Abstract
Background
Histological differentiation of mammary papillary lesions can be difficult. The evaluation of myoepithelial cells can be helpful, with benign papilloma showing a continuous myoepithelial cell layer, which becomes attenuated or absent in malignant papillary lesions.
Methods
A large series of 100 papillomas (28 papillomas with florid epithelial hyperplasia) and 68 papillary carcinomas (9 invasive, 44 in situ, and 15 ductal carcinomas in situ (DCIS) involving papillomas) of the breast were stained for myoepithelial cells by immunohistochemistry using antibodies to smooth‐muscle actin (SMA), p63, CD10 and cytokeratin (CK) 14.
Results
In the papillomas, using these four antibodies, myoepithelial cells were positive in 88%, 99%, 91% and 95% of cases, respectively, with SMA showing marked stromal component cell staining and CD10 showing epithelial and stromal staining. CK14 also showed epithelial staining in 71% of papillomas and 96% of papillomas with florid epithelial hyperplasia. In the papillary carcinomas, 36 (53%) cases showed staining of myoepithelial cells that were scattered, discontinuous and diminished in number and the remaining 32 (47%) cases did not show myoepithelial cells. Invasive papillary carcinoma has the lowest proportion (33%) with myoepithelial cells, and DCIS involving papillomas had the highest proportion (87%).
Conclusions
p63 had the highest sensitivity and did not cross‐react with stromal cells and only rarely with epithelial cells. CK14 has the added ability to distinguish between florid epithelial hyperplasia involving papilloma and DCIS involving papillomas. CK14 and p63 may be used as an adjunct in assessing difficult papillary lesions of the breast.
Papillomas and papillary carcinomas of the breast are often encountered by surgical pathologists, and accurate distinction between these entities is critical, as the management and outcomes are different. Although histological criteria have been established in several classic papers,1,2,3 difficulties sometimes arise, particularly in the distinction between papilloma and papillary carcinoma in situ, as both lesions may show an elaborate arborising pattern and overlapping histological appearances. The differential diagnosis between papilloma with florid epithelial hyperplasia and papilloma that is involved in low‐grade ductal carcinoma in situ (DCIS) can also be problematic. The morphological patterns of these entities are similar, and they have been aptly described collectively as the “macropapillary” lesions by Oyama and Koerner,4 which include papilloma, papillary carcinoma and papilloma harbouring carcinoma. In general, it is agreed that in papilloma, a continuous myoepithelial cell layer is present, whereas in papillary carcinoma, the myoepithelial cells may be absent or focal.5,6,7
Evaluation of myoepithelial markers in the elucidation of the nature of a papillary lesion has not been widely reported in the literature, with only a few studies considering the role of myoepithelial markers in histology6,8,9 and cytology.10 Other studies have also assessed the differences in the epithelial component of papilloma and papillary carcinomas, on the basis of histomorphology11 and expression of cytokeratins (CKs),12 cyclin D1,11 p5311 and CD 44.13,14 Some of these markers were reported to be useful, but the findings were not definitive.
On the basis of the premise that myoepithelial cells are absent or under‐represented in malignant papillary lesions, we evaluated a panel of myoepithelial markers including smooth‐muscle actin (SMA), p63, CD10 and CK14 in the detection of myoepithelial cells in a large series of papillary lesions of the breast, paying particular attention to the differentiation between papilloma and papillary carcinoma in situ, and between papilloma with florid epithelial hyperplasia and papilloma that is involved in low‐grade DCIS. Of these markers, p63 has recently gained prominence as a myoepithelial marker, and many authors consider p63 to be the modern gold standard for myoepithelial staining in breast lesions.15,16,17,18,19 The advantage of using p63 as a myoepithelial marker is that the staining is nuclear, hence the interpretation of positivity is hence easier. Furthermore, it is specific to myoepithelial cells, and unlike actin, does not cross‐react with the stromal myofibroblasts.20
Materials and methods
The histopathology files from the participating institutions were searched for papillomas, intraduct papillomas, papillary carcinoma and papillary DCIS over a period of up to 12 years. All the cases were formalin fixed and routinely processed, and haematoxylin and eosin staining was performed on 4‐μm‐thick sections. All the slides were retrieved and the stained slides were reviewed by two of the authors to confirm the diagnosis. Papilloma was diagnosed when the lesion showed an intraduct proliferation of epithelial cells showing an arborising pattern with well‐defined fibrovascular cores, and with an identifiable, continuous myoepithelial cell layer and minimal epithelial atypia. When there were foci of epithelial hyperplasia, with the typical morphology of florid epithelial hyperplasia, including slightly spindled nuclei with prominent streaming pattern, and when these foci of epithelial hyperplasia accounted for >20% of the area, a diagnosis of papilloma with florid epithelial hyperplasia was made.
Papillary carcinomas were further subclassified as follows:
Invasive papillary carcinoma was diagnosed on the basis of the presence of clusters of tumour cells penetrating the pseudocapsules of the tumours and into the stroma, without myoepithelial cells.
Papillary DCIS was diagnosed on the basis of the typical morphological features of papillary configuration with well‐defined fibrovascular cores, and overlying neoplastic epithelium with cellular homogeneity, without an identifiable or with attenuated, scanty myoepithelial cells.
DCIS involving a papilloma was diagnosed on the basis of the presence of confluent epithelial proliferation with features of solid or cribriform‐type DCIS as a focal finding within an otherwise typical papilloma. Lesions with epithelial hyperplasia showing features typical of atypical duct hyperplasia within a papilloma, the atypical focus measuring >0.3 cm, were also grouped into this category as DCIS involving papillomas.
From each case, one representative section was selected for staining modified avidin–biotin with microwave antigen retrieval. The immunostaining for the slides of all the cases were performed in one centre under the same conditions. The immunostaining of all cases was scored by one investigator.
The antibodies used were:
1. SMA (NeoMarkers, Fremont USA; clone SMMS‐1, 1:80)
2. p63 (Dako, California, USA; clone 4A4, 1:100)
3. CD10 (Novocastra, Newcastle upon Tyne, UK; clone 56C6, 1:60)
4. CK14 (NeoMarkers; clone LL002, 1:100)
For each of the antibodies, staining was assessed within the lesion for the presence of myoepithelial cells, and for the degree of staining in the epithelial cells and the stromal cells (including endothelial cells, pericytes and stromal myofibroblasts). The staining was scored from 0 to 3 (0, negative; 1, weak; 2, moderate; 3, strong) and the percentage of cells showing the staining was also recorded. The staining of myoepithelial cells in the adjacent normal breast tissue, if available, was taken as a positive internal control (of an intensity of 3). For SMA and CD10 the staining pattern was cytoplasmic, and for CK14 the staining pattern was cytoplasmic and membranous. In p63, only nuclear staining was considered.
Moderate to strong staining of >10 % of stromal pericytes and myofibroblasts, epithelial cells in all cases, and in myoepithelial cells in papillomas was considered positive. In papillary carcinoma, moderate to strong staining of any myoepithelial cells was considered positive, and the papillary carcinoma was taken as containing myoepithelial cells if any one or more of the markers showed positive staining.
Statistical analysis
Student's t test was used to determine whether the differences in age, tumour size and staining between the different groups were significant, which was established at p<0.05.
Results
In total, 100 papillomas and 68 papillary carcinomas from the five participating institutions (Prince of Wales Hospital, Hong Kong, North District Hospital, Hong Kong, Alize Ho Nethensole Hospital, Hong Kong, Singapore, General Hospital, Singapore, Vancouver General Hospital, Canada) were included in this study. The 100 papillomas were from 99 patients. All patients were women, and the age range was 22–89 years (mean age 47 years). Excised tumours were in the size range 0.1–2.2 cm (mean size 0.71 cm). In this group, 72 were papillomas and 28 were papillomas with florid epithelial hyperplasia.
The 68 papillary carcinomas were from 68 patients. All patients were women, and the age range was 29–89 years (mean age 61 years). Excised carcinomas were in the size range 0.2–2.4 cm (mean size 1.05 cm). In this group, 9 were invasive papillary carcinoma, 44 were papillary carcinoma in situ and 15 were DCIS involving papillomas.
In general, patients with papillomas were younger than patients with papillary carcinoma (p<0.001), and the lesion sizes were smaller in the first group than in the second (p<0.001).
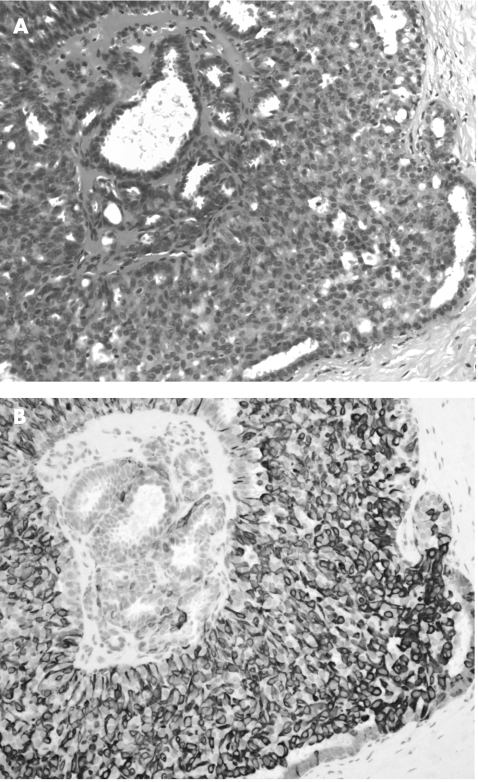
Within the benign group (papilloma and papilloma with florid epithelial hyperplasia), myoepithelial cell staining was identified in all cases. Table 1 lists the staining patterns with the four antibodies. All the benign lesions showed myoepithelial cell staining, with 80 (80%) cases showing staining with all four markers, 14 (14%) cases with three markers, 6 (6%) cases with two markers and none (0%) with only one marker. SMA was positive in 88 (88%) cases, but 43 (43%) cases also showed considerable staining of the stromal cells. p63 was positive in myoepithelial cells in 99 (99%) lesions, with virtually no staining of ductal luminal epithelial cells or stromal cells (fig 1). CD10 was positive in myoepithelial cells in 91 (91%) lesions, with 20 (20%) and 28 (28%) cases, respectively, showing considerable epithelial and stromal staining. CK14 was positive in myoepithelial cells in 95 (95%) lesions, with 36 (36%) cases showing marked stromal staining. For epithelial staining by CK14, after segregation of the lesions into papilloma and papilloma with florid epithelial hyperplasia, we obtained 71% (51/72) papilloma‐positive cases and 96% (25/26) of cases positive for papilloma with florid epithelial hyperplasia (fig 2).
Table 1 Staining pattern of all cases of papilloma.
| SMA | p63 | CD10 | CK14 | |
|---|---|---|---|---|
| Papilloma | ||||
| Myoepithelial | 62/72 | 71/72 | 63/72 | 68/72 |
| Epithelial | 2/72 | 0/72 | 16/72 | 51/72 |
| Stromal | 32/72 | 7/72 | 14/72 | 22/72 |
| Papilloma with florid epithelial hyperplasia | ||||
| Myoepithelial | 26/28 | 28/28 | 28/28 | 27/27* |
| Epithelial | 0/28 | 0/28 | 4/28 | 25/27* |
| Stromal | 11/28 | 0/28 | 14/28 | 4/27* |
SMA, smooth‐muscle actin.
*In one case, the immunostaining results were not interpretable, and no additional material was available for repeat assessment.
Figure 1 (A) Benign papilloma (haematoxylin and eosin staining, 100×); (B) with p63 staining highlighting abundant myoepithelial cells.
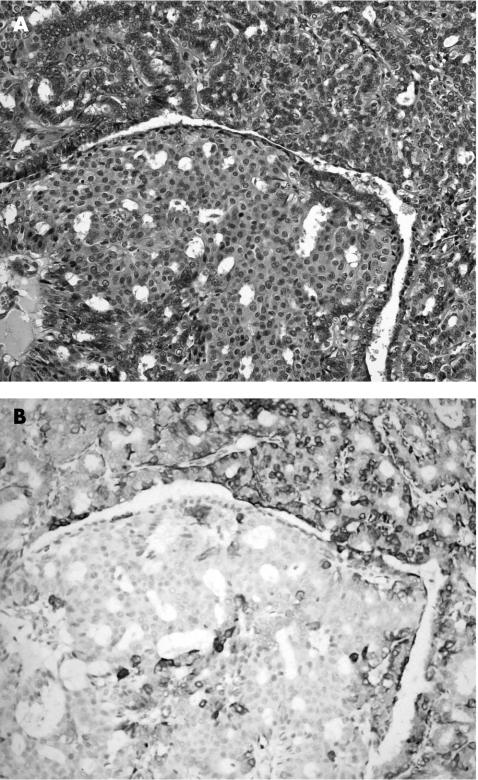
Figure 2 (A) Benign papilloma with extensive florid epithelial hyperplasia (haematoxylin and eosin staining, 200×). (B) Cytokeratin 14 showed strong staining of the epithelium.
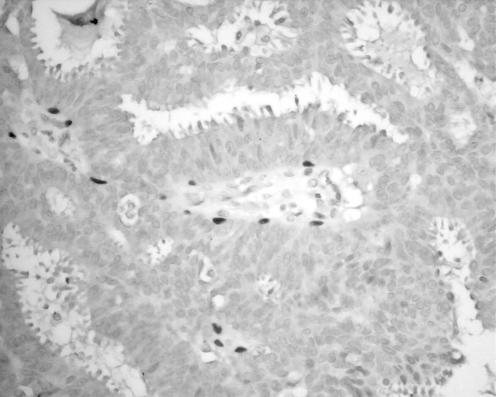
Table 2 lists the results for the malignant group (invasive papillary carcinoma, papillary carcinoma in situ and DCIS involving papilloma). In all, 3 of 9 (33%) cases of invasive papillary carcinoma, 20 of 44 (45%) cases of papillary carcinoma in situ and 13 of 15 (87%) cases of DCIS involving a papilloma showed staining of myoepithelial cells with one or more of the four immunomarkers. Table 3 lists the sensitivity of each of these markers in detecting myoepithelial cells. Compared with benign papillary lesions, the number of myoepithelial cells was markedly reduced (fig 3). This was particularly so in the category of papillary carcinoma in situ, in which scanty myoepithelial cells were seen along the elaborate fibrovascular cores, and the layer of myoepithelial cells was discontinuous. Not unexpectedly, myoepithelial cells were more prominent in the group of DCIS involving papillomas, showing a similar pattern to that seen in papillomas, especially at the fibrovascular cores.
Table 2 Staining pattern of all cases of papillary carcinoma.
| SMA | p63 | CD10 | CK14 | |
|---|---|---|---|---|
| Invasive papillary carcinoma | ||||
| Myoepithelial | 1/9 | 2/9 | 2/9 | 3/9 |
| Epithelial | 0/9 | 0/9 | 1/9 | 1/9 |
| Stromal | 4/9 | 0/9 | 2/9 | 1/9 |
| Papillary carcinoma in situ | ||||
| Myoepithelial | 14/44 | 15/44 | 11/44 | 15/44 |
| Epithelial | 0/44 | 1/44 | 0/44 | 11/44 |
| Stromal | 26/44 | 0/44 | 5/44 | 1/44 |
| DCIS involving papilloma | ||||
| Myoepithelial | 9/15 | 12/15 | 6/15 | 10/15 |
| Epithelial | 0/15 | 1/15 | 0/15 | 2/15 |
| Stromal | 7/15 | 0/15 | 2/15 | 0/15 |
DCIS, ductal carcinoma in situ; SMA, smooth‐muscle actin.
Table 3 Sensitivity of individual myoepithelial markers in detecting myoepithelial cells in papillary carcinoma showing myoepithelial cells with any one or more of the four markers.
| SMA | p63 | CD10 | CK14 | |
|---|---|---|---|---|
| Invasive papillary carcinoma | 1/3 | 2/3 | 2/3 | 3/3 |
| Papillary carcinoma in situ | 14/20 | 15/20 | 11/20 | 15/20 |
| DCIS involving papilloma | 9/15 | 12/15 | 6/15 | 10/15 |
DCIS, ductal carcinoma in situ; SMA, smooth‐muscle actin.
Figure 3 Papillary carcinoma in situ showing markedly diminished myoepithelial cells when stained with p63 (400×).
Importantly, SMA and CD10 also stained the stromal cellular component in 37 of 68 (54%) cases and 9 of 68 (13%) cases of papillary carcinoma, respectively, whereas staining of stromal cells was <6 % for CK14 and there was no staining of stromal cells with p63. Marked staining of the ductal luminal epithelial cells occurred with CK14, and was detected in 18 of 68 (26%) cases, whereas for all other markers, the ductal luminal epithelial staining was minimal.
Comparing the myoepithelial cell staining pattern of papilloma and papillary carcinoma in situ, most papillomas showed the presence of a continuous myoepithelial cell layer, readily highlighted by any of the four myoepithelial cell markers, but for papillary carcinoma in situ and invasive papillary carcinoma, a high proportion (30 of 53, 57% ) was negative for any of the myoepithelial cell markers, and the remainder (23 of 53, 43%) showed only focal and discontinuous staining of the myoepithelial layer. The difference in the positive staining rate was significant (p<0.001).
The morphological patterns of papilloma with florid epithelial hyperplasia and DCIS involving papilloma may occasionally be problematic to distinguish. If we consider the epithelial staining pattern of CK14 in these lesions, the staining was positive in 23 of 27 (85%) cases of papillomas with florid epithelial hyperplasia, and in 6 of 15 (40%) cases of DCIS involving papilloma. If we use a more stringent cut‐off so that moderate to strong staining of at least half (50%) of the epithelial cell population is required to be considered positive, then only 19 of 27 cases of papilloma with florid epithelial hyperplasia showed this degree of CK14 positivity compared with none of the cases of papilloma with DCIS. This difference was significant (p<0.001).
Discussion
Correct histological assessment of papillary lesions of the breast is sometimes fraught with difficulties and even fallacies. Nevertheless, this is important as the management of benign and malignant (either in situ or invasive) papillary lesions is different, with vastly different prognostic implications. The observation that papillomas are frequently involved in other epithelial proliferative processes, ranging from florid epithelial hyperplasia and atypical duct hyperplasia to DCIS further complicates this issue. The major problems lie in the differentiation between papilloma and papillary carcinoma in situ, as well as between papillomas that are involved in either florid epithelial hyperplasia or DCIS (particularly low‐grade disease). In the literature, apart from the well‐established histological criteria,9,11,21 immunohistochemistry to investigate myoepithelial cell numbers is the most common approach that has been reported, under the presumption that there are far fewer or no myoepithelial cells in malignant papillary lesions compared with their benign counterparts.6,21 As an increasing number of needle core biopsies are performed for each breast lesion, the correct diagnosis of papillary lesions becomes more difficult as the amount of material is reduced. Although many authors have reported a good degree of accuracy in diagnosing benign papillary lesions in needle core biopsy specimens,22,23,24,25 both benign and malignant papillary lesions may show up as papilloma with atypical epithelial features in needle core biopsy specimens.24 Immunohistochemistry becomes a useful tool in assessing such specimens.
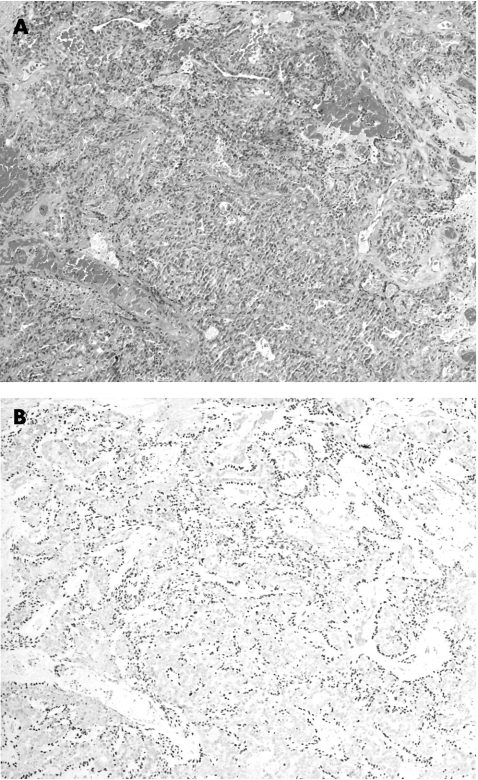
SMA, in addition to staining smooth muscle or myoepithelial cells, also stains stromal myofibroblasts and pericytes, and this is particularly problematic in cases at the base of an enlarged epithelial cluster, where stromal myofibroblasts may be compressed to form an apparently continuous layer, mimicking a myoepithelial cell layer. In this series, we found strong staining of the stromal cells, thus reducing the specificity of the stain as a myoepithelial marker. This problem is accentuated in papillary carcinoma in situ, in which the presence of myoepithelial cells is sparse, and cross‐reactivity with stromal myofibroblasts may give an erroneous impression of abundant myoepithelial cells (fig 4).
Figure 4 Papillary carcinoma in situ (A) Haematoxylin and eosin staining, 200×), highlighting that smooth‐muscle actin staining of the stromal cells may give a false impression of myoepithelial cell layer (B).
p63 is one of the p53 homologues and related genes, and it seems to play a crucial part in the regulation of epithelial proliferation and differentiation. Mice that are p63−/−have no hair follicles, teeth or mammary, lachrymal and salivary glands.26,27 In humans, immunohistochemical analysis shows p63 expression in the epithelial cells of stratified epithelium, including skin, oesophagus, exocervix, tonsil and bladder, and the basal cells in glandular organs including breast, bronchi and prostate.28 Furthermore p63 is expressed in the myoepithelial cells in the breast15,16 and as a marker of “undifferentiation,”29 and may be useful as a myoepithelial marker in assessing breast lesions.17,18,19 Recent reports30,31 suggest that p63 is also expressed in sarcomatoid or metaplastic carcinoma of the breast and may be a marker for this specific mammary carcinoma subtype. In our study, p63 proved to be the most sensitive marker for the detection of myoepithelial cells, and thus a negative or sparse p63 staining is quite specific for malignant papillary lesion. Interestingly, epithelial staining was present in a single case of papillary carcinoma in situ in our series, and this fact has also been observed in other series, in which epithelial staining was present in a small proportion of the papillary lesions.21 Howoever, evaluation in combination with the morphological characteristic obtained on haematoxylin an eosin staining, is unlikely to create confusion in the assessment of myoepithelial cells. This probably relates to the fact that p63 is also a marker of undifferentiation,29 thus the more basal epithelial cells may be positive for the stain.
CD10, or the common acute lymphoblastic leukaemia antigen, is a cell‐surface neutral endopeptidase, and is expressed by lymphoid precursor cells and some B lymphoid cells.32 It has been recently demonstrated in normal tissues including myoepithelial cells of the breast and salivary glands, in apocrine metaplastic cells of the breast33 and in endometrial stromal sarcoma,34,35 and is useful to differentiating endometrial stromal sarcoma from leiomyoma.34 In the breast, CD10 has been assessed as a myoepithelial cell marker,33,36 and overexpressed in malignant mammary phyllodes tumours.37 In our series, CD10 was shown to be a sensitive myoepithelial cell marker, although there seems to be considerable cross‐reactivity with epithelial and stromal cells.
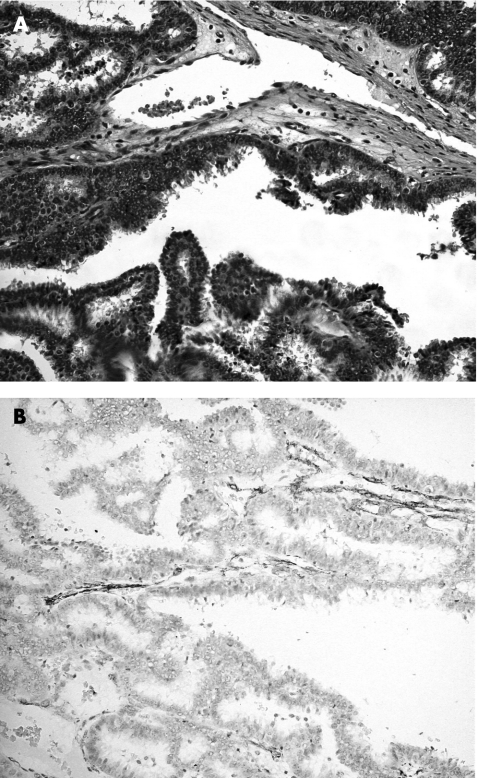
CKs belong to one of five classes of intermediate filaments; they form a dense network radiating from the nucleus to the plasma membrane, and act as cytoplasmic scaffolding. The CK family is a highly complex multigene family of polypeptides; some are simple epithelial keratins (CK7, CK8, CK18 and CK19), expressed by the ductal epithelium of the breast, and the myoepithelial cells express basal‐type cytokeratins (CK5, CK14 and CK17).38,39 More recently, the expression of basal cell keratins (CK5/6 and CK14) has been reported as indicative of a worse prognosis in invasive breast cancer.40 Furthermore, CK14 was also reported to be useful in differentiating between benign and malignant epithelial lesions of the breast and in papilloma.12,41,42 In our series, we found CK14 to be useful in two respects. Firstly, it can identify myoepithelial cells with high sensitivity, thus rendering it a useful marker in assisting differentiation between benign and malignant papillary lesions. Secondly, in the group of papillary lesions with prominent epithelial proliferation, which includes papillomas with florid epithelial hyperplasia or DCIS involving papillomas, CK14 has the additional benefit of selectively staining the epithelium of the hyperplastic (fig 2) but not malignant lesions (fig 5). As morphological differentiation is occasionally difficult, CK14 provides an additional criterion for this differentiation. If we use a cut‐off of moderate to strong staining of ⩾50% of the epithelial cells, then CK14 becomes 100% specific in identifying benign epithelial proliferation within a papilloma.
Figure 5 Ductal carcinoma in situ involving papilloma. (A) Haematoxylin and eosin staining, 200×) showing negative staining of the epithelial component by cytokeratin 14, (B) in contradistinction with the epithelial cells in florid epithelial hyperplasia involving papillomas (fig 2).
Take‐home messages
Immunohistochemistry is useful in differentiating mammary papilloma and papillary carcinoma.
P63 and CD10 are useful to highlight myoepithelium with papillary lesions.
CK14 can differentiate benign and neoplastic epithelium within a papilloma.
In this study, we have shown that all myoepithelial markers —SMA, p63, CD10 and CK14—were sensitive in identifying myoepithelial cells in papillary lesions. They are thus useful in assisting the differentiation of benign (more myoepithelial cells with continuous staining) from malignant (much less or absent staining, discontinuous if present) lesions. p63 is probably superior to SMA and CD10 because the staining is nuclear, and there is no cross‐reactivity with the stromal cells or stromal myofibroblasts. However, we should remember that p63 may rarely be expressed by epithelial cells. In those cases with prominent epithelial proliferation, when either florid epithelial hyperplasia or DCIS involving pre‐existing papillomas enters into the differential diagnosis, CK14 may provide an added advantage in highlighting the benign epithelial proliferation.
To conclude, a simple panel of p63 and CK14 may be a useful and sufficient immunohistochemical tool for assisting the characterisation of papillary lesions of the breast.
Abbreviations
DCIS - ductal carcinomas in situ
SMA - smooth‐muscle actin
Footnotes
Competing interests: None declared.
References
- 1.Haagensen C D, Stout A P, Philips J S. The papillary neoplasms of the breast. I. Benign intraductal papilloma. Ann Surg 195113318–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kraus F T, Neubecker R D. The differential diagnosis of papillary tumours of the breast. Cancer 196215444–455. [DOI] [PubMed] [Google Scholar]
- 3.Murad T M, Contesso G, Mouriesse H. Papillary tumours of large lactiferous ducts. Cancer 198148122–133. [DOI] [PubMed] [Google Scholar]
- 4.Oyama T, Koerner F C. Noninvasive papillary proliferations. Semin Diagn Pathol 20042132–41. [DOI] [PubMed] [Google Scholar]
- 5.Ellis I O, Elston C W, Pinder S E. Papillary lesions. In: Elston CW, Ellis IO, eds. The breast. Systemic pathology, 3rd edn, Vol 13. Edinburgh, UK: Churchill Livingston, 1998133–146.
- 6.Hill C B, Yeh I T. Myoepithelial cell staining patterns of papillary breast lesions: from intraductal papillomas to invasive papillary carcinomas. Am J Clin Pathol 200512336–44. [DOI] [PubMed] [Google Scholar]
- 7.Page D L, Anderson T J. Papilloma and related lesions. In: Page DL, Anderson TJ, eds. Diagnostic histopathology of the breast. Edinburgh, UK: Churchill Livingston, 1987104–119.
- 8.Papotti M, Eusebi V, Gugliotta P.et al Immunohistochemical analysis of benign and malignant papillary lesions of the breast. Am J Surg Pathol 19837451–461. [DOI] [PubMed] [Google Scholar]
- 9.Raju U B, Lee M W, Zarbo R J.et al Papillary neoplasia of the breast: immunohistochemically defined myoepithelial cells in the diagnosis of benign and malignant papillary breast neoplasms. Mod Pathol 19892569–576. [PubMed] [Google Scholar]
- 10.Mosunjac M B, Lewis M M, Lawson D.et al Use of a novel marker, calponin, for myoepithelial cells in fine‐needle aspirates of papillary breast lesions. Diagn Cytopathol 200023151–155. [DOI] [PubMed] [Google Scholar]
- 11.Zhang C, Zhang P, Hao J.et al High nuclear grade, frequent mitotic activity, cyclin D1 and p53 overexpression are associated with stromal invasion in mammary intracystic papillary carcinoma. Breast J 2005112–8. [DOI] [PubMed] [Google Scholar]
- 12.Tan P H, Aw M Y, Yip G.et al Cytokeratins in papillary lesions of the breast: is there a role in distinguishing intraductal papilloma from papillary ductal carcinoma in situ? Am J Surg Pathol 200529625–632. [DOI] [PubMed] [Google Scholar]
- 13.Saddik M, Lai R. CD44s as a surrogate marker for distinguishing intraductal papilloma from papillary carcinoma of the breast. J Clin Pathol 199952862–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tse G M, Tan P H, Ma T K.et al CD44 is useful in the differentiation of benign and malignant papillary lesions of the breast. J Clin Pathol 2005581185–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbareschi M, Pecciarini L, Cangi M G.et al p63, a p53 homologue, is a selective nuclear marker of myoepithelial cells of the human breast. Am J Surg Pathol 2001251054–1060. [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro‐Silva A, Zambelli Ramalho L N, Britto Garcia S.et al The relationship between p63 and p53 expression in normal and neoplastic breast tissue. Arch Pathol Lab Med 2003127336–340. [DOI] [PubMed] [Google Scholar]
- 17.Reis‐Filho J S, Milanezi F, Amendoeira I.et al Distribution of p63, a novel myoepithelial marker, in fine‐needle aspiration biopsies of the breast: an analysis of 82 samples. Cancer 200399172–179. [DOI] [PubMed] [Google Scholar]
- 18.Reis‐Filho J S, Milanezi F, Amendoeira I.et al p63 Staining of myoepithelial cells in breast fine needle aspirates: a study of its role in differentiating in situ from invasive ductal carcinomas of the breast. J Clin Pathol 200255936–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribeiro‐Silva A, Zamzelli Ramalho L N, Garcia S B.et al Is p63 reliable in detecting microinvasion in ductal carcinoma in situ of the breast? Pathol Oncol Res 2003920–23. [DOI] [PubMed] [Google Scholar]
- 20.Stefanou D, Batistatou A, Nonni A.et al p63 expression in benign and malignant breast lesions. Histol Histopathol 200419465–471. [DOI] [PubMed] [Google Scholar]
- 21.Hoda S A, Rosen P P. Practical considerations in the pathologic diagnosis of needle core biopsies of breast. Am J Clin Pathol 2002118101–108. [DOI] [PubMed] [Google Scholar]
- 22.Agoff S N, Lawton T J. Papillary lesions of the breast with and without atypical ductal hyperplasia: can we accurately predict benign behavior from core needle biopsy? Am J Clin Pathol 2004122440–443. [DOI] [PubMed] [Google Scholar]
- 23.Carder P J, Garvican J, Haigh I.et al Needle core biopsy can reliably distinguish between benign and malignant papillary lesions of the breast. Histopathology 200546320–327. [DOI] [PubMed] [Google Scholar]
- 24.Ivan D, Selinko V, Sahin A A.et al Accuracy of core needle biopsy diagnosis in assessing papillary breast lesions: histological predictors of malignancy. Mod Pathol 200417165–171. [DOI] [PubMed] [Google Scholar]
- 25.Renshaw A A, Derhagopian R P, Tizol‐Blanco D M.et al Papillomas and atypical papillomas in breast core needle biopsy specimens: risk of carcinoma in subsequent excision. Am J Clin Pathol 2004122217–221. [DOI] [PubMed] [Google Scholar]
- 26.Mills A A, Zheng B, Wang X J.et al p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 1999398708–713. [DOI] [PubMed] [Google Scholar]
- 27.Yang A, Schweitzer R, Sun D.et al p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 1999398714–718. [DOI] [PubMed] [Google Scholar]
- 28.Di Como C J, Urist M J, Babayan I.et al p63 expression profiles in human normal and tumour tissues. Clin Cancer Res 20028494–501. [PubMed] [Google Scholar]
- 29.Kaufmann O, Fietze E, Mengs J.et al Value of p63 and cytokeratin 5/6 as immunohistochemical markers for the differential diagnosis of poorly differentiated and undifferentiated carcinomas. Am J Clin Pathol 2001116823–830. [DOI] [PubMed] [Google Scholar]
- 30.Koker M M, Kleer C G. p63 expression in breast cancer: a highly sensitive and specific marker of metaplastic carcinoma. Am J Surg Pathol 2004281506–1512. [DOI] [PubMed] [Google Scholar]
- 31.Tse G M, Tan P H, Chaiwun B.et al p63 is useful in the diagnosis of mammary metplastic carcinomas. Pathology 20063816–20. [DOI] [PubMed] [Google Scholar]
- 32.Greaves M F, Brown G, Rapson N T.et al Antisera to acute lymphoblastic leukemia cells. Clin Immunol Immunopathol 1975467–84. [DOI] [PubMed] [Google Scholar]
- 33.Chu P, Arber D A. Paraffin section detection of CD10 in 505 nonhematopoietic neoplasms. Am J Clin Pathol 2000113374–382. [DOI] [PubMed] [Google Scholar]
- 34.Chu P, Arber D A, Weiss L M.et al Utility of CD10 in distinguishing between endometrial stromal sarcoma and uterine smooth muscle tumours: an immunohistochemical comparison of 34 cases. Mod Pathol 200114465–471. [DOI] [PubMed] [Google Scholar]
- 35.McCluggage W G, Sumathi V P, Maxwell P. CD10 is a sensitive and diagnostically useful immunohistochemical marker of normal endometrial stroma and of endometrial stromal neoplasms. Histopathology 200139273–278. [DOI] [PubMed] [Google Scholar]
- 36.Moritani S, Kushima R, Sugihara H.et al Availability of CD10 immunohistochemistry as a marker of breast myoepithelial cells on paraffin sections.Mod Pathol 200215397–405. [DOI] [PubMed] [Google Scholar]
- 37.Tse G M, Tsang A K, Putti T C.et al Stromal CD10 expression in mammary fibroadenomas and phyllodes tumours. J Clin Pathol 200558185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bocker W, Bier B, Freytag G.et al An immunohistochemical study of the breast using antibodies to basal and luminal keratins, alpha‐smooth muscle actin, vimentin, collagen IV and laminin. Part I: normal breast and benign proliferative lesions. Virchows Arch A Pathol Anat Histopathol 1992421315–322. [DOI] [PubMed] [Google Scholar]
- 39.Tsubura A, Okada H, Senzaki H.et al Keratin expression in the normal breast and in breast carcinoma. Histopathology 199118517–522. [DOI] [PubMed] [Google Scholar]
- 40.Abd El‐Rehim D M, Pinder S E, Paish C E.et al Expression of luminal and basal cytokeratins in human breast carcinoma. J Pathol 2004203661–671. [DOI] [PubMed] [Google Scholar]
- 41.Otterbach F, Bankfalvi A, Bergner S.et al Cytokeratin 5/6 immunohistochemistry assists the differential diagnosis of atypical proliferations of the breast. Histopathology 200037232–240. [DOI] [PubMed] [Google Scholar]
- 42.Wetzels R H, Kuijpers H J, Lane E B.et al Basal cell‐specific and hyperproliferation‐related keratins in human breast cancer. Am J Pathol 1991138751–763. [PMC free article] [PubMed] [Google Scholar]