present with clinical or biochemical evidence of hormone excess. Nevertheless, they produce follicle‐stimulating hormone (FSH) or luteinising hormone, and they express the transcription factors that prove gonadotroph differentiation. They have a highly characteristic histological pattern, in which solid sheets, nests and even sinusoidal patterns are interrupted by pseudopapillae and striking pseudorosettes around vascular channels (fig 9). They usually have two types of cell: tall columnar cells line pseudopapillae and rosettes, whereas polygonal cells comprise the bulk of the lesion. Oncocytic change can be observed in all patterns. Gonadotroph adenomas express α‐subunit, β‐FSH and β‐leutinising hormone in scattered patterns and to variable degrees; they also express steroidogenic factor‐1 with strong nuclear reactivity.
Figure 9 Gonadotroph adenoma. Most clinically non‐functioning adenomas are of gonadotroph differentiation. They have a characteristic architecture with solid nests of round cells surrounded by a palisade of elongated cells with distinct polarity at the periphery, which can form pseudorosettes around vascular channels.
Occasional clinically silent adenomas are positive for Pit‐1, and for growth hormone, PRL or β‐TSH; these lesions should be classified as adenomas of the appropriate type as indicated earlier, with the additional qualification of “silent” adenoma. The pathophysiology of their lack of clinical symptomatology is not known. In contrast, silent corticotroph adenomas are thought to arise from cells that fail to process the ACTH precursor, pro‐opiomelanocortin, into the biologically active 1–39 ACTH. These lesions express Tpit, ACTH‐like immunoreactivity and keratins; they resemble functioning corticotroph adenomas of the two types, sparsely and densely granulated variants; however, they are invariably macroadenomas and there is no associated Crooke's hyaline change in non‐tumorous corticotrophs. These lesions are generally much more aggressive than other silent adenomas, and recurrence is extremely common.
As immunohistochemical markers become more sophisticated, the number of truly unclassified adenomas is falling. The rare tumour that is completely negative for all hormones and transcription factors is classified as a “null‐cell adenoma” and usually behaves like a gonadotroph adenoma.
The question of plurihormonality
The existence of unusual plurihormonal adenomas is a problem in this field. Several reports of various combinations of hormones in a single tumour are found in the literature.
The application of highly specific monoclonal antibodies and the understanding of cell differentiation have clarified many of the controversies. Reports of adenomas expressing growth hormone or PRL with gonadotropins are now recognised to reflect an aberration that was identified clearly by a group of meticulous pathologists as non‐specific cross‐reactivity.47 The fact that cells of the Pit‐1 lineage express the α‐subunit and that many antisera raised against FSH or leutinising hormone recognised the α‐subunit explains many of these anomalies. In our experience, the application of monoclonal antisera has made the occurrence of truly atypical plurihormonal adenomas exceptionally rare. Some of these lesions represent double adenomas or “collision” tumours.48 Most lesions respect the lines of differentiation attributable to the three transcription factor lineages. Even the rare silent subtype 3 adenoma is usually positive for Pit‐1, PRL, growth hormone and β‐TSH, with other reactivities possibly reflecting α‐subunit cross‐reactivity. This lesion is characterised by intense stromal fibrosis and high vascularity. Other distinct features are identified by electron microscopy.
The role of electron microscopy
The classification of pituitary adenomas is based on careful studies that used immunohistochemistry, electron microscopy and immunoelectron microscopy to identify structure–function correlations.49 Many of the ultrastructural features that were recognised as characterising specific tumour types are now identified by immunohistochemistry. For example, fibrous bodies were considered to be the hallmark of the sparsely granulated somatotroph adenoma, and these are now readily identified with the Cam 5.2 immunostain.
There remain situations in which the histology and immunohistochemical profile are atypical and these cases require electron microscopy for accurate classification. This is particularly true of the silent subtype 3 adenoma.
The best approach to the use of this diagnostic tool is to fix and embed a small fragment of all pituitary tumours at the time of receipt in the event that electron microscopy may be required, recognising that only a small number of specimens will ever require sectioning and ultrastructural examination. If this is impractical, certainly specimens from patients with atypical histories deserve this type of handling, as they will be the cases most likely to require this ancillary study.
Prognosis
Prognostication remains a major challenge in pituitary pathology. The proliferative activity50,51,52 using markers such as proliferating cell nuclear antigen, Ki‐67/MIB‐1, and anti‐apoptotic Bcl‐2 has unfortunately shown no consistent correlation with tumour invasiveness or recurrence.51 Although invasive pituitary adenomas and carcinomas show a high DNA topoisomerase IIα index, this indicator has no major advantage over MIB‐1 as a prognostic marker.53 Cyclo‐oxygenase‐2 expression correlates with patient age, but not with tumour size or invasiveness.54 Detection of telomerase expression may predict recurrence in pituitary adenomas.55 Galectin‐3, a β‐galactoside‐binding protein implicated in cellular differentiation and proliferation as well as angiogenesis, tumour progression and metastasis, may have a role in pituitary tumour progression.56
Unfortunately, none of these is a true marker of biological behaviour. The best predictive marker remains the tumour classification based on hormone content and cell structure. For example, among people with acromegaly who fail surgical resection, response to long‐acting somatostatin analogues is best predicted by the subtype of somatotroph adenoma as densely or sparsely granulated.45,46 This finding renders the value of a Cam 5.2 keratin stain more important than almost any other immunostain in this setting. A silent corticotroph adenoma will recur more often and more aggressively than a silent gonadotroph adenoma. And a silent subtype 3 adenoma will almost certainly behave invasively, infiltrating the base of the skull, whereas a silent adenoma of the gonadotroph lineage will usually grow by expansion upwards.
Pituitary carcinoma, by definition a lesion that shows distant cerebrospinal or systemic metastasis, is an exceptionally rare lesion that cannot be defined by morphological parameters of the primary tumour.
Conclusion
The approach to pituitary pathology is complex and requires recognition of many pathological entities. Familiarity with inflammatory and neurological diseases must be coupled with a detailed understanding of pituitary hyperplasia and adenoma classification. In the past, a diagnosis of “adenoma” was considered sufficient for many patients, but the advances in pituitary medicine demand a more thorough clinicopathological diagnosis that will guide patient management.
Abbreviations
ACTH - adrenocorticotrophic hormone
PRL - prolactin
TSH - thyrotropin
Footnotes
Competing interests: None declared.
References
- 1.Asa S L. Tumors of the pituitary gland. In: Rosai J, ed. Atlas of tumor pathology. 3rd series, fascicle 22. Washington, DC: Armed Forces Institute of Pathology, 1998
- 2.DeLellis R A, Lloyd R V, Heitz P U, Eng C. eds. World Health Organization classification of tumours. Lyon: IARC, 2004
- 3.Ezzat S, Asa S L, Couldwell W T.et al The prevalence of pituitary adenomas: a systematic review. Cancer 2004101613–619. [DOI] [PubMed] [Google Scholar]
- 4.Shin J L, Asa S L, Woodhouse L J.et al Cystic lesions of the pituitary: clinicopathological features distinguishing craniopharyngioma, Rathke's cleft cyst, and arachnoid cyst. J Clin Endocrinol Metab 1999843972–3982. [DOI] [PubMed] [Google Scholar]
- 5.Cheung C C, Ezzat S, Smyth H S.et al The spectrum and significance of primary hypophysitis. J Clin Endocrinol Metab 2001861048–1053. [DOI] [PubMed] [Google Scholar]
- 6.Puchner M J A, Lüdecke D K, Saeger W.et al Gangliocytomas of the sellar region—a review. Exp Clin Endocrinol 1995103129–149. [DOI] [PubMed] [Google Scholar]
- 7.Sarubi J C, Bei H, Adams E F.et al Clonal composition of human adamantinomatous craniopharyngiomas and somatic mutation analyses of the patched (PTCH), Gsalpha and Gi2alpha genes. Neurosci Lett 20013105–8. [DOI] [PubMed] [Google Scholar]
- 8.Sekine S, Shibata T, Kokubu A.et al Craniopharyngiomas of adamantinomatous type harbor beta‐catenin gene mutations. Am J Pathol 20021611997–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jennings M T, Gelman R, Hochberg F. Intracranial germ‐cell tumors: natural history and pathogenesis. J Neurosurg 198563155–167. [DOI] [PubMed] [Google Scholar]
- 10.Samaratunga H, Perry‐Keene D, Apel R L. Primary lymphoma of the pituitary gland: a neoplasm of acquired MALT? Endocr Pathol 19978335–341. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn D, Buchfelder M, Brabletz T.et al Intrasellar malignant lymphoma developing within pituitary adenoma. Acta Neuropathol (Berlin) 199997311–316. [DOI] [PubMed] [Google Scholar]
- 12.Landman R E, Wardlaw S L, McConnell R J.et al Pituitary lymphoma presenting as fever of unknown origin. J Clin Endocrinol Metab 2001861470–1476. [DOI] [PubMed] [Google Scholar]
- 13.Roessmann U, Kaufman B, Friede R L. Metastatic lesions in the sella turcica and pituitary gland. Cancer 197025478–480. [DOI] [PubMed] [Google Scholar]
- 14.Kovacs K. Metastatic cancer of the pituitary gland. Oncology 197327533–542. [DOI] [PubMed] [Google Scholar]
- 15.McCormick P C, Post K D, Kandji A D.et al Metastatic carcinoma to the pituitary gland. Br J Neurosurg 1989371–79. [DOI] [PubMed] [Google Scholar]
- 16.Fassett D R, Couldwell W T. Metastases to the pituitary gland. Neurosurg Focus 200416E8. [PubMed] [Google Scholar]
- 17.Branch C L, Jr, Laws E R., Jr Metastatic tumors of the sella turcica masquerading as primary pituitary tumors. J Clin Endocrinol Metab 198765469–474. [DOI] [PubMed] [Google Scholar]
- 18.Khalil A, Kovacs K, Sima A A F.et al Pituitary thyrotroph hyperplasia mimicking prolactin‐secreting adenoma. J Endocrinol Invest 19847399–404. [DOI] [PubMed] [Google Scholar]
- 19.Kubota T, Hayashi M, Kabuto M.et al Corticotroph cell hyperplasia in a patient with Addison disease: case report. Surg Neurol 199237441–447. [DOI] [PubMed] [Google Scholar]
- 20.Sano T, Asa S L, Kovacs K. Growth hormone‐releasing hormone‐producing tumors: clinical, biochemical, and morphological manifestations. Endocr Rev 19889357–373. [DOI] [PubMed] [Google Scholar]
- 21.Ezzat S, Asa S L, Stefaneanu L.et al Somatotroph hyperplasia without pituitary adenoma associated with a long standing growth hormone‐releasing hormone‐producing bronchial carcinoid. J Clin Endocrinol Metab 199478555–560. [DOI] [PubMed] [Google Scholar]
- 22.Asa S L, Ezzat S. The cytogenesis and pathogenesis of pituitary adenomas. Endocr Rev 199819798–827. [DOI] [PubMed] [Google Scholar]
- 23.Asa S L, Ezzat S. Molecular basis of pituitary development and cytogenesis. Front Horm Res 2004321–19. [DOI] [PubMed] [Google Scholar]
- 24.Asa S L, Puy L A, Lew A M.et al Cell type‐specific expression of the pituitary transcription activator Pit‐1 in the human pituitary and pituitary adenomas. J Clin Endocrinol Metab 1993771275–1280. [DOI] [PubMed] [Google Scholar]
- 25.Friend K E, Chiou Y ‐ K, Laws E R., Jret al Pit‐1 messenger ribonucleic acid is differentially expressed in human pituitary adenomas. J Clin Endocrinol Metab 1993771281–1286. [DOI] [PubMed] [Google Scholar]
- 26.Friend K E, Chiou Y K, Lopes M B S.et al Estrogen receptor expression in human pituitary: correlation with immunohistochemistry in normal tissue, and immunohistochemistry and morphology in macroadenomas. J Clin Endocrinol Metab 1994781497–1504. [DOI] [PubMed] [Google Scholar]
- 27.Zafar M, Ezzat S, Ramyar L.et al Cell‐specific expression of estrogen receptor in the human pituitary and its adenomas. J Clin Endocrinol Metab 1995803621–3627. [DOI] [PubMed] [Google Scholar]
- 28.Asa S L, Bamberger A ‐ M, Cao B.et al The transcription activator steroidogenic factor‐1 is preferentially expressed in the human pituitary gonadotroph. J Clin Endocrinol Metab 1996812165–2170. [DOI] [PubMed] [Google Scholar]
- 29.Lamolet B, Pulichino A M, Lamonerie T.et al A pituitary cell‐restricted T box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell 2001104849–859. [DOI] [PubMed] [Google Scholar]
- 30.Lamonerie T, Tremblay J J, Lanctot C.et al Ptx1, a bicoid‐related homeo box transcription factor involved in transcription of the pro‐opiomelanocortin gene. Genes Dev 1996101284–1295. [DOI] [PubMed] [Google Scholar]
- 31.Poulin G, Turgeon B, Drouin J. NeuroD1/beta2 contributes to cell‐specific transcription of the proopiomelanocortin gene. Mol Cell Biol 1997176673–6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ingraham H A, Chen R, Mangalam H J.et al A tissue‐specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell 198855519–529. [DOI] [PubMed] [Google Scholar]
- 33.Mangalam H J, Albert V R, Ingraham H A.et al A pituitary POU domain protein, Pit‐1, activates both growth hormone and prolactin promoters transcriptionally. Genes Dev 19893946–958. [DOI] [PubMed] [Google Scholar]
- 34.Li S, Crenshaw EB I I I, Rawson E J.et al Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU‐domain gene pit‐1. Nature 1990347528–533. [DOI] [PubMed] [Google Scholar]
- 35.Ingraham H A, Albert V R, Chen R.et al A family of POU‐domain and Pit‐1 tissue‐specific transcription factors in pituitary and neuroendocrine development. Annu Rev Physiol 199052773–791. [DOI] [PubMed] [Google Scholar]
- 36.Rosenfeld M G. POU‐domain transcription factors: pou‐er‐ful developmental regulators. Genes Dev 19915897–907. [DOI] [PubMed] [Google Scholar]
- 37.Yan G, Pan W T, Bancroft C. Thyrotropin‐releasing hormone action on the prolactin promotor is mediated by the POU protein Pit‐1. Mol Endocrinol 19915535–541. [DOI] [PubMed] [Google Scholar]
- 38.Day R N, Koike S, Sakai M.et al Both Pit‐1 and the estrogen receptor are required for estrogen responsiveness of the rat prolactin gene. Mol Endocrinol 199041964–1971. [DOI] [PubMed] [Google Scholar]
- 39.Drolet D W, Scully K M, Simmons D M.et al TEF, a transcription factor expressed specifically in the anterior pituitary during embryogenesis, defines a new class of leucine zipper proteins. Genes Dev 199151739–1753. [DOI] [PubMed] [Google Scholar]
- 40.Scully K M, Rosenfeld M G. Pituitary development: regulatory codes in mammalian organogenesis. Science 20022952231–2235. [DOI] [PubMed] [Google Scholar]
- 41.Frawley L S, Boockfor F R. Mammosomatotropes: presence and functions in normal and neoplastic pituitary tissue. Endocr Rev 199112337–355. [DOI] [PubMed] [Google Scholar]
- 42.Horvath E, Lloyd R V, Kovacs K. Propylthiouracyl‐induced hypothyroidism results in reversible transdifferentiation of somatotrophs into thyroidectomy cells. A morphologic study of the rat pituitary including immunoelectron microscopy. Lab Invest 199063511–520. [PubMed] [Google Scholar]
- 43.Trouillas J, Guigard M P, Fonlupt P.et al Mapping of corticotropic cells in the normal human pituitary. J Histochem Cytochem 199644473–479. [DOI] [PubMed] [Google Scholar]
- 44.McNicol A M. Patterns of corticotropic cells in the adult human pituitary in Cushing's disease. Diag Histopathol 19814335–341. [PubMed] [Google Scholar]
- 45.Ezzat S, Kontogeorgos G, Redelmeier D A.et al In vivo responsiveness of morphological variants of growth hormone‐producing pituitary adenomas to octreotide. Eur J Endocrinol 1995133686–690. [DOI] [PubMed] [Google Scholar]
- 46.Bhayana S, Booth G, Asa S L.et al The implication of somatotroph adenoma phenotype to somatostatin analogue responsiveness in acromegaly. J Clin Endocrinol Metab 2005906290–6295. [DOI] [PubMed] [Google Scholar]
- 47.Labat‐Moleur F, Trouillas J, Seret‐Begue D.et al Evaluation of 29 monoclonal and polyclonal antibodies used in the diagnosis of pituitary adenomas. A collaborative study from pathologists of the Club Français de l'Hypophyse. Pathol Res Pract 1991187534–538. [DOI] [PubMed] [Google Scholar]
- 48.Jastania R A, Alsaad K O, Al_Shraim M.et al Double adenomas of the pituitary: transcription factors Pit‐1, T‐pit, and SF‐1 identify cytogenesis and differentiation. Endocr Pathol 200516187–194. [DOI] [PubMed] [Google Scholar]
- 49.Kovacs K, Horvath E. Tumors of the pituitary gland. In: Hartmann WH, ed. Atlas of tumor pathology. 2nd series, fascicle 21. Washington, DC: Armed Forces Institute of Pathology, 1986
- 50.Knosp E, Kitz K, Perneczky A. Proliferation activity in pituitary adenomas: measurement by monoclonal antibody Ki‐67. Neurosurgery 198925927–930. [PubMed] [Google Scholar]
- 51.Amar A P, Hinton D R, Krieger M D.et al Invasive pituitary adenomas: significance of proliferation parameters. Pituitary 19992117–122. [DOI] [PubMed] [Google Scholar]
- 52.Thapar K, Kovacs K, Scheithauer B W.et al Proliferative activity and invasiveness among pituitary adenomas and carcinomas: an analysis using the MIB‐1 antibody. Neurosurgery 19963899–107. [DOI] [PubMed] [Google Scholar]
- 53.Vidal S, Kovacs K, Horvath E.et al Topoisomerase IIalpha expression in pituitary adenomas and carcinomas: relationship to tumor behavior. Mod Pathol 2002151205–1212. [DOI] [PubMed] [Google Scholar]
- 54.Vidal S, Kovacs K, Bell D.et al Cyclooxygenase‐2 expression in human pituitary tumors. Cancer 2003972814–2821. [DOI] [PubMed] [Google Scholar]
- 55.Yoshino A, Katayama Y, Fukushima T.et al Telomerase activity in pituitary adenomas: significance of telomerase expression in predicting pituitary adenoma recurrence. J Neurooncol 200363155–162. [DOI] [PubMed] [Google Scholar]
- 56.Riss D, Jin L, Qian X.et al Differential expression of galectin‐3 in pituitary tumors. Cancer Res 2003632251–2255. [PubMed] [Google Scholar]