Abstract
Background
The reasons for recurrent adenotonsillitis are poorly understood.
Methods
The in situ composition of microbiota of nasal (5 children, 25 adults) and of hypertrophied adenoid and tonsillar tissue (50 children, 20 adults) was investigated using a broad range of fluorescent oligonucleotide probes targeted to bacterial rRNA. None of the patients had clinical signs of infection at the time of surgery.
Results
Multiple foci of ongoing purulent infections were found within hypertrophied adenoid and tonsillar tissue in 83% of patients, including islands and lawns of bacteria adherent to the epithelium, with concomitant marked inflammatory response, fissures filled with bacteria and pus, and diffuse infiltration of the tonsils by bacteria, microabscesses, and macrophages containing phagocytosed microorganisms. Haemophilusinfluenzae mainly diffusely infiltrated the tissue, Streptococcus and Bacteroides were typically found in fissures, and Fusobacteria,Pseudomonas and Burkholderia were exclusively located within adherent bacterial layers and infiltrates. The microbiota were always polymicrobial.
Conclusions
Purulent processes persist during asymptomatic periods of adenotonsillitis. Most bacteria involved in this process are covered by a thick inflammatory infiltrate, are deeply invading, or are located within macrophages. The distribution of the bacteria within tonsils may be responsible for the failure of antibiotic treatment.
Potentially pathogenic bacteria are often isolated from the nasopharynx of healthy children as transient or regular constituents of the nasopharyngeal flora. These include Neisseria, Streptococcuspyogenes, Haemophilus influenzae, Staphylococcus aureus, Actinomyces, Bacteroides, Prevotella,Porphyromonas, Peptostreptococci and Fusobacterium spp.1 The detection of pathogenic bacteria is therefore of uncertain significance, and microbial analysis has a marginal role in the treatment of chronic infections of the adenoids and tonsils.2 Despite the widespread use of antibiotics, adenotonsillitis is often recalcitrant and recurrent, leading to frustration for parents and medical staff, which finally ends in the decision for surgery.3,4 The tenacity of the disease has led to the assumption that the infection may derive from the normal nasopharyngeal flora1 or result from biofilms that are resistant to antibiotics.4,5 However, the mechanisms used by bacteria to persist on epithelial surfaces are poorly understood, and no data reporting the composition and spatial organisation of biofilms in the nasopharynx are available.
We aimed to study the bacterial community structure and the spatial organisation of microbiota in surgically removed material from the nasopharynx, adenoids and tonsils using fluorescence in situ hybridisation (FISH) with a broad range of bacterial group‐specific rRNA‐targeted probes.
Patients and methods
Patients
Tissues from adenoids and tonsils were obtained during surgery from 70 patients, 13 months to 38 years of age (42 male and 28 female patients), consecutively operated for recurrent infections or airway obstruction. Nasal tissues were obtained from 30 patients, 1 month to 62 years of age (20 male and 10 female patients). All samples represented material that would otherwise have been discarded. The study was approved by the Institutional Human Research Committee of the Charité Hospital (Berlin, Germany).
Surgical indications included recurrent acute adenotonsillitis (5 episodes a year, 4 episodes in 2 years or 3 episodes in 3 years), adenotonsillar hypertrophy with sleep disturbance (snoring, apnoea), severe dysphagia, recurrent serous otitis media after a trial of myringotomy tubes, and nasal obstruction of the ostia resulting in sinusitis. Patients with acute surgery, upper airway obstruction or cor pulmonale were excluded.
Children <4 years of age underwent an adenoidotomy or tonsillotomy. Older children underwent an adenoidectomy or bilateral extracapsular tonsillectomy under general anaesthesia.
The tissue from the nasal epithelium was obtained by conchotomy performed for hyperplastic nasal turbinates. Four of these patients had a history of chronic sinusitis. The surgery was elective in each case. None of the patients had clinical signs of infection at the time of surgery. None had received antibiotics during the 4 weeks before surgery.
Tissue preparation
The tissues were fixed in Carnoy's solution6 (6/6/1 vol. ethanol/glacial acetic acid/chloroform) and embedded in paraffin‐wax blocks using standard techniques. Sections of 4‐µm thickness were placed on SuperFrost slides (R Langenbrinck, Emmendingen, Germany) for FISH studies.
Fluorescence in situ hybridisation
Oligonucleotide probes were synthesised with a carbocyanine dye (Cy3), fluorescein isothiocyanate (FITC) or Cy5 fluorescent dye at the 5′ ends (MWG Biotech, Ebersberg, Germany). A broad range of domain‐specific, group‐specific and species‐specific FISH probes were chosen for the orolaryngogastrointestinal origin of the expected bacteria including potential respiratory pathogens (table 1). At least 60 single hybridisations using combinations of specific and universal FISH probes were performed with tissue sections of each patient, thus giving a reliable picture of the three dimensional spread of the biofilms over the surface.
Table 1 FISH probes.
| Name | Target | Reference |
|---|---|---|
| Eub338 | Virtually all bacteria, Kingdom Eubacteria | 7 |
| Gamma‐proteobacteria | ||
| Aer66 | Aeromonas | 8 |
| Ebac | Enterobacteriaceae | 9 |
| Ec1531 | Escherichia coli | 10 |
| Haeinf | Haemophilus influenzae | 11 |
| Stemal | Stenotrophomonas maltophilia | 11 |
| Ps | Pseudomonas | 12 |
| Pseaer A | Pseudomonas aeruginosa | 11 |
| Pseaer B | Pseudomonas aeruginosa | 11 |
| AcAc | Actinobacillus actinomycetemcomitans | 13 |
| Beta‐proteobacteria | ||
| Bet42a | Beta‐proteobacteria incl Neisseria | 14 |
| Burkho | Burkholderia | 11 |
| Burcep | Burkholderia cepacia | 11 |
| Epsilon‐proteobacteria | ||
| Arc1430 | Arcobacter, Epsilon‐proteobacteria | 15 |
| Hpy‐1 | Helicobacter pylori, Epsilon‐proteobacteria | 16 |
| Firmicutes | ||
| LGC | Firmicutes | 17 |
| Erec482 | Clostridium coccoides ‐ Eubacterium rectale group | 18 |
| Lach | Subgroup of Erec (incl. Lachnospira multipara) | 19 |
| Chis150 | Clostridium histolyticum group | 18 |
| Clit135 | Clostridium lituseburense group (incl. Clostridiumdifficile) | 18 |
| Ehal | subgroup of Erec (incl. Eubacterium hallii) | 19 |
| Ecyl | Eubacterium cylindroides and others | 19 |
| Efaec | Enterococcus faecalis, Enterococcus sulfuricus | 20 |
| Strc493 | Streptococcus group | 18 |
| Lab158 | Lactobacillus and Enterococcus group | 21 |
| Strpyo | Streptococcus pyogenes | 11 |
| Enc131 | Enterococcus | 22 |
| Staaur | Staphylococcus aureus | 11 |
| Veil | Veillonella group | 19 |
| Vepa | Veillonella parvula | 13 |
| Fprau | Fusobacterium prausnitzii group | 23 |
| Sfb | Segmented filamentous bacteria | 24 |
| Lis 637,1255 | Listeria, Brochothrix | 25 |
| UroA, UroB | Ruminococcusobeum‐like bacteria (subgroup of Erec) | 26 |
| Phasco | Phascolarctobacterium faecium group | 19 |
| Rbro, Rfla | Ruminococcus bromii, Ruminococcus flavefaciens and others | 19 |
| Actinobacteria | ||
| HGC | Actinobacterium | 27 |
| Cor653 | Coriobacterium | 28 |
| Ato291 | Atopobium, Coriobacterium, Eggerthella and Collinsella | 28 |
| Bif164, 662 | Bifidobacteriaceae | 29 |
| Cytophaga‐Flavobacterium‐Bacteroides | ||
| Bac303 | Bacteroides/Prevotella | 30 |
| CF319a | Cytophaga‐Flavobacteria | 30 |
| Bdis656 | Bacteroides distasonis | 18 |
| Bfra | Bacteroides fragilis | 18 |
| Prin | Prevotella intermedia | 13 |
| Pogi | Porphyromonas gingivalis | 13 |
| B(T)AFO | Tannerella forsythia | 13 |
| Spirochaetes | ||
| Ser1410 | Brachyspira | 31 |
| Borr4 | Borrelia | 32 |
| Fusobacteria | ||
| Fuso | Fusobacterium spp | 13 |
| Non338 | Nonsense probe used to test for non‐specific binding | 14 |
Formamide concentration and hybridisation temperature were chosen to achieve the optimum stringency as described in the references for each probe set. Additional hybridisations using a permeation step with lysozyme for 15, 30 and 60 min were performed in parallel for detection of Gram‐positive bacteria.
In situ quantification of mucosal bacteria
Bacteria were visualised by FISH and DAPI stains using a Nikon e600 fluorescence microscope (Nikon, Tokyo, Japan) and photo graphed with a Nikon DXM1200 camera and software (Nikon).
High‐power (×1000 magnification) photographs were made consecutively from the entire tissue surface. Separate photo series were made for bacteria adherent to the epithelial surface, bacteria within crypts, fissures filled with bacteria, abscesses and regions of tissues infiltrated by bacteria.
The quantification of bacteria was based on the assumption that a 10‐μl sample with a cell concentration of 107 cells/ml contains 40 cells per average microscopic field at a magnification of ×1000.6 The autofluorescence background of the human tissue allowed tissue structures to be seen. Furthermore, for each biopsy, at least one or two additional stains (periodic acid Schiff, haematoxylin and eosin, or Gram stain) were performed.
Statistics
Mean values and standard deviations (SD) were calculated from the bacterial counts. Analysis of variance and χ2 test were used, and a value of p<0.05 was considered significant.
Results
Occurrence, concentrations and spatial organisation of bacterial communities
Normal nasal mucosa
No confluent bacterial biofilms were detected on the surface of any of the 30 samples of nasal epithelium, including those from four patients with chronic sinusitis. In addition, no bacteria were seen in the subepithelial tissue.
Single bacteria were scattered over the epithelial surface in 19 patients, with one bacterium in 10 consecutive microscopic fields. One bacterium was seen on average in six microscopic fields in eight patients. One bacterium was seen on average in five fields in three patients, corresponding to a concentration of <104 bacteria/ml. Bacteria were mostly coccoid or short rods. Owing to the overall low bacterial concentrations, no species or group specification was possible. Single‐group or species‐specific FISH probes hybridised with a maximum of one bacterium in <30 fields.
Tonsil and adenoid tissue
No bacterial conglomerates attached to the mucosa in the absence of an inflammatory response that might represent “normal biofilms” were observed in any patient. Instead, 83% of the patients showed multiple signs of focal infections in the adenoids or tonsils. These included lawns of highly concentrated bacteria attached to the epithelial surface and infiltrating superficial epithelial cell rows, deep fistulas and fissures disrupting the tonsillar tissue, with loose bacterial conglomerates following these branched purulent passages and infiltrating the adjacent parenchyma, and diffuse infiltration of tonsillar tissue by bacteria without apparent spatial relationship with fistulas or adherent lawns.
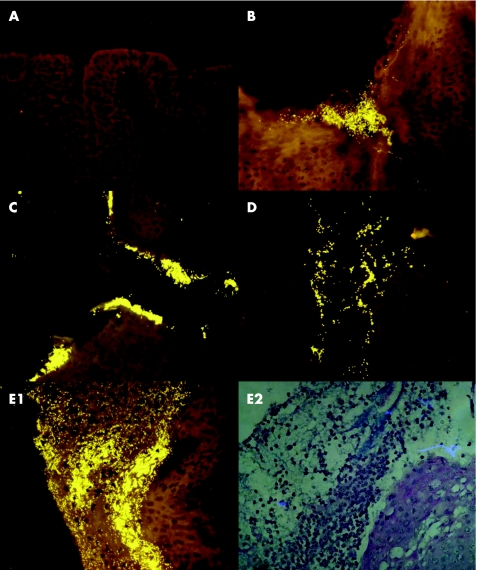
The different manifestations of infectious processes could be combined with each other, could be concomitant with microabscesses and macrophages filled with phagocytosed bacteria, or could appear separately. About 12 of 70 tonsils had no signs of bacterial infection (fig 1A, table 2). They had single coccoid bacteria in low concentrations irregularly scattered over the surface.
Figure 1 Bacteria on the epithelial surface of tonsils visualised with a universal bacterial probe (Eub 338, Cy3 orange signals). (A) No biofilm. (B) Adherent bacteria in and around a crypt opening. (C) Adherent bacteria entering the crypt. (D) Adherent bacteria present throughout the crypt. (E1) Prolific bacterial infiltrate attached to the surface of the epithelium and covered with inflammatory cells. E2 is the same as E1 but shown with periodic acid‐Schiff (PAs) stain (periodic acid‐Schiff stain, right).
Table 2 Focal manifestations of infection, age dependence and involved bacterial groups.
| Age (years) | <2 | 2–<4 | 4–<15 | 15–<25 | ⩾25 | |||
|---|---|---|---|---|---|---|---|---|
| Number of patients | 10 | 20 | 20 | 10 | 10 | |||
| Tissue | T | A | T | A | T | A | T | T |
| Number of samples | 10 | 10 | 20 | 20 | 20 | 10 | 10 | 10 |
| No signs of infection | 1 | 1 | 2 | 5 | 4 | 8 | 1 | 4 |
| Adherent infiltrates | ||||||||
| No adherence (single bacteria irregularly attached to the epithelium) | 5 | 8 | 12 | 16 | 4 | 9 | 1 | 5 |
| Adherence exclusively to crypt entrance and within crypts | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 4 |
| Islands of adherence covering <5% of the epithelial surface | 4 | 2 | 6 | 4 | 10 | 4 | 4 | 1 |
| Lawns of bacteria covering >5% of the epithelial surface | 1 | 0 | 2 | 0 | 5 | 1 | 3 | 0 |
| Fissures | ||||||||
| No fissures | 5 | 7 | 8 | 15 | 5 | 7 | 7 | 10 |
| 1–3 | 4 | 1 | 10 | 5 | 6 | 7 | 3 | 0 |
| >3 | 1 | 2 | 2 | 0 | 5 | 0 | 0 | 0 |
| Branched fissures | 0 | 0 | 0 | 0 | 4 | 1 | 0 | 0 |
| Abscesses | 1 | 0 | 4 | 1 | 7 | 2 | 0 | 0 |
| Diffuse infiltration of tissue | 7 | 6 | 12 | 7 | 6 | 3 | 0 | 0 |
| Occurrence of bacterial groups | ||||||||
| Haemophilus influenzae (Haeinf) | 7 | 6 | 14 | 6 | 9 | 2 | 2 | 0 |
| Beta‐Proteobacteria incl. Neisseria (Bet42a) | 2 | 0 | 2 | 0 | 3 | 0 | 1 | 2 |
| Actinobacteria (HGC) | 6 | 5 | 9 | 3 | 6 | 4 | 0 | 2 |
| Atopobium and other (Ato291) | 0 | 0 | 1 | 0 | 2 | 0 | 1 | 0 |
| Coriobacterium group (Cor653) | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 1 |
| Firmicutes (LGC) | 3 | 3 | 11 | 8 | 13 | 7 | 4 | 3 |
| Fusobacteria (Fuso) | 0 | 0 | 5 | 2 | 4 | 1 | 1 | 0 |
| Streptococcus (Strc493) | 4 | 2 | 8 | 7 | 13 | 5 | 6 | 7 |
| Streptococcus pyogenes (Strpyo) | 1 | 0 | 0 | 0 | 3 | 0 | 2 | 2 |
| Staphylococcus aureus (Staaur) | 3 | 1 | 0 | 0 | 0 | 0 | 2 | 3 |
| Lactobacillus and Enterococcus (Lab) | 3 | 0 | 4 | 2 | 7 | 0 | 0 | 1 |
| Clostridium coccoides—Eubacterium rectale group (EREC) | 2 | 0 | 5 | 2 | 5 | 0 | 2 | 1 |
| Listeria, Brochothrix (Lis 637,1255) | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 1 |
| Ruminococcus bromii, Ruminococcus flavefaciens (Rbro, Rfla) | 0 | 0 | 1 | 1 | 3 | 0 | 0 | 0 |
| Veillonella group incl. Veillonella parvula (Veil,Vepa) | 1 | 0 | 2 | 1 | 5 | 0 | 4 | 3 |
| Bacteroides/Prevotella group (Bac303) | 4 | 2 | 7 | 2 | 4 | 1 | 4 | 3 |
| Cytophaga‐Flavobacteria (CF 319) | 2 | 1 | 2 | 1 | 8 | 3 | 4 | 4 |
| Prevotella intermedia (Prin) | 1 | 0 | 4 | 1 | 2 | 0 | 0 | 0 |
| Burkholderia (Burcep, Burkho) | 0 | 0 | 5 | 3 | 4 | 2 | 0 | 1 |
| Pseudomonas (Ps, Pseaer A, Pseaer B) | 2 | 1 | 5 | 5 | 4 | 0 | 3 | 4 |
A, adenoids; T, tonsils
Adherent bacterial infiltrates
Patches or lawns of bacteria that covered the epithelium of tonsils or adenoids without disrupting its integrity or reaching the basal epithelial layer were often observed in children aged 4–15 years of age (table 2), and were less often seen in young adults or infants <2 years (p<0.01). Bacterial adherence was mainly observed around crypt entrances and within crypts in adults >25 years of age (fig 1B,C). The adherent infiltrates covered 1–20% of the epithelial surface in 33 patients. The bacteria adhered to >50% of the epithelial surface in three patients, aged 4, 8 and 9 years. The adherence was always accompanied by a pronounced inflammatory response with broad layers of leucocytes covering the bacteria (fig 1E right). The concentrations of bacteria within adherent infiltrates reached up to 1012 bacteria/ml.
Fissures
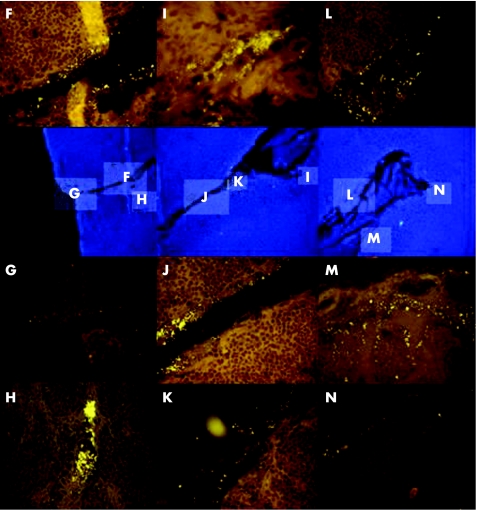
Once the epithelial layer was disrupted, bacteria invaded deep into the adenoid or tonsillar tissue, leading to fistulas and fissures (fig 2). The walls of fissures differed from those of crypts, as they were not covered by epithelium. True fissures were filled with bacteria in concentrations between 108 and 1010 bacteria/ml in contrast to mechanical artefacts that resulted from tissue preparation. Bacteria infiltrated the tissue adjacent to the fistulas, building thin branches of secondary order (fig 2H,M,N). In some cases, the inflammatory response resulted in formation of abscesses. The fissures were common in children 2–<15 years of age (table 2). They were not seen in adults, and were less common in children <2 years of age and in patients 15–<25 years of age (p<0.001).
Figure 2 The blue 4′,6‐diamidino‐2‐phenylindole (DAPI)‐stained images in the second row of the figure show at low magnification (×100) a deep fissure crossing the tonsil. The indicated regions are shown at higher magnification relative to DAPI image after hybridisation with the Eub338‐Cy3 (orange) probe. Bacteria fill the fissure (F–N) and infiltrate the adjacent parenchyma in fissure branches of secondary order (H,M,N).
Diffuse infiltration
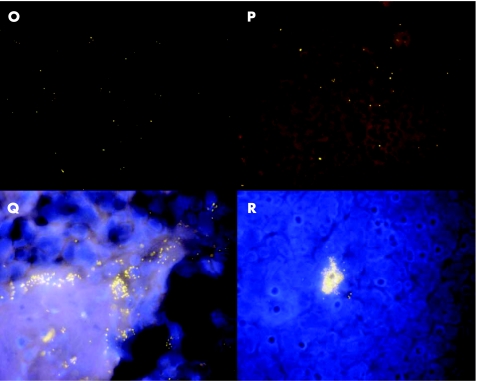
Diffuse infiltration of adenoid or tonsillar tissues by bacteria was characteristic for children <4 years of age. It was less often seen in children 4–15 years of age, and never observed in adults (p<0.001). Bacterial concentrations were relatively low (<108 bacteria/ml), but the bacteria could be spread over 30% of the sample surface (figs 3O,P and 4).
Figure 3 Bacteria (universal probe Cy3 orange) diffusely infiltrate the tonsils (O,P). Clusters of bacteria surround the nuclei of eukaryotic cells (DAPI stain, blue) and correspond to phagocytosed bacteria (Q,R).
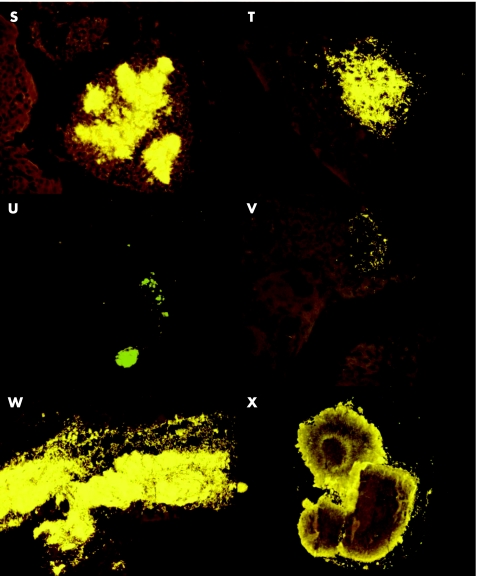
Figure 4 Some examples of microabscesses (universal probe Cy3 orange) found in the tonsils of different children (S–X).
Macrophages
Macrophages containing bacteria were intermixed with inflammatory cells covering adherent bacteria. They were also often seen within fistulas (fig 3H) and within tonsillar parenchyma (fig 3R) and within abcesses (fig 4).
Composition of the adherent and invading bacteria
The adherent and invading bacteria were always polymicrobial. Altogether, 60–80% of bacteria positively hybridising with universal Eub 338 probe could be further affiliated to different bacterial groups (tables 2 and 3).
Table 3 Specific probes that hybridised with bacteria within different compartments*.
| Adherent | Fissures | Diffuse infiltration |
|---|---|---|
| Fusobacteria spp (Fuso) | Staphylococcus aureus (Staaur) | Haemophilus influenzae (Haeinf) |
| Burkholderia (Burcep, Burkho) | Coriobacterium group (Cor653) | Actinobacteria (HGC) |
| Pseudomonas (Ps, Pseaer A, Pseaer B) | Clostridium coccoides—Eubacterium rectale (EREC) | Firmicutes (LGC) |
| Listeria, Brochothrix (Lis 637, 1255) | Atopobium and other (Ato291) | |
| Veillonella group incl Veillonella parvula (Veil, Vepa) | Bacteroides/Prevotella (Bac303) | |
| Cytophaga‐Flavobacteria (CF 319) | ||
| Streptococcus pyogenes (Strpyo) | ||
| Streptococcus (Strc493) |
*Bacterial groups diffusely infiltrating tonsils were also found in fissures and adherent infiltrates. Similarly, bacteria within fissures could be identified within adherent infiltrates, but the reverse was not true.
The diversity of bacteria was higher and often consisted of >10 different bacterial groups at sites with greater bacterial concentrations. Identification at species or group level was difficult when the number of bacteria was low but, even in the case of diffuse invasive bacteria with overall low local concentrations, up to six different groups could be found in each patient. The distribution of bacteria on the epithelial surface or within tonsils was mosaic. Bacterial communities could markedly differ in their composition even within the same microscopic field.
Some of the groups, such as Fusobacterium, Burkholderia and Pseudomonas were found exclusively attached to the mucosa. Others could also be detected within fissures and diffusely infiltrating tissue (table 3).
The following bacteria were found singular within adherent infiltrates, composing <1% of the bacterial mass: Bifidobacteriacae (Bifi), Clostridium histolyticum (Chis) and Clostridium lituseburense (Clit).
We found no bacteria that positively hybridised with Helicobacter pylori (Hpy‐1) or the following probes: Ebac, Ec 1531, AcAc, B(T)AFO, Bdis, Bfra, Borr4, Ecyl, Enc, Fprau, Pogi, Phasco, Sfb, Stemal, Ser 1410, Rbro, Rfla and UroA,B. However, specific bacteria in concentrations <105/ml could not be clearly identified by FISH within complex eukaryotic tissues.
Discussion
Inflammation of the tonsils and adenoids with subsequent obstructive hypertrophy is one of the most ancient and common paediatric problems. Attempts at surgical treatment have been documented throughout the past 2000 years.33 The frequency of adenotonsillectomy increased rapidly in the early part of the last century as the “focus of infection” theory attributed “rheumatism” and other systemic disorders to diseased tonsils and adenoids. Broad‐scale surgery was performed on entire populations of school children. With the spontaneous decline of acute rheumatic fever in Western countries, introduction of antibiotics, and the falling incidence of chronic tonsillitis and its complications, surgery became less frequent, but is still the major ambulatory surgical procedure among children in the USA.33,34,35
The reasons for the chronic recurrent course of an otherwise benign disease are poorly understood, and microbial diagnosis is of low value. The microbial cultures from oronasopharyngeal samples show a large portion of irrelevant contaminants. The clinical relevance of potentially causative agents is often questionable. Both treatment with medicine and surgery rely on statistics rather than on individual findings.1,2,36
As surgery is mostly performed electively and rheumatic fever is no longer a problem, the question arises as to what is actually achieved with a tonsillotomy/ectomy other than the mechanical reduction of the hypertrophied tissue? Our findings clearly show that the removal of chronic purulent foci is still the major potential benefit of tonsillectomy. The tonsils and adenoids from 83% of the patients in our study showed signs of ongoing purulent infections, whereas none of the patients reported symptoms of infection or had received antibiotics in the month before surgery. The concentrations of bacteria within infectious foci could reach values up to 1012 bacteria/ml. However, these bacteria were shielded either by inflammatory infiltrates that covered them or by lymphatic tissue that surrounded them, or were incorporated into macrophages and thus were not accessible for microbial diagnosis by swab. This shielding of bacterial biofilms could also be shown by electron microscopy,4 which may be responsible for the poor efficiency of antibiotics that leads to surgery.
The distribution and composition of bacteria was age‐dependent. Diffuse infiltration of the tonsils was predominantly observed in infants. The occurrence rate of bacteria that diffusely infiltrated the lymphoid tissue decreased after 4 years of age, whereas the frequency of fissures and fistulas increased. Both diffuse infiltration and fissures were not observed, but only small patches of adherence and crypt communities were observed in patients over 25 years of age. Although FISH does not presently allow each bacterial species to be tracked absolutely specifically, the spectrum of microorganisms clearly changed in parallel with the displacement of the content from the interior of the organ to the periphery. The frequency of bacteria that hybridised with the probe for H influenzae and were often found within diffuse infiltrates was high in infants, declined with increasing age of the children and was zero in adults. The frequency of Streptococci showed the opposite pattern of distribution. In each case, different bacterial groups were found in different locations within tissue, leading to a mosaic picture with up to 10 different bacterial groups involved in the infectious process.
We found no consistent adherent biofilm propagated on the surface of the nasopharyngeal epithelium to support the hypothesis that infections of adenoid or tonsillar tissues develop from the normal flora. Our FISH studies show that chronic adenotonsillitis is more likely a result of multiple overlapping infections caused by different pathogens. This leads to a morphologically highly diverse picture involving multiple pathogenic, opportunistic and commensal microorganisms that mimics tolerance to some bacteria or simulates the pathogenicity of others. Owing to their location within parenchyma or inflammatory infiltrates, most bacteria within the adenoids and tonsils are difficult to assess quantitatively by microbial culture. The abundance of purulent foci despite clinically undetectable infection emphasises the importance of either consequent conservative treatment or surgery, and implies the necessity to intensify our search for new therapeutic and diagnostic strategies.
Abbreviations
Cy3/Cy5 - different carbocyanine dyes used in fluorescence microscopy to label the oligonucleotide probes
DAPI - 4′,6‐diamidino‐2‐phenylindole
FISH - fluorescence in situ hybridisation
FITC - fluorescein isothiocyanate
Footnotes
Competing interests: None declared.
References
- 1.Brook I. The role of anaerobic bacteria in tonsillitis. Int J Pediatr Otorhinolaryngol 2005699–19. [DOI] [PubMed] [Google Scholar]
- 2.Van Staau B K, Van Den Akker E H, De Haas Van Dorsser E H M.et al Does the tonsillar surface flora differ in children with and without tonsillar disease? Acta Otolaryngol 2003123873–878. [DOI] [PubMed] [Google Scholar]
- 3.Sclafani A P, Ginsburg J, Shah M K.et al Treatment of symptomatic adenotonsillar hypertrophy with amoxixillin/clavulane potassium: short‐ and long‐term results. Pediatrics 1998101675–681. [DOI] [PubMed] [Google Scholar]
- 4.Chole R A, Faddis B T. Anatomical evidence of microbial biofilms in tonsillar tissues: a possible mechanism to explain chronicity. Arch Otolaryngol Head Neck Surg 2003129634–636. [DOI] [PubMed] [Google Scholar]
- 5.Perkins J, Mouzakes J, Pereira R.et al Bacterial biofilm presence in pediatric tracheotomy tubes. Arch Otolaryngol Head Neck Surg 2004130339–343. [DOI] [PubMed] [Google Scholar]
- 6.Swidsinski A, Weber J, Loening‐Baucke V.et al Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. JCM 2005433380–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amann R, Krumholz L, Stahl D A. Fluorescent‐oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J Bacteriol 1990172762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kämpfer P, Erhart R, Beimfohr C.et al Characterization of bacterial communities from activated sludge: culture‐dependent numerical identification versus in situ identification using group‐ and genus‐specific rRNA‐targeted oligonucleotide probes. Microb Ecol 199632101–121. [DOI] [PubMed] [Google Scholar]
- 9.Bohnert J, Hübner B, Botzenhart K. Rapid identification of Enterobacteriaceae using a novel 23S rRNA‐targeted oligonucleotide probe. Int J Hyg Environ Health 200220377–82. [DOI] [PubMed] [Google Scholar]
- 10.Poulsen L K, Licht T R, Rang C.et al Physiological state of Escherichia coli BJ4 growing in the large intestines of streptomycin‐treated mice. J Bacteriol 19951775840–5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hogardt M, Trebesius K, Geiger A M.et al Specific and rapid detection by fluorescent in situ hybridization of bacteria in clinical samples obtained from cystic fibrosis patients. J Clin Microbiol 200038818–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geisenberger O, Ammendola A, Christensen B B.et al Monitoring the conjugal transfer of plasmid RP4 in activated sludge and in situ identification of the transconjugants. FEMS Microbiol Lett 19991749–17. [DOI] [PubMed] [Google Scholar]
- 13.Sunde P T, Olsen I, Gobel U B.et al Fluorescence in situ hybridization (FISH) for direct visualization of bacteria in periapical lesions of asymptomatic root‐filled teeth. Microbiology 20031491095–1102. [DOI] [PubMed] [Google Scholar]
- 14.Manz W, Amann R, Ludwig W.et al Phylogenetic oligodeoxynucleotide probes for the major subclasses of Proteobacteria: problems and solutions. Syst Appl Microbiol 199215593–600. [Google Scholar]
- 15.Snaidr J, Amann R, Huber I.et al Phylogenetic analysis and in‐situ identification of bacteria in activated sludge. Appl Environ Microbiol 1997632884–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feydt‐Schmidt A, Rüssmann H, Lehn N.et al Fluorescence in situ hybridization vs. epsilometer test for detection of clarithromycin‐susceptible and clarithromycin‐resistant Helicobacter pylori strains in gastric biopsies from children. Aliment Pharmacol Ther 2002162073–2079. [DOI] [PubMed] [Google Scholar]
- 17.Meier H, Amann R, Ludwig W.et al Specific oligonucleotide probes for in situ detection of a major group of gram‐positive bacteria with low DNA G + C content. Syst Appl Microbiol 199922186–196. [DOI] [PubMed] [Google Scholar]
- 18.Franks A H, Harmsen H J, Raangs G C.et al Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group‐specific 16S rRNA‐targeted oligonucleotide probes. Appl Environ Microbiol 1998643336–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harmsen H J, Raangs G C, He T.et al Extensive set of 16S rRNA‐based probes for detection of bacteria in human feces. Appl Environ Microbiol 2002682982–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jansen G J, Mooibroek M, Idema J.et al Rapid identification of bacteria in blood cultures by using fluorescently labeled oligonucleotide probes. J Clin Microbiol 200038814–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harmsen H J, Elfferich P, Schut F.et al A 16S rRNA‐targeted probe for detection of lactobacilli and enterococci in fecal samples by fluorescent in situ hybridization. Microbiol Ecol Health Dis 1999113–12. [Google Scholar]
- 22.Frahm E, Heiber I, Hoffman S.et al Application of 23S rDNA‐targeted oligonucleotide probes specific for Enterococci to water hygiene control. Syst Appl Microbiol 199821450–453. [DOI] [PubMed] [Google Scholar]
- 23.Suau A, Rochet V, Sghir A.et al Fusobacterium prausnitzii and related species represent a dominant group within the human fecal flora. Syst Appl Microbiol 200124139–145. [DOI] [PubMed] [Google Scholar]
- 24.Urdaci M C, Regnault B, Grimont P A D. Identification by in situ hybridization of segmented filamentous bacteria in the intestine of diarrheic trout (Oncorhynchus mykiss). Res Microbiol 200115267–73. [DOI] [PubMed] [Google Scholar]
- 25.Schmid M, Walcher M, Bubert A.et al Nucleic acid‐based, cultivation‐independent detection of Listeria spp and genotypes of L monocytogenes. FEMS Immunol Med Microbiol 200335215–225. [DOI] [PubMed] [Google Scholar]
- 26.Zoetendal E G, Ben‐Amor K, Harmsen H J.et al Quantification of uncultured Ruminococcus obeum‐like bacteria in human fecal samples by fluorescent in situ hybridization and flow cytometry using 16S rRNA‐targeted probes. Appl Environ Microbiol 2002684225–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roller C, Wagner M, Amann R.et al In situ probing of gram‐positive bacteria with high DNA G + C content using 23S rRNA‐targeted oligonucleotides. Microbiology 19941402849–2858. [DOI] [PubMed] [Google Scholar]
- 28.Harmsen H J, Wildeboer‐Veloo A C, Grijpstra J.et al Development of 16S rRNA‐based probes for the Coriobacterium group and the Atopobium cluster and their application for enumeration of Coriobacteriaceae in human feces from volunteers of different age groups. Appl Environ Microbiol 2000664523–4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langendijk P S, Schut F, Jansen G J.et al Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus‐specific 16SrRNA‐targeted probes and its application in fecal samples. Appl Environ Microbiol 1995613069–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manz W, Amann R, Ludwig W.et al Application of a suite of 16S rRNA‐specific oligonucleotide probes designed to investigate bacteria of the phylum cytophaga‐flavobacter‐bacteroides in the natural environment. Microbiology 19961421097–1106. [DOI] [PubMed] [Google Scholar]
- 31.Jensen T K, Boye M, Ahrens P.et al Diagnostic examination of human intestinal spirochetosis by fluorescent in situ hybridization for Brachyspira aalborgi, Brachyspira pilosicoli, and other species of the genus Brachyspira (Serpulina). J Clin Microbiol 2001394111–4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammer B, Moter A, Kahl O.et al Visualization of Borrelia burgdorferi sensu lato by fluorescence in situ hybridization (FISH) on whole‐body sections of Ixodes ricinus ticks and gerbil skin biopsies. Microbiology 20011471425–1436. [DOI] [PubMed] [Google Scholar]
- 33.Younis R T, Lazar R H. History and current practice of tonsillectomy. Laryngoscope 2002112(Suppl 100)3–5. [DOI] [PubMed] [Google Scholar]
- 34.Wang E C, Choe M C, Meara J G.et al Inequality of access to surgical specialty health care: why children with government‐funded insurance have less access than those with private insurance in Southern California. Pediatrics 2004114584–590. [DOI] [PubMed] [Google Scholar]
- 35.Paradise J L, Bluestone C D, Colborn D K.et al Tonsillectomy and adenotonsillectomy for recurrent throat infection in moderately affected children. Pediatrics 20021107–15. [DOI] [PubMed] [Google Scholar]
- 36.Gerber M A, Tanz R R, Kabat W.et al Potential mechanisms for failure to eradicate Group A Streptococci from the pharynx. Pediatrics 1999104911–917. [DOI] [PubMed] [Google Scholar]