Abstract
Background
Versican, an extracellular matrix proteoglycan, has been noted to be expressed in several malignant tumours and has been suggested to play an important role in cancer development and tumour growth.
Aims
To investigate whether the versican expression level in the peritumoural stromal tissue of primary oral squamous cell carcinoma (OSCC) predicts relapse‐free or disease‐specific survival. Also, to study the associations between versican expression and several other clinicopathological variables, as well as tumour cell proliferation.
Methods
Immunohistochemistry was used to study the expression of versican and tumour cell proliferative activity in 139 OSCCs. All pertinent clinical data were collected retrospectively from the hospital records.
Results
In this cohort, versican expression did not correlate with the clinicopathological factors or tumour cell proliferation. In univariate analyses, higher risk for disease recurrence was associated with higher stromal versican expression score (p = 0.02), positive neck node status (p = 0.02), lower Karnofsky performance status (p = 0.03) and higher tumour cell proliferation index (p = 0.04). Increased disease‐specific risk of death was associated with high stromal versican expression score (p = 0.005) higher T class (p = 0.002), positive neck node status (p<0.001), higher stage (p<0.001), poorer histological differentiation (p = 0.005), worse general condition of the patient (p = 0.049) and increased tumour cell proliferative index (p = 0.02). In multivariate disease‐specific survival analysis, high stromal versican expression score (p = 0.048), poorer histological differentiation (p = 0.047) and higher stage (p = 0.002) independently predicted poorer disease outcome.
Conclusions
In this cohort, increased stromal versican expression correlated with both increased risk for disease recurrence and shortened survival. High stromal versican expression may thus be considered an independent and adverse prognostic marker in OSCC.
Oral and pharyngeal cancer is the sixth most common cancer worldwide.1 In the UK and US, however, only 4% of malignant tumours originate in the oral cavity.1 Over 90% of all oral cavity malignomas are squamous cell carcinomas.1,2 The incidence of oral squamous cell carcinoma (OSCC) is about 2–3 times higher in men than in women, although the difference seems to be decreasing.1 OSCC may present with ulcerative, infiltrative or exophytic growth patterns.1 Specific OSCC sites include buccal and lip mucosa, floor of the mouth, alveolar ridge, retromolar trigone, anterior two thirds of the tongue and hard palate. In developed countries the oral tongue is the most common site of OSCC, representing about 50% of all cases.2
The extracellular matrix (ECM) is an organised intricate molecular network made up of diverse collagen superfamily molecules and non‐collagenous molecules, including hyaluronan, proteoglycans and glycoproteins.3,4 It is constantly remodelled during various physiological and pathological processes.3,4,5 In these processes, several cytokines, including transforming growth factor β1, platelet‐derived growth factors and epidermal growth factor, seem to play central regulatory roles.3
Versican, a member of the aggrecan gene family, is a large chondroitin sulphate proteoglycan carrying several active domains that enable versatile interactions in a variety of biological and pathological processes. Versican plays a role in ECM assembly, anti‐adhesion, cell proliferation, migration and extracellular matrix remodelling.5,6,7,8 It is expressed in several malignant tumours, suggesting its involvement in the development and progression of cancer. Apparently, versican promotes tumour growth by destabilising focal cell contacts, which repress cell adhesion, stimulate cell proliferation and regulate angiogenesis.9,10,11,12,13 Increased levels of versican have been reported in adenocarcinomas14,15,16,17,18,19,20 as well as in squamous cell carcinomas,14,21 sarcomas,22 mesotheliomas9 and in malignant melanomas.23 Data on versican expression have been reported in head and neck tumours only in oropharyngeal and hypopharyngeal carcinomas and, recently, also in oral premalignant and malignant lesions.21,24 Even though the role of versican remains uncertain in OSCC, Banerjee et al24 suggested its appearance in premalignant oral lesions to be a potential marker of invasiveness and impending malignant tumour behaviour, which emphasises the role of versican as a clinically extremely interesting tumour progression‐related molecule.
Even though treatment of OSCC has developed over the past few decades, the long‐term survival rates have not shown notable improvement. The unpredictable nature of OSCC seems to ensue from its characteristic heterogenic tumour behaviour among these carcinomas. For prognosis and selecting treatment, more independently of the clinical variables used at present, a search for new biological markers is crucial.25,26,27 This study was undertaken to investigate the prognostic significance of versican and its relationship to clinicopathological variables as well as to tumour cell proliferation in OSCC.
Materials and methods
Cohort and clinicopathological data
This retrospective cohort included 139 patients with OSCC with sufficient tumour material available for immunohistochemistry. Comprehensive clinicopathological data of the patients, tumours and their treatment (table 1) were obtained from the previous study of the same original patient group that included 239 patients with OSCC.28 The patients were diagnosed as having squamous cell carcinoma of the oral cavity and treated in Kuopio University Hospital or Jyväskylä Central Hospital, Finland, between 1979 and 1998. All clinical data were reviewed by an otorhinolaryngologist (AK) and an oncologist (EK). Staging of the tumours was based on otorhinolaryngological status, endoscopy and chest x ray, and was performed according to International Union Against Cancer (UICC) classification.29 Karnofsky performance status at the time of diagnosis was also determined.30 All 139 patients were followed‐up until death or June 2002.
Table 1 Clinicopathological data and results of univariate survival analyses (log rank).
| Variable | Patients, n (%) | Power of different variables in predicting survival, univariate analyses | |||
|---|---|---|---|---|---|
| DFS | DSS | ||||
| Log rank | p Value | Log rank | p Value | ||
| Sex | 1.43 | 0.2 | 1.91 | 0.2 | |
| Male | 72 (51) | ||||
| Female | 67 (49) | ||||
| Age (years) | 3.2 | 0.07 | 0.38 | 0.5 | |
| <65 | 73 (52) | ||||
| ⩾65 | 66 (48) | ||||
| Site | 3.58 | 0.6 | 6.32 | 0.3 | |
| Mucosa of the cheek or lip | 16 (11) | ||||
| Upper alveolus or gingival | 10 (8) | ||||
| Lower alveolus or gingival | 18 (13) | ||||
| Hard palate | 8 (6) | ||||
| Tongue | 62 (44) | ||||
| Floor of mouth | 25 (18) | ||||
| T class | 3.32 | 0.3 | 14.9 | 0.002 | |
| T1 | 50 (37) | ||||
| T2 | 55 (39) | ||||
| T3 | 17 (12) | ||||
| T4 | 17 (12) | ||||
| N class | 5.47 | 0.02 | 23.7 | <0.001 | |
| N0 | 107 (77) | ||||
| N1–3 | 32 (23) | ||||
| Stage | 2.76 | 0.4 | 18.95 | <0.001 | |
| I | 45 (33) | ||||
| II | 43 (31) | ||||
| III | 27 (19) | ||||
| IV | 24 (17) | ||||
| Histological differentiation | 3.54 | 0.06 | 7.74 | 0.005 | |
| Good | 83 (60) | ||||
| Moderate or poor | 56 (40) | ||||
| Karnofsky performance status | 4.76 | 0.03 | 3.88 | 0.049 | |
| ⩾80 | 44 (32) | ||||
| <80 | 95 (68) | ||||
| Tumour cell proliferation index | 4.3 | 0.04 | 5.59 | 0.02 | |
| ⩽40 | 70 (50) | ||||
| >40 | 64 (46) | ||||
| Data not available | 5 (4) | ||||
| Stromal versican expression score | 5.46 | 0.02 | 7.89 | 0.005 | |
| ⩽100 | 75 (54) | ||||
| >100 | 64 (46) | ||||
DFS, disease‐free survival; DSS, disease‐specific survival; N, node; T, tumour.
Table 1 summarises the pertinent clinical and immunohistological data. The median age of the patients at the time of diagnosis was 64 years (range 10–88). This cohort included almost equal numbers of both sexes. Distribution of the baseline characteristics (sex, age, Karnofsky performance status, histological differentiation, primary location and stage) of the present cohort (n = 139) did not differ significantly from those of the original patient group (n = 239; p>0.19). In all, 32 (23%) patients presented with neck metastasis and 1 (1%) with distant lung metastasis. The goal of primary treatment was curative in 129 (93%) patients. The treatment modalities were surgery in 65 (47%), surgery and radiotherapy in 54 (38%), and radiotherapy in 10 (8%) patients. Owing to advanced disease or poor general condition, 4 (2%) patients received palliative, and 6 (5%) only basic treatment. In curatively treated patients, the disease recurred in 54 (38%): recurrence was local in 29 (21%), regional in 21 (15%) and distant in 4 (2%) patients. The median follow‐up time was 53 months (range 0.6–272).
Histopathology
Representative samples of primary tumours were fixed in 10% buffered formalin (pH 7), routinely processed and embedded in paraffin wax. All tumours were reassessed for the diagnosis and histological grade by two senior histopathologists according to the World Health Organization classification.31 The most representative blocks were selected and cut into 5 μm‐thick sections for immunohistochemical analyses.
Immunohistochemistry
The sections cut from paraffin wax‐embedded tumour blocks were mounted on organosilan‐coated (3‐aminopropyltriethoxysilane; Sigma, St Louis, Missouri, USA) object slides, incubated for 30 min at 58°C, deparaffined in xylene, and rehydrated in graded alcohols and distilled water. To optimise antigen accessibility, the epitopes were unmasked by boiling the samples in a microwave oven (800 W, 3×5 min) in citrate buffer (0.01 mol/l, pH 6). The evaporated buffer was replaced with distilled water. Rehydrated sections were left standing for 18 min in the last buffer, and were then washed in phosphate‐buffered saline (PBS; 0.1 mol/l, pH 7.4). Endogenous peroxidase activity was blocked with 5% H2O2 in distilled water for 5 min and any unspecific binding of the primary antibody with 1.5% normal horse serum (Vectastain Elite ABC Kit, Vector Laboratories, Burlingame, California, USA) in PBS at 20°C for 35 min. The sections were treated with a monoclonal mouse IgG anti‐human versican antibody, that recognises all versican isoforms (clone 2‐B‐1, Seikagaku Corporation, Tokyo, Japan)22 at 1:500 dilution in 1.5% normal horse serum (Vectastain Elite ABC Kit) for 20 h at 4°C. For Ki‐67 immunostaining, the blocking of endogenous peroxidase activity lasted for 25 min, and to demonstrate cell proliferation in the sections mouse IgG, anti‐human Ki‐67 antibody (MIB1; Immunotech, Marseilles, France) was used at a dilution of 1:200 in PBS with 1% bovine serum albumin. Subsequently, the samples were incubated with biotinylated horse anti‐mouse IgG secondary antibody (Vectastain Elite ABC Kit) for 45 min and bathed in avidin–biotin peroxidase reagent (Vectastain Elite ABC Kit) for 50 min at room temperature. Before and after both steps, the samples were washed in PBS (2× 5 min). The bound secondary antibody was visualised by incubating the samples for 5 min in 0.05% diaminobenzidine tetrahydrochloride (Sigma). The slides were rinsed in tap water for 3 min and in distilled water for 2 min, counterstained with Mayer's haematoxylin, dehydrated, cleared and mounted with DePex (BDH, Poole, Dorset, UK). All immunostainings were performed with Coverplate technology on a Shandon Sequenza workstation (Shandon Scientific, Pittsburgh, Pennsylvania, USA).
In each staining batch, dermal tissue samples from the same tissue block with known strong stromal versican positivity around normal hair follicles were used as positive controls.32 In Ki‐67 stainings strongly positive melanoma samples served as positive controls. Corresponding samples together with a tumour sample from the present series without the use of primary antibody were used as negative controls.
Scoring of immunohistological stainings
All immunohistochemical stainings were reviewed by two investigators (AK and KR) unaware of the clinical data. In the case of discrepancy (in all <10%), they re‐evaluated samples with a dual‐headed microscope and settled the final scores. To assess stromal versican expression, the stained stromal proportion of the whole stromal area around the invasive carcinoma was assessed on a continuous (0–100%) scale. Simultaneously, the most prevailing versican staining intensity around invasive carcinoma nests was visually graded into three groups: 1, weak; 2, moderate; or 3, strong. A semiquantitative stromal versican expression score was obtained by multiplying the percentage by the intensity. The proliferative activity of the tumour cells (proliferation index) was determined similarly by assessing visually the percentage of Ki‐67‐stained tumour cell nuclei on a continuous scale (0–100%). For statistics, versican expression score was divided into two categories according to the median score: low (⩽100) and high (>100). Similarly, the tumour cell proliferation index obtained was divided into two groups by median: low (⩽40) and high (>40).
Statistical analyses
The representativeness of this cohort (n = 139) of the original patient group (n = 239) was checked with a non‐parametric χ2 test for the categorical variables, and with the one‐sample t test for continuous variables. To exclude the possibility that the 20‐year study period itself or variations in tissue processing during this time might have some effect on immunohistological staining (versican and Ki‐67), the cohort was divided by date of primary diagnosis into four subgroups, each including a 5‐year study period. The percentual distribution of tripartite stromal versican staining intensity (weak, moderate, strong) as well as low versus high versican expression score, and low versus high tumour cell proliferation index among the subgroups was tested by a non‐parametric χ2 goodness‐of‐fit test and the resemblance of the distributions by a non‐parametric Kruskal–Wallis test. The associations between stromal versican staining pattern and clinicopathological variables were analysed with the χ2 test. Univariate analysis of disease‐free survival (DFS) and disease‐specific survival (DSS) was performed by the Kaplan–Meier method.33 Only patients treated with curative intention and who achieved complete remission were included in the analysis of DFS, which was calculated from the date of primary diagnostic biopsy to the date of discovering disease recurrence. DSS was calculated from the date of primary diagnostic biopsy to the end of follow‐up or death. Only deaths from OSCC were considered as events in the DSS analyses. Multivariate DSS analyses (Cox proportional hazards model) were performed in a stepwise manner (entry limit, p<0.05; removal limit, p⩾0.1).34 Baseline covariates used in the Cox proportional hazards model were age, sex, histological differentiation (good vs moderate or poor), stage (categorical, reference class stage I), Karnofsky status (<80 vs ⩾80), stromal versican expression score (high vs low) and tumour cell proliferation index (high vs low). SPSS for Windows V.10 was used for all statistical analyses and p<0.05 was regarded as significant.
Ethics
The research was approved by the research ethics committee of Kuopio University and Kuopio University Hospital. We had no conflicts of interests in relation to the study methodology, results, or other sectors of the study.
Results
Stromal versican expression score and tumour cell proliferation index
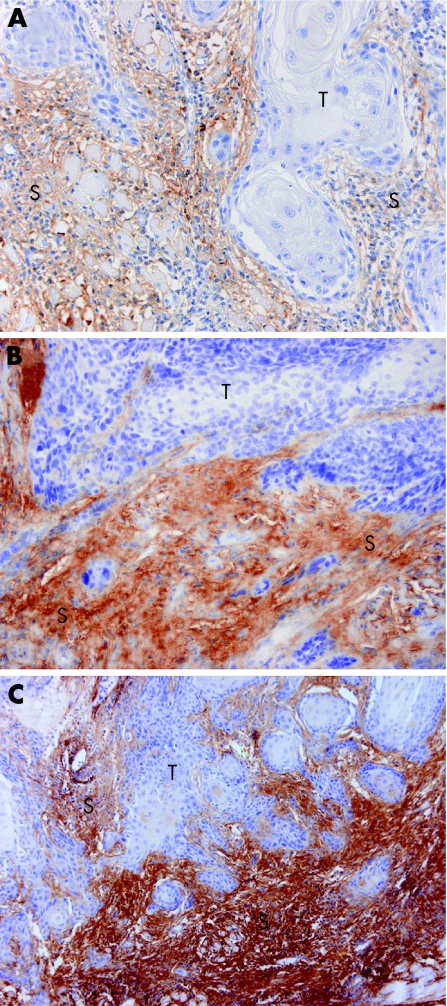
In addition to stromal versican staining around the invasive carcinoma nests, occasional immunoreactivity for versican was seen in the walls of the blood vessels. In our group of patients with OSCC, the stromal versican staining intensity was weak in 41 (30%; fig 1A), moderate in 58 (42%; fig 1B) and strong in 40 (28%; fig 1C) primary tumours. The median stromal versican staining percentage was 50 (range 10–90) and the median stromal versican expression score 100 (range 10–270). The median tumour cell proliferation index in this series was 40 (range 10–90). Higher stromal versican expression score was clearly associated with high tumour cell proliferation index (Pearson's χ2 = 2.9; p = 0.09) and disease recurrence (Pearson's χ2 = 3.28; p = 0.07), although not significantly. A similar trend was not evident in association with any other variable tested (sex, age, Karnofsky status, T class, N class, stage, histological differentiation) (Pearson's χ2<2.76; p>0.1). Hyaluronan expression data from our previous study were available for 138 patients in this cohort (data not shown).26 Reduced (irregular) pericellular hyaluronan expression, which linked with poorer DSS, was strongly associated with high stromal versican expression score (Pearson's χ2 19.55; p<0.001). The time of diagnosis did not have any effect on distributions of stromal versican staining intensity, versican expression score or tumour cell proliferation index (χ2<1.81; p>0.4). All distributions inside the chronological subgroups were also essentially similar to original distributions (χ2<1.16; p>0.8).
Figure 1 Stromal versican staining intensity in oral squamous cell carcinoma (OSCC) primary tumour demonstrated by immunohistochemistry. (A) Weak staining intensity; magnification ×400. (B) Moderate staining intensity; magnification ×400. (C) Strong staining intensity; magnification ×200. T, tumour; S, stroma.
Survival analyses
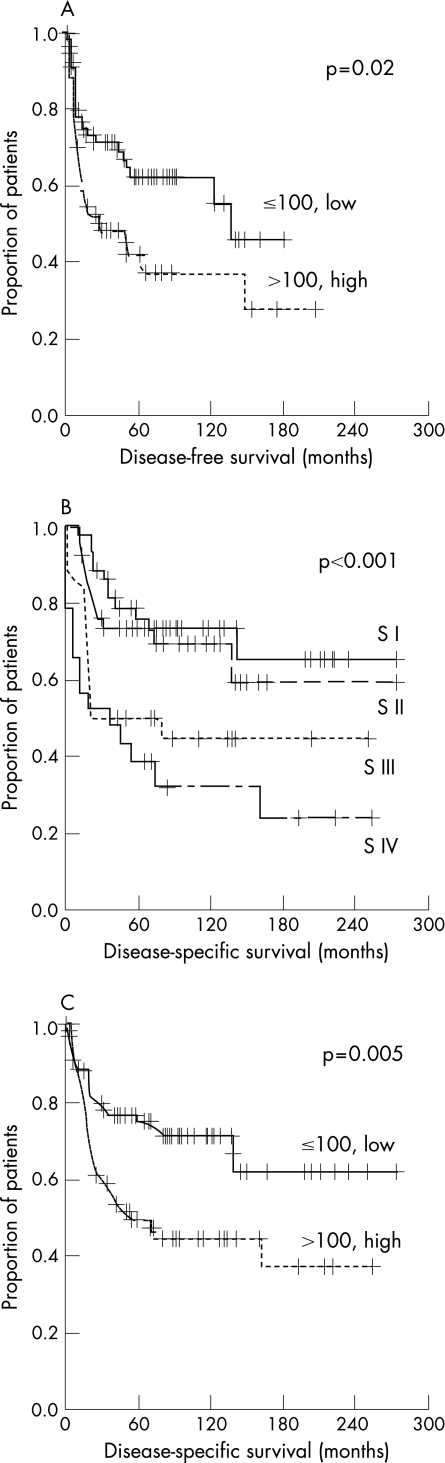
Table 1 presents the details of all DFS and DSS data of univariate analyses. In this cohort the median DFS was 9 months (range 0.9–146; 95% CI 13 to 29) and DSS 17 months (range 0.6–160; 95% CI 18 to 37). In univariate analyses, increased risk of disease recurrence was significantly associated with positive neck node status, lower Karnofsky performance status, higher tumour cell proliferation index and higher stromal versican expression score (fig 2A). In this cohort, the risk of death due to OSCC was significantly higher in association with larger primary tumour, positive neck node status, higher stage (fig 2B), poorer histological differentiation of the tumour, worse general condition of the patient, increased tumour cell proliferative index and higher stromal versican expression score (fig 2C). In multivariate DSS analysis, poorer histological differentiation, higher stage and high stromal versican expression score remained as independent predictors for unfavourable prognosis (table 2).
Figure 2 Kaplan–Meier plots for disease‐free survival (DFS) and disease‐specific survival (DSS) in oral squamous cell carcinoma (OSCC). (A) Univariate analysis for DFS by stromal versican expression score (high vs low), log rank statistic 5.46; p = 0.02. (B) Univariate analysis for DSS by tumour stage, log rank statistic 18.95; p<0.001.(C) Univariate analysis for DSS by stromal versican expression score (high vs low), log rank statistic 7.89; p = 0.005. S, stage.
Table 2 Multivariate analysis of disease‐specific survival.
| Variable | Wald | Relative risk | 95% CI | p Value |
|---|---|---|---|---|
| Age (years)* | 3.57 | 1.9 | 0.98 to 3.7 | 0.06 |
| Sex | 1.78 | 0.7 | 0.4 to 1.2 | 0.2 |
| Karnofsky performance status* | 0.23 | 0.8 | 0.4 to 1.7 | 0.6 |
| Histological differentiation* | 3.96 | 1.9 | 1.01 to 3.5 | 0.047 |
| Stage† | 15.37 | 0.002 | ||
| SII | 0.26 | 1.3 | 0.5 to 3 | 0.6 |
| SIII | 5.56 | 2.7 | 1.2 to 6 | 0.02 |
| SIV | 12.21 | 4.1 | 1.9 to 9.2 | <0.001 |
| Tumour cell proliferation index* | 1.28 | 1.4 | 0.8 to 2.7 | 0.3 |
| Stromal versican expression score* | 3.92 | 1.8 | 1.006 to 3.3 | 0.048 |
*For cut‐offs, see table 1.
†Categorical variable, reference class stage I.
Discussion
The results from this study support the hypothesis that strong stromal versican expression is an adverse prognostic sign in OSCC. In several other cancers, intense versican expression has been shown to be associated with both more aggressive tumour behaviour and unfavourable clinical course of the disease.14,15,17,18,20,35 In oral carcinogenesis, versican gene overexpression has been proposed to be a distinctive molecular marker of malignant transformation and impending invasive potential of the tumour cells.24 However, in our previous studies of non‐small cell lung cancer and pharyngeal squamous cell carcinomas, strong stromal versican expression was not associated with prognosis.14,21 So far, only a few investigators have reported data for the prospective power of stromal versican expression in clinical cohorts. The small number of studies together with possible variations in the role of versican in different cancers might explain the existing discrepancies, and necessitates further cancer‐specific studies.
Earlier in vitro findings suggest that stromal versican expression might be accompanied by more active tumour cell proliferation.9,11,23,36 In this material from patients with OSCC, such an association was, however, not evident. The potential ability of stromal versican to stimulate tumour cell proliferation in clinical materials has previously been studied only in non‐small cell lung cancer, in which high stromal versican expression did not correlate with tumour cell proliferation.14 Transforming growth factor β1 secreted by tumour cells has been suggested to induce versican production in tumour stroma.37,38 This paracrine induction of versican synthesis has been suggested to increase and remodel the pericellular matrix around tumour cell islands and expand ECM, creating a highly pliable environment for cell proliferation and invasion.3,4,5,24,38 The exact effect of versican on cell proliferation and invasion during tumour development and progression still remains unclear. Accordingly, other versican‐related mechanisms promoting OSCC progression should also be sought. These include restrained cell adhesion and apoptosis, as well as induced migration and invasion.9,11,12,13,36 Furthermore, versican may enhance tumour growth through its angiogenic effect.39
In this OSCC material, versican was expressed in tumour stroma inside and around the invasive tumour nests. This finding is supported by several previous reports.9,16,17,18,19,20,22,23,35 In different cancers, versican deposits have been demonstrated both in tumour stroma19 and in tumour cells.9,23,24,40 Analyses of mRNA have also shown that tumour‐associated versican is synthesised both in the peritumoural stroma and in tumour cells.9,19 Banerjee et al24 have reported versican expression in the invasive oral cancer cells. However, in this OSCC cohort, versican expression resided only in the tumour stroma without any supplementary intracellular versican accumulation, which is in line with previous reports on breast cancer.15 This finding suggests that, in OSCC, versican is probably synthesised mostly in tumour stroma by stromal fibroblasts.
In vitro studies have shown that the amino‐terminal domain of versican binds hyaluronan with high affinity.8 The increased amounts of versican together with ECM macromolecules, such as polysaccharide hyaluronan, increase pericellular matrix volume and distend the ECM, thereby permitting tumour invasion.5 Versican may also stimulate stroma synthesis in tumours through induced fibroblast proliferation, which has been illustrated in experimental cell models in vitro.11 Apart from a tumour‐promoting effect, Cattaruzza et al36 have recently shown that hyaluronan may inhibit versican‐induced cell proliferation in sarcoma cells. In this OSCC cohort, high stromal versican expression was inter‐related with reduced stromal hyaluronan expression, suggesting that, in OSCC, mechanisms eliciting increased levels of versican in tumour stroma do not induce hyaluronan expression and might even suppress it. In breast cancer, by contrast, stromal versican and stromal hyaluronan expression were not correlated.15 Furthermore, in ovarian and lung adenocarcinomas, stronger stromal versican expression was linked with more intense stromal hyaluronan expression.14,17 This inconsistency once more possibly reflects differences in the development and progression of various types of cancers. Versican has also been shown to bind CD44, which is a group of carbohydrate‐binding molecules also binding hyaluronan.41 Unpublished data of CD44 expression in this cohort were also available (data not shown). However, in this material, the expression of these two molecules were not inter‐related. The ambiguous role of versican in various biological processes has been noticed earlier and has been explained by diverse effects of different isoforms, the modular structure of versican, several potential binding partners, such as hyaluronan, as well as by divergence in activating various signalling pathways in different cells and tissues.4
Apart from survival and tumour cell proliferation, stromal versican expression were not related to any other clinicopathological variable studied in this cohort (sex, age, Karnofsky performance, T class, N class, stage, and histological differentiation), which is completely in line with the results reported for breast adenocarcinoma.15 Our previous results in oropharyngeal and hypopharyngeal tumours were otherwise analogous, but in those tumours stronger versican expression was associated with lower stage.21 In more advanced stages of both epithelial ovarian cancer17 and lung adenocarcinoma,14 stromal versican is more abundantly expressed. In prostate adenocarcinoma, stromal versican expression is more active in less well‐differentiated tumours,20 whereas, in breast adenocarcinoma, versican expression and tumour differentiation do not seem to be inter‐related.15,18 Owing to the small number of published reports so far, the association between stromal versican expression and clinicopathological tumour characteristics remain unclear.
Take‐home messages
Extracellular matrix (ECM) is constantly remodelled during various physiological and pathological processes.
Versican has an important role in ECM assembly, anti‐adhesion, cell proliferation, migration and ECM remodelling
Versican is expressed in several malignant tumours, suggesting its involvement in the development and progression of cancer.
Increased stromal versican expression in oral squamous cell carcinoma (OSCC) correlates with both increased risk of disease recurrence and shortened survival.
High stromal versican expression is an independent and adverse prognostic marker in OSCC.
Versican seems to have numerous efficient ways to contribute to cancer progression. However, further studies will be required to clarify both the general role and exact mechanisms of action of versican in human neoplasia, including OSCC. We conclude that stromal versican expression seems to be an independent prognostic marker in OSCC.
Acknowledgements
We thank Mrs A Parkkinen for her skilful immunohistochemical assistance.
Abbreviations
DFS - disease‐free survival
DSS - disease‐specific survival
ECM - extracellular matrix
OSCC - oral squamous cell carcinoma
PBS - phosphate‐buffered saline
Footnotes
Funding: This study was financially supported by Kuopio University Hospital EVO Funds, The Savo Cancer Fund and The Finnish Foundation for Cancer Research.
Competing interests: None.
References
- 1.Soames J V, Southam J C. Oral epithelial tumours, melanocytic naevi, and malignant melanoma. In: Oral pathology. 4th edn. Oxford, UK: Oxford University Press, 2005133–149.
- 2.Silverman S., Jr ed. Epidemiology. Oral Cancer. 5th edn. Hamilton, Canada: BC Decker, 20031–6.
- 3.Tiedemann K, Malmstrom A, Westergren‐Thorsson G. Cytokine regulation of proteoglycan production in fibroblasts: separate and synergistic effects. Matrix Biol 199715469–478. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y J, La Pierre D P, Wu J.et al The interaction of versican with its binding partners. Cell Res 200515483–494. [DOI] [PubMed] [Google Scholar]
- 5.Wight T N. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol 200214617–623. [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann D R, Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J 198982975–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz N B, Pirok EW I I I, Mensch J R., Jret al Domain organization, genomic structure, evolution, and regulation of expression of the aggrecan gene family. Prog Nucleic Acid Res Mol Biol 199962177–225. [DOI] [PubMed] [Google Scholar]
- 8.LeBaron R G, Zimmermann D R, Ruoslahti E. Hyaluronate binding properties of versican. J Biol Chem 199226710003–10010. [PubMed] [Google Scholar]
- 9.Gulyas M, Hjerpe A. Proteoglycans and WT1 as markers for distinguishing adenocarcinoma, epithelioid mesothelioma, and benign mesothelium. J Pathol 2003199479–487. [DOI] [PubMed] [Google Scholar]
- 10.Cattaruzza S, Schiappacassi M, Ljungberg‐Rose A.et al Distribution of PG‐M/versican variants in human tissues and de novo expression of isoform V3 upon endothelial cell activation, migration, and neoangiogenesis in vitro. J Biol Chem 200227747626–47635. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Cao L, Yang B L.et al The G3 domain of versican enhances cell proliferation via epidermial growth factor‐like motifs. J Biol Chem 199827321342–21351. [DOI] [PubMed] [Google Scholar]
- 12.Ang L C, Zhang Y, Cao L.et al Versican enhances locomotion of astrocytoma cells and reduces cell adhesion through its G1 domain. J Neuropathol Exp Neurol 199958597–605. [DOI] [PubMed] [Google Scholar]
- 13.Yang B L, Zhang Y, Cao L.et al Cell adhesion and proliferation mediated through the G1 domain of versican. J Cell Biochem 199972210–220. [DOI] [PubMed] [Google Scholar]
- 14.Pirinen R, Leinonen T, Bohm J.et al Versican in nonsmall cell lung cancer: relation to hyaluronan, clinicopathologic factors, and prognosis. Hum Pathol 20053644–50. [DOI] [PubMed] [Google Scholar]
- 15.Suwiwat S, Ricciardelli C, Tammi R.et al Expression of extracellular matrix components versican, chondroitin sulfate, tenascin, and hyaluronan, and their association with disease outcome in node‐negative breast cancer. Clin Cancer Res 2004102491–2498. [DOI] [PubMed] [Google Scholar]
- 16.Theocharis A D, Vynios D H, Papageorgakopoulou N.et al Altered content composition and structure of glycosaminoglycans and proteoglycans in gastric carcinoma. Int J Biochem Cell Biol 200335376–390. [DOI] [PubMed] [Google Scholar]
- 17.Voutilainen K, Anttila M, Sillanpaa S.et al Versican in epithelial ovarian cancer: relation to hyaluronan, clinicopathologic factors and prognosis. Int J Cancer 2003107359–364. [DOI] [PubMed] [Google Scholar]
- 18.Ricciardelli C, Brooks J H, Suwiwat S.et al Regulation of stromal versican expression by breast cancer cells and importance to relapse‐free survival in patients with node‐negative primary breast cancer. Clin Cancer Res 200281054–1060. [PubMed] [Google Scholar]
- 19.Brown L F, Guidi A J, Schnitt S J.et al Vascular stroma formation in carcinoma in situ, invasive carcinoma, and metastatic carcinoma of the breast. Clin Cancer Res 199951041–1056. [PubMed] [Google Scholar]
- 20.Ricciardelli C, Mayne K, Sykes P J.et al Elevated levels of versican but not decorin predict disease progression in early‐stage prostate cancer. Clin Cancer Res 19984963–971. [PubMed] [Google Scholar]
- 21.Pukkila M J, Kosunen A S, Virtaniemi J A.et al Versican expression in pharyngeal squamous cell carcinoma: an immunohistochemical study. J Clin Pathol 200457735–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isogai Z, Shinomura T, Yamakawa N.et al 2B1 antigen characteristically expressed on extracellular matrices of human malignant tumors is a large chondroitin sulfate proteoglycan, PG‐M/versican. Cancer Res 1996563902–3908. [PubMed] [Google Scholar]
- 23.Touab M, Villena J, Barranco C.et al Versican is differentially expressed in human melanoma and may play a role in tumor development. Am J Pathol 2002160549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banerjee A G, Bhattacharyya I, Vishwanatha J K. Identification of genes and molecular pathways involved in the progression of premalignant oral epithelia. Mol Cancer Ther 20054865–875. [DOI] [PubMed] [Google Scholar]
- 25.Bettendorf O, Piffko J, Bankfalvi A. Prognostic and predictive factors in oral squamous cell cancer: important tools for planning individual therapy? Oral Oncol 200440110–119. [DOI] [PubMed] [Google Scholar]
- 26.Hardisson D. Molecular pathogenesis of head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol 2003260502–508. [DOI] [PubMed] [Google Scholar]
- 27.Williams H K. Molecular pathogenesis of oral squamous carcinoma. Mol Pathol 200053165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosunen A, Ropponen K, Kellokoski J.et al Reduced expression of hyaluronan is a strong indicator of poor survival in oral squamous cell carcinoma. Oral Oncol 200440257–263. [DOI] [PubMed] [Google Scholar]
- 29.Sobin L H, Wittekind C. eds. Head and neck tumours. TNM classification of malignant tumours. 5th edn. New York: John Wiley & Sons, 199717–50.
- 30.Karnofsky D A, Abelmann W H, Craver L F.et al The use of the nitrogen mustards in the palliative treatment of carcinoma. Cancer 19481634–656. [Google Scholar]
- 31.Shanmugaratnam K, Sobin L H. In: Shanmugaratnam K, ed. Histological typing of tumours of the upper respiratory tract and ear. 2nd edn. Heidelberg: Springer‐Verlag, 1991
- 32.Karvinen S, Kosma V M, Tammi M I.et al Hyaluronan, CD44 and versican in epidermal keratinocyte tumours. Br J Dermatol 200314886–94. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan E L, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc 195853457–481. [Google Scholar]
- 34.Cox D R. Regression models and life‐tables (with discussion). J R Stat Soc B 197234187–220. [Google Scholar]
- 35.Paulus W, Baur I, Dours‐Zimmermann M T.et al Differential expression of versican isoforms in brain tumors. J Neuropathol Exp Neurol 199655528–533. [DOI] [PubMed] [Google Scholar]
- 36.Cattaruzza S, Schiappacassi M, Kimata K.et al The globular domains of PG‐M/versican modulate the proliferation‐apoptosis equilibrium and invasive capabilities of tumor cells. FASEB J 200418779–781. [DOI] [PubMed] [Google Scholar]
- 37.Sakko A J, Ricciardelli C, Mayne K.et al Modulation of prostate cancer cell attachment to matrix by versican. Cancer Res 2003634786–4791. [PubMed] [Google Scholar]
- 38.Sakko A J, Ricciardelli C, Mayne K.et al Versican accumulation in human prostatic fibroblast cultures is enhanced by prostate cancer cell‐derived transforming growth factor beta1. Cancer Res 200161926–930. [PubMed] [Google Scholar]
- 39.Zheng P S, Wen J, Ang L C.et al Versican/PG‐M G3 domain promotes tumor growth and angiogenesis. FASEB J 200418754–756. [DOI] [PubMed] [Google Scholar]
- 40.Touab M, Arumi‐Uria M, Barranco C.et al Expression of the proteoglycans versican and mel‐CSPG in dysplastic nevi. Am J Clin Pathol 2003119587–593. [DOI] [PubMed] [Google Scholar]
- 41.Kawashima H, Hirose M, Hirose J.et al Binding of a large chondroitin sulfate/dermatan sulfate proteoglycan, versican, to L‐selectin, P‐selectin, and CD44. J Biol Chem 200027535448–35456. [DOI] [PubMed] [Google Scholar]