Abstract
Background
Recent advances in fibrosis biology have identified transforming growth factor (TGF)‐β type I receptor‐mediated activation of Smads as playing a central part in the development of fibrosis. However, to date, there have been few studies that examined the localisation and distribution of receptor‐activated Smads protein (R‐Smads: Smad2 and 3) during the fibrosis progression.
Aims
To histopathologically assess the time‐course change of the localisation and distribution of the Smads protein in pulmonary fibrosis.
Methods
Pulmonary fibrosis was induced by intranasal injection of bleomycin (0.3 U/mouse). Lungs were isolated 2, 5, 7, 9 and 14 days after bleomycin treatment. Histological changes in the lungs were evaluated by haematoxylin‐eosin stain or Masson's trichrome stain, and scored. TGF‐β1, Smad3 and phosphorylated Smad2 localisations in lung tissues were determined by immunohistochemistry.
Results
The bleomycin treatment led to considerable pulmonary fibrotic changes accompanied by marked increase in TGF‐β1 expression in infiltrating macrophages. With the progression in fibrosis (day 7–14), marked increases in Smad3‐positive and pSmad2‐positive cells were observed. There were intense Smad3‐positive and pSmad2‐positive signals localised to the nuclei of the infiltrating macrophages and to type II epithelial cells, and less intense signals in fibroblasts and hyperplastic alveolar/bronchiolar epithelial cells.
Conclusions
The time‐course data of TGF‐β1 and R‐Smads indicate that progressive enhancement of TGF‐β1 signalling via R‐Smad is activated in the process of fibrosis progression.
Pulmonary fibrosis is characterised by inflammation, excessive proliferation of fibroblasts and deposition of extracellular matrix (ECM) in the distal airspace, and may be initiated by acute or chronic lung injury.1,2,3,4 Until now, growing evidence indicates the importance of transforming growth factor (TGF)‐β1 signalling in the developmental process of various types of pulmonary fibrosis.5,6
TGF‐β1 is an extremely potent inducer of synthesis of procollagen and other matrix elements.5,6 Levels of TGF‐β1 mRNA and protein are upregulated in animal models of pulmonary fibrosis.7,8 Neutralisation of TGF‐β1 by systemic treatment with its antibodies or soluble TGF‐β type II receptor prevented bleomycin‐induced pulmonary fibrosis in mice.9,10 Overexpression of active TGF‐β1 by adenoviral vector transfection in the lung of a rat can induce severe pulmonary fibrosis.11 Thus, TGF‐β1 is believed to have an essential role in the pathogenesis of pulmonary fibrosis. Furthermore, it is well known that TGF‐β1 is exclusively produced by infiltrating macrophages in the lung of patients with advanced idiopathic pulmonary fibrosis and in bleomycin‐induced animal models.7,12,13
TGF‐β1 signalling is mediated by type I and II receptor serine/threonine kinase. The activated type I receptor phosphorylates a subset of downstream signalling molecules, two receptor‐activated Smads (R‐Smads: Smad2 and Smad3), which then enable their binding to the common mediator Smad4. This complex translocates into the nucleus and regulates the transcription of TGF‐β responsive genes, thereby inducing fibrosis‐related changes.14 As TGF‐β1 and TGF‐β receptors are overexpressed in pulmonary fibrosis, concomitant changes in R‐Smad activation or expression may account for the mechanism underlying pulmonary fibrosis.15 The importance of type I receptor‐mediated activation of R‐Smads was evident from in vitro and in vivo studies.16,17,18,19 In the Smad3‐deficient mice, pulmonary fibrosis induced by bleomycin or TGF‐β1 was markedly attenuated compared with wild‐type mice.17,18 Moreover, a recent biochemical study showed that an increase in Smad 2/3 protein in the nuclear fraction of the lung tissue extract was seen in bleomycin‐induced animal models.19 However, direct evidence indicating tissue localisation of R‐Smads during pulmonary fibrosis development is still lacking.
In this study, we used the bleomycin‐induced pulmonary fibrosis as a model system to investigate the localisation of R‐Smads in the process of pulmonary fibrosis development. Using the bleomycin‐induced model, we analysed the time course of R‐Smads localisation from the early inflammatory phase to the fibrosis progression phase.
Materials and methods
Animals
Male 9–10 week‐old BALB/c mice (Charles River Japan, Yokohama, Japan) were used. All animals were housed under controlled environmental conditions with free access to standard laboratory food and water according to the Ethical Guideline for Animal Experiments, Tsukuba Research Laboratories, GlaxoSmithKline (Ibaraki, Japan).
Animal treatment and tissue sampling
Mice were injected intranasally with 20 μl of a bleomycin hydrochloride solution containing 0.3 U of bleomycin dissolved in sterile saline under deep anaesthesia induced by intramuscular injection of the mixture of ketamine (50 mg/kg) and xylazine (10 mg/kg). Control mice were intranasally injected with saline. At 2, 5, 7, 9 and 14 days after bleomycin treatment, the mice were killed by exsanguination from the abdominal aorta under deep anaesthesia. Isolated left lobes of the lungs were fixed in 10% neutral buffered formalin, embedded in paraffin wax, sectioned and stained with either haematoxylin‐eosin or Masson's trichrome stain.
Histopathological examination
The intensity and extent of fibrotic changes in the lung were scored in accordance with the method of Ashcroft et al,20 with minor modifications. The scores used in determining the degree of fibrosis are defined as follows: 0, normal lung; 1, minimal fibrotic thickening of alveolar or bronchial walls; 2, moderate thickening of walls without obvious damage to the lung architecture; 3, increased fibrosis with definite damage to the lung structure and formation of fibrous bands or small fibrous masses; 4, severe distortion of structure and large fibrous areas; the “honeycomb lung” is placed in this category. Macrophage/monocyte infiltration and neutrophil infiltration were scored as follows: 0, normal; 1, slight; 2, mild; 3, moderate; 4, severe.
Immunohistochemical study
Tissue sections were de‐paraffinised with xylene, and rehydrated through graded concentrations of ethanol. For antigen retrieval, deparaffinised sections were microwaved for 10 min in citrate buffer, pH 6. The immunohistochemical procedure, thereafter, was performed using the Autostainer system (DAKO cytomation Japan, Kyoto, Japan). Briefly, endogenous peroxidase activity was inactivated by peroxidase blocking solution (DAKO) and tissue non‐specific binding sites were blocked using normal goat or rabbit serum. Tissue sections were overlaid with the 1:4000 dilution of phosphor‐Smad2 (Cell Signalling Technology, Beverly, Massachusetts, USA) or 1:750 dilution of either polyclonal (rabbit) anti‐TGF‐β1 (Santa Cruz, Santa Cruz, California, USA) or Smad3 (Zymed, South San Francisco, California, USA) or control (rabbit), and then incubated for 30 min. The tissue sections were washed with TRIS‐buffered saline and then incubated for 30 min with secondary goat anti‐rabbit biotinylated antibodies (Vector Laboratories, Burlingame, California, USA). The tissue sections were then washed in TRIS‐buffered saline and then incubated with Vectastain ABC reagent (Vector laboratories) followed by the peroxidase substrate, DAB reagent (DAKO). After optimal colour development, tissue sections were immersed in sterile water, counterstained with haematoxylin, and coverslipped. The increasing levels of TGF‐β1 and nuclear R‐Smad‐positive signals were evaluated as follows: (+/−) slightly increased and (+) mildly increased, (++) moderately increased and (+++) markedly increased as compared with results of the vehicle group.
All histopathological scoring and immunohistochemical evaluation were done by blind evaluation unaware of the bleomycin treatment.
Results
Sequential histopathological changes in bleomycin‐induced pulmonary fibrosis
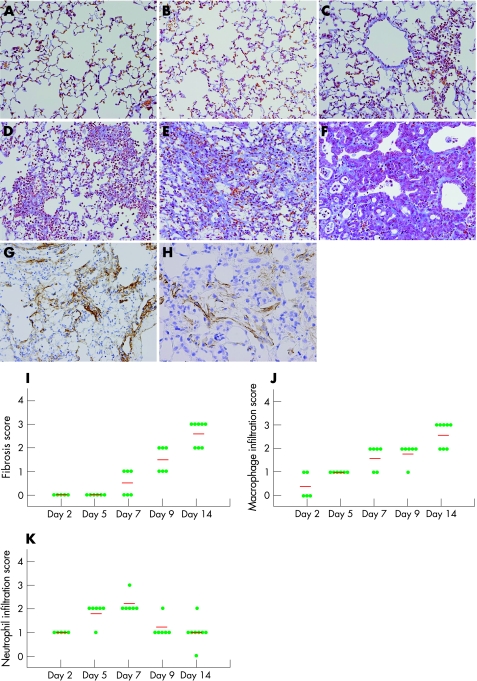
On day 2, slight inflammatory cell infiltration dominated by neurotrophils and macrophages was observed sparsely in the perivascular area (fig 1B). On day 5, the cellularity of focal inflammatory cell infiltration increased and spread to the interstitial and alveolar spaces (fig 1C). On day 7, massive inflammatory cell infiltration spread widely throughout the lung (fig 1D). The number of infiltrating neutrophils peaked on day 7 and decreased thereafter. On the other hand, the number of infiltrating macrophages tended to increase until day 7 and remained markedly increased up to day 14. In addition, on day 7, a slight fibrotic change, thickening of alveolar or bronchial walls, was noted in half of the bleomycin‐treated animals, and fibrosis further progressed thereafter.
Figure 1 A histopathological time course of pulmonary fibrosis induced by bleomycin. Representative results of Masson's trichrome staining of the lung from the saline‐treated group on day 9 (A), bleomycin (0.3 U/mice)‐treated group on days 2, 5, 7, 9 and 14 after intranasal injection (B, C, D, E and F, respectively), and immunohistochemical results for α‐smooth muscle actin and type I collagen form in the bleomycin‐treated group on day 9 (G and H, respectively). (A) No significant histological changes were observed in the lungs of the saline‐treated mice at all time points examined. (B) On day 2, slight inflammatory cell infiltration dominated by neurotrophils and macrophages was observed. (C) On day 5, inflammatory cell infiltration progressed and diffusely spread in the interstitial and alveolar spaces. (D) On day 7, massive inflammatory cell infiltration spread widely throughout the lung. A slight fibrotic change, thickening of alveolar or bronchial walls, was observed. (E) On day 9, thickening of the alveolar/bronchial walls, collapse of alveolar spaces, proliferation of fibroblasts and deposition of extracellular matrix were strongly induced. (F) On day 14, the level of fibrosis further progressed, and hyperplasia of the alveolar/bronchiolar epithelium was evident in affected lesions. (G) On day 9, deposition of type I collagen was induced in the interstitial region. (H) On day 9, proliferation of myofibroblasts (arrow heads; α‐smooth muscle actin positive) was induced. Original magnification ×200. (I–K) Results of histopathological evaluation for pulmonary fibrosis (I), macrophage/monocyte infiltration (J) and neutrophil infiltration (K). The intensity and extent of fibrotic changes in the lung were scored in accordance with the method of Ashcroft et al,20 with minor modifications. Macrophage/monocyte infiltration and neutrophil infiltration were scored as follows: 0, normal; 1,slight; 2, mild; 3, moderate; 4, severe. Horizontal bars in the figure express the mean scores of 5–8 mice. Each circle corresponds to the score from one mouse.
On day 9, interstitial fibrosis was evident in all bleomycin‐treated animals. Histopathological findings of pulmonary fibrosis, including thickening of the alveolar/bronchial walls, collapse of alveolar spaces, proliferation of fibroblasts and deposition of ECM, were strongly induced (fig 1E). Masson's trichrome staining clearly showed the formation of fibrous bands and masses. On day 14, the level of fibrosis further progressed, along with a regenerative change in the epithelium—that is, hyperplasia of alveolar/bronchiolar epithelial cells was evident in affected lesions (fig 1F). Immunohistochemical examination showed type I collagen deposition and myofibroblast proliferation (fig 1G, and H, respectively).
In the saline‐treated control animal, such morphological changes were not observed at any time point examined (fig 1A).
Time‐course changes of TGF‐β1 localisation in bleomycin‐induced pulmonary fibrosis
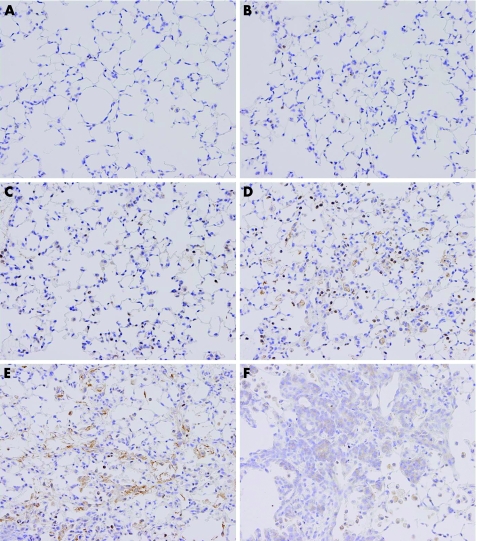
At the early time points, from day 2 to day 7, positive signals for TGF‐β1 were observed predominantly on infiltrating macrophages and a small number of type II epithelial cells (fig 2B–D). The number of TGF‐β1‐positive macrophages progressively increased up to day 14, which mirrors the elevation of macrophage infiltration. On day 7, positive signals for TGF‐β1 were observed in almost all infiltrating macrophages (fig 2D).
Figure 2 Time‐course changes of TGF‐β1 localisation in bleomycin‐induced pulmonary fibrosis. Representative immunohistochemical results for TGF‐β1 for the saline‐treated group on day 9 (A) and for the bleomycin‐treated group on days 2, 5, 7, 9 and 14 after intranasal injection (B, C, D, E and F, respectively). (A) In the vehicle group, positive signals for TGF‐β1 were sparsely observed on a small number of alveolar macrophages. (B–D) On days 2–7, positive signals for TGF‐β1 were predominantly detected in the cytoplasm of infiltrating macrophages and some neutrophils. The number of TGF‐β1‐positive macrophages progressively increased with the elevation of infiltration. (E) On day 9, in addition to infiltrating macrophages, positive signals for TGF‐β1 were weakly observed on fibroblasts/myofibroblasts in the fibroblastic foci. Extracellularly localised TGF‐β1 was prominently observed in the interstitial and alveolar spaces (F). On day 14, weak positive signals for TGF‐β1 were observed on hyperplastic alveolar/bronchiolar epithelial cells.
At the later time points, from day 9 to 14, in addition to the infiltrating macrophages, positive signals for TGF‐β1 were weakly observed on fibroblasts/myofibroblasts in the fibrotic foci and hyperplastic alveolar/bronchiolar epithelial cells (figs 2E, F). These results confirmed that TGF‐β1 production was markedly increased before the progression of pulmonary fibrosis. In the saline‐treated control animal, positive signals for TGF‐β1 were observed weakly in the cytoplasm of a small number of bronchial epithelial cells and alveolar macrophages (fig 2A).
Time‐course changes of R‐Smad (Smad3 and pSmad2) localisation in pulmonary fibrosis
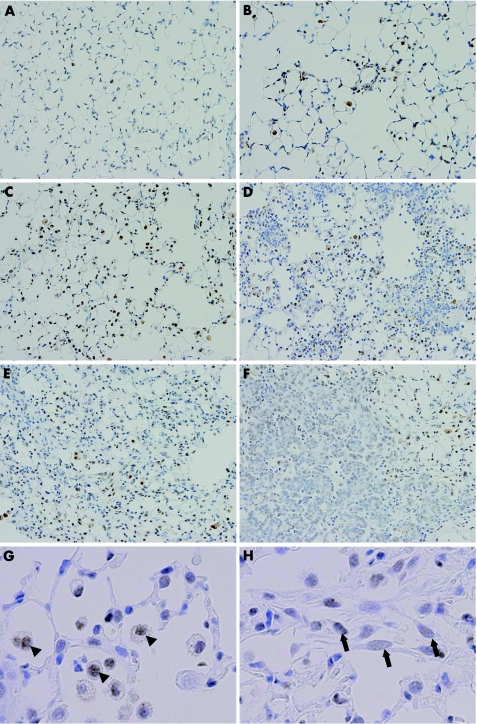
At the early time points, from day 2 to 7, positive signals for Smad3 were observed preferentially on infiltrating macrophages and some type II alveolar epithelial cells (fig 3B–D). Intense Smad3‐positive signals were exclusively localised to the nucleus, suggesting that Smad3 activation/translocation occurred in these cells. The cellularity of Smad3‐positive macrophages progressively increased along with an increase in macrophage infiltration.
Figure 3 Time‐course changes of Smad3 localisation in bleomycin‐induced pulmonary fibrosis. Representative immunohistochemical results for Smad3 for the saline‐treated group on day 9 (A) and for the bleomycin‐treated group on days 2, 5, 7, 9 and 14 after intranasal injection (B, C, D, E and F, respectively). Higher‐magnification views of specimens from bleomycin‐treated mice on day 9 (G,H). (A) In the vehicle group, positive signals for Smad3 were sparsely observed on a small number of bronchial epithelial cells and alveolar macrophages. (B–D) On days 2–7, positive signals for Smad3 were observed preferentially on infiltrating macrophages and some type II alveolar epithelial cells. Smad3‐positive signals were exclusively localised to the nucleus. The cellularity of Smad3‐positive macrophages progressively increased along with an increase in macrophage infiltration. (E) On day 9, the numbers of nuclear Smad3‐positive macrophages and type II epithelial cells were still high. Positive signals for Smad3 were observed weakly on the nucleus of proliferating fibroblasts in fibroblastic foci. (F) On day 14, weakly positive signals for Smad3 were observed on hyperplastic alveolar/bronchiolar epithelial cells. (G) A higher magnification image on day 9. Arrowheads indicate Smad3‐positive signals on macrophages. Note the intense Smad3‐positive signals observed in the nucleus of infiltrating macrophages. (H) A higher magnification image on day 9. Arrows indicate Smad3‐positive signals on fibroblasts. Note the faint Smad3‐positive signals observed in the nucleus of fibroblasts in fibroblastic foci, ×400.
On day 9, histopathologically prominent fibrosis developed, and the numbers of nuclear Smad3‐positive macrophages and type II epithelial cells were still large (fig 3E). Positive signals for Smad3 were also observed in the nucleus of proliferating fibroblasts in fibroblastic foci, although signal intensity was much weaker than that of macrophages (fig 3G,H). On day 14, positive nuclear Smad3 signals were also observed in the regenerating alveolar/bronchiolar epithelial cells (fig 3F). In the saline‐treated control animal, positive signals for Smad3 were observed weakly in the cytoplasm of a small number of bronchial epithelial cells and alveolar macrophages (fig 3A).
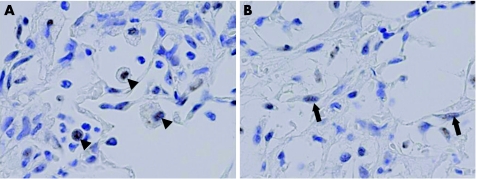
To examine Smad2 activation, we immunohistochemically examined the presence of pSmad2. pSmad2‐positive cell types were similar to Smad3‐positive cell types (fig 4). pSmad2 was exclusively localised to the nucleus, although positive signals were less intense compared with Smad3 signals. The only minor difference observed was that the number of pSmad2‐positive fibroblasts was much smaller than that of Smad3‐positive cells.
Figure 4 Time‐course changes of pSmad2 localisation in bleomycin‐induced pulmonary fibrosis. Representative immunohistochemical results for pSmad2 for the bleomycin‐treated group on day 9 (A,B). (A) Arrowheads indicate pSmad2‐positive signals on macrophages. Note the intense pSmad2‐positive signals observed in the nucleus of infiltrating macrophages. (B) Arrows indicate pSmad2‐positive signals on fibroblasts. Note that faintly pSmad2‐positive signals were observed in the nucleus of fibroblasts in fibroblastic foci.
Table 1 summarises the histopathological and immunohistochemical results.
Table 1 A summary of histopathological time‐course changes and immunohistochemical localisation of transforming growth factor‐β1 and R‐Smads in bleomycin‐induced pulmonary fibrosis.
| Time points | Day2 | Day5 | Day7 | Day9 | Day14 | |
|---|---|---|---|---|---|---|
| Fibrosis (1) | Fibrosis (1 or 2) | Fibrosis (2 or 3) | ||||
| Histopathological findings (score) | Neutrophil infiltration (1) | Neutrophil infiltration (1∼2) | Neutrophil infiltration (2–3) | Macrophage infiltration (1–2) | Macrophage infiltration (2–3) | |
| Macrophage infiltration (0∼1) | Macrophage infiltration (1) | Macrophage infiltration (1–2) | Neutrophil infiltration (1–2) | Neutrophil infiltration (1–2) | ||
| Cell types | ||||||
| Monocytes/macrophages | TGF‐β1 | (++) | (++) | (+++) | (+++) | (+++) |
| Smad3 | (++) | (++) | (+++) | (+++) | (+++) | |
| pSmad2 | (++) | (++) | (+++) | (+++) | ||
| Neutrophils | TGF‐β1 | (+) | (++) | |||
| Smad3 | (+) | (++) | ||||
| pSmad2 | (+) | (++) | ||||
| Type II epithelial cells | TGF‐β1 | (+) | (+) | (++) | (++) | (++) |
| Smad3 | (+) | (+) | (++) | (++) | (++) | |
| pSmad2 | (+) | (+) | (++) | (++) | (++) | |
| Bronchial epithelial cells | TGF‐β1 | (+) | (+) | (+) | (+) | (+) |
| Smad3 | (+) | (+) | (+) | (+) | (+) | |
| pSmad2 | (+) | (+) | (+) | (+) | (+) | |
| Fibroblasts | TGF‐β1 | (+) | (+) | (+) | ||
| Smad3 | (+) | (+) | (+) | |||
| pSmad2 | (+/−) | (+/−) | (+/−) | |||
| Regenerating epithelial cells | TGF‐β1 | (++) | (++) | |||
| Smad3 | (++) | (++) | ||||
| pSmad2 | (++) | (++) | ||||
The level of increase in transforming growth factor‐β1 and nuclear R‐Smad‐positive signals was evaluated as follows: (+/−) slightly increased, (+) mildly increased, (++) moderately increased, (+++) markedly increased as compared with the results in the vehicle group. The numbers in parentheses express the range of scores at each time point.
Discussion
TGF‐β1 is the cytokine causatively associated with disorders characterised by inflammation and fibrosis. TGF‐β1 stimulates fibroblast proliferation and migration, induces synthesis of ECM proteins and promotes myofibroblast differentiation.5,6 Production of TGF‐β1 is amplified in various types of pulmonary fibrosis.5,6 In agreement with previous reports,7,8 our present results confirmed that a progressive increase in TGF‐β1 expression was preferentially observed in infiltrating macrophages from the early inflammatory phase to the fibrosis progression phase. This indicated that infiltrating macrophages are the main source of TGF‐β1 in pulmonary fibrosis and have a regulatory function of fibrosis initiation.
The Smad protein family has been identified as an essential component of the intracellular signalling system for TGF‐β family proteins. The importance of R‐Smad activation in the development of pulmonary fibrosis has been shown by recent in vivo and in vitro studies.16,17,18,19 In this study, our immunohistochemical data showed that Smad2 and Smad3 activation occurred in infiltrating macrophages, type II epithelial cells, fibroblasts and hyperplastic alveolar/bronchiolar epithelial cells. These results are understandable, because these Smad2 and Smad3 positive cell types are known to express high levels of TGF‐β receptors.13,21 Furthermore, the time‐course changes of Smad2 and Smad3 activation were parallel to both elevation of TGF‐β1 and fibrosis progression, implying that TGF‐β1 drives fibrosis development via activation of these two R‐Smad in vivo.
Infiltrating macrophages are regarded as the main producers of TGF‐β1 and are believed to have a central role in regulating pulmonary fibrosis.6,12,13 Our results showed that TGF‐β1 expression and R‐Smad activation were observed in most of the infiltrating macrophages from the disease initiation until the fibrosis progression stage, suggesting involvement of the autoinduction of TGF‐β1 in macrophages.
On the other hand, it was reported that R‐Smad activation in lung fibroblasts results in the induction of COL1A2, COL3A1, COL6A1, COL6A3 and the tissue inhibitor of metalloprotease‐1 mRNA.22 In this study, we detected R‐Smad‐positive signals on the nuclei of proliferating fibroblasts in the fibrotic foci, suggesting active induction of ECM production in the affected area.
Although both Smad2 and Smad3 mediate signals from TGF‐β1, these R‐Smads are believed to have non‐redundant functions.23 TGF‐β1‐mediated induction of matrix metalloprotease‐2 was Smad2‐dependent, whereas the induction of c‐fos, Smad7 and TGF‐β1 was Smad3‐dependent.23 Further studies on the transcriptional regulation of R‐Smads in each cell type will show the molecular mechanism underlying the pathogenesis of pulmonary fibrosis.
In summary, we showed that the TGF‐β1/Smad‐mediated signalling pathway was activated during the progression of pulmonary fibrosis. Furthermore, we identified the cell types expressing TGF‐β1, Smad2 and Smad3 at various time points from the early inflammatory phase to the fibrosis progression phase. Our data emphasise that strategies to downregulate TGF‐β1/Smad signalling in each cell type could provide a valuable adjunctive treatment for fibrotic conditions.
Take‐home messages
Transforming growth factor (TGF)β1 is a growth factor playing a central part in pulmonary fibrosis. The receptor‐activated Smads proteins (R‐Smads: Smad2 and Smad3) are intracellular mediators of TGF‐β1 signalling.
TGF‐β1, Smad3 and pSmad2 protein localisations were analysed at various time points from the early inflammatory phase to the fibrosis progression phase using the bleomycin‐induced model.
Considerable pulmonary fibrotic changes accompanied by marked increases in TGF‐β1, Smad3 and pSmad2 positive signals were observed.
TGFβ‐1‐positive signals were preferentially localised in the cytoplasm of infiltrating macrophages.
Smad3 and pSmad2 positive signals were observed in the nuclei of infiltrating macrophages and type II alveolar epithelial cells early in the course of pulmonary fibrosis, and subsequently in the nuclei of fibroblasts.
Abbreviations
ECM - extracellular matrix
TGF - transforming growth factor
Footnotes
Competing interests: None.
References
- 1.Crouch E. Pathobiology of pulmonary fibrosis. Am J Physiol 1990259(Pt 1)L159–L184. [DOI] [PubMed] [Google Scholar]
- 2.Thannickal V J, Toews G B, White E S.et al Mechanisms of pulmonary fibrosis. Annu Rev Med 200455395–417. [DOI] [PubMed] [Google Scholar]
- 3.Garantziotis S, Steele M P, Schwartz D A. Pulmonary fibrosis: thinking outside of the lung. J Clin Invest 2004114319–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper J A., Jr Pulmonary fibrosis: pathways are slowly coming into light. Am J Respir Cell Mol Biol 200022520–523. [DOI] [PubMed] [Google Scholar]
- 5.Bartram U, Speer C P. The role of transforming growth factor beta in lung development and disease. Chest 2004125754–765. [DOI] [PubMed] [Google Scholar]
- 6.Leask A, Abraham D J. TGF‐beta signaling and the fibrotic response. FASEB J 200418816–827. [DOI] [PubMed] [Google Scholar]
- 7.Broekelmann T J, Limper A H, Colby T V.et al Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci USA 1991886642–6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoyt D G, Lazo J S. Alterations in pulmonary mRNA encoding procollagens, fibronectin and transforming growth factor‐beta precede bleomycin‐induced pulmonary fibrosis in mice. J Pharmacol Exp Ther 1988246765–771. [PubMed] [Google Scholar]
- 9.Giri S N, Hyde D M, Hollinger M A. Effect of antibody to transforming growth factor beta on bleomycin induced accumulation of lung collagen in mice. Thorax 199348959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q, Wang Y, Hyde D M.et al Reduction of bleomycin‐induced lung fibrosis by transforming growth factor beta soluble receptor in hamsters. Thorax 199954805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sime P J, Xing Z, Graham F L.et al Adenovector‐mediated gene transfer of active transforming growth factor‐beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest 1997100768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denholm E M, Rollins S M. Expression and secretion of transforming growth factor‐beta by bleomycin‐stimulated rat alveolar macrophages. Am J Physiol 1993264(Pt 1)L36–L42. [DOI] [PubMed] [Google Scholar]
- 13.Khalil N, Parekh T V, O'Connor R.et al Regulation of the effects of TGF‐beta 1 by activation of latent TGF‐beta 1 and differential expression of TGF‐beta receptors (T beta R‐I and T beta R‐II) in idiopathic pulmonary fibrosis. Thorax 200156907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groneberg D A, Witt H, Adcock I M.et al Smads as intracellular mediators of airway inflammation. Exp Lung Res 200430223–250. [DOI] [PubMed] [Google Scholar]
- 15.Flanders K C. Smad3 as a mediator of the fibrotic response. Int J Exp Pathol 20048547–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Y, Geverd D A. Regulation of Smad3 expression in bleomycin‐induced pulmonary fibrosis: a negative feedback loop of TGF‐beta signaling. Biochem Biophys Res Commun 2002294319–323. [DOI] [PubMed] [Google Scholar]
- 17.Bonniaud P, Kolb M, Galt T.et al Smad3 null mice develop airspace enlargement and are resistant to TGF‐beta‐mediated pulmonary fibrosis. J Immunol 20041732099–2108. [DOI] [PubMed] [Google Scholar]
- 18.Zhao J, Shi W, Wang Y L.et al Smad3 deficiency attenuates bleomycin‐induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol 2002282L585–L593. [DOI] [PubMed] [Google Scholar]
- 19.Venkatesan N, Pini L, Ludwig M S. Changes in Smad expression and subcellular localization in bleomycin‐induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2004287L1342–L1347. [DOI] [PubMed] [Google Scholar]
- 20.Ashcroft T, Simpson J M, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol 198841467–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Y, Shah D U. Expression of transforming growth factor‐beta type I and type II receptors is altered in rat lungs undergoing bleomycin‐induced pulmonary fibrosis. Exp Mol Pathol 20006967–78. [DOI] [PubMed] [Google Scholar]
- 22.Verrecchia F, Chu M L, Mauviel A. Identification of novel TGF‐beta/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem 200127617058–17062. [DOI] [PubMed] [Google Scholar]
- 23.Piek E, Ju W J, Heyer J.et al Functional characterization of transforming growth factor beta signaling in Smad2‐ and Smad3‐deficient fibroblasts. J Biol Chem 200127619945–19953. [DOI] [PubMed] [Google Scholar]