Abstract
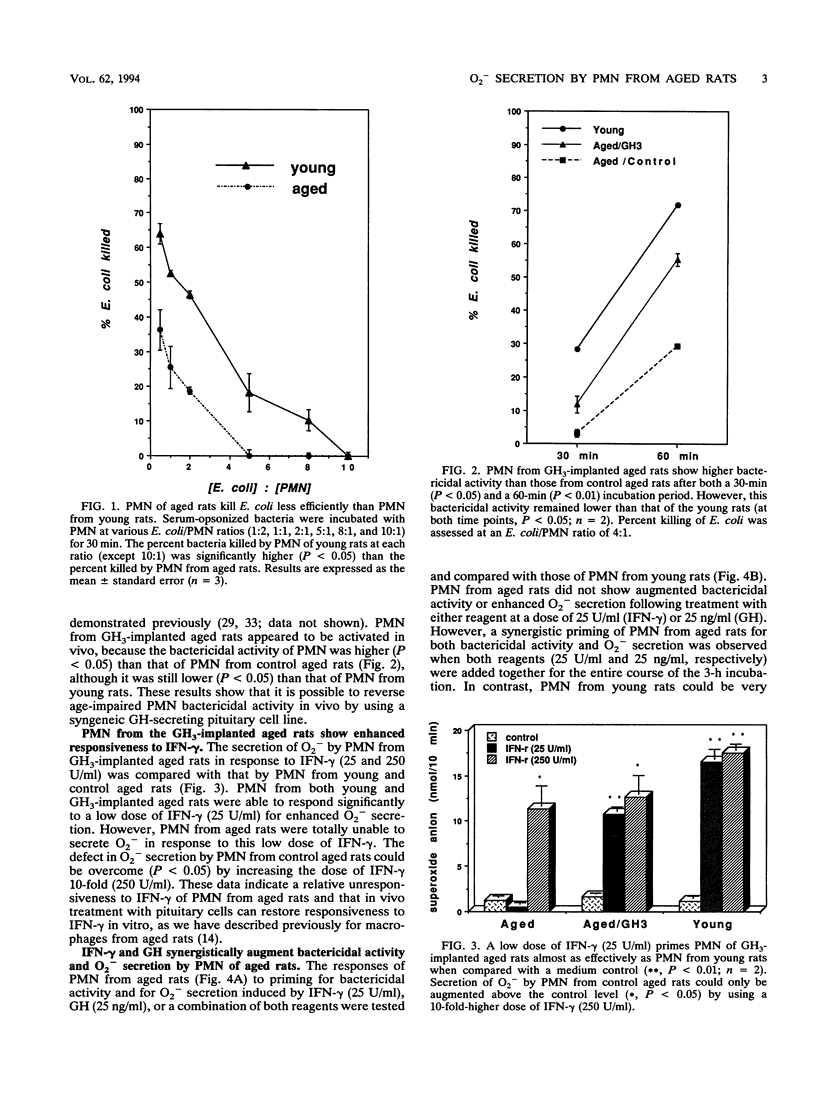
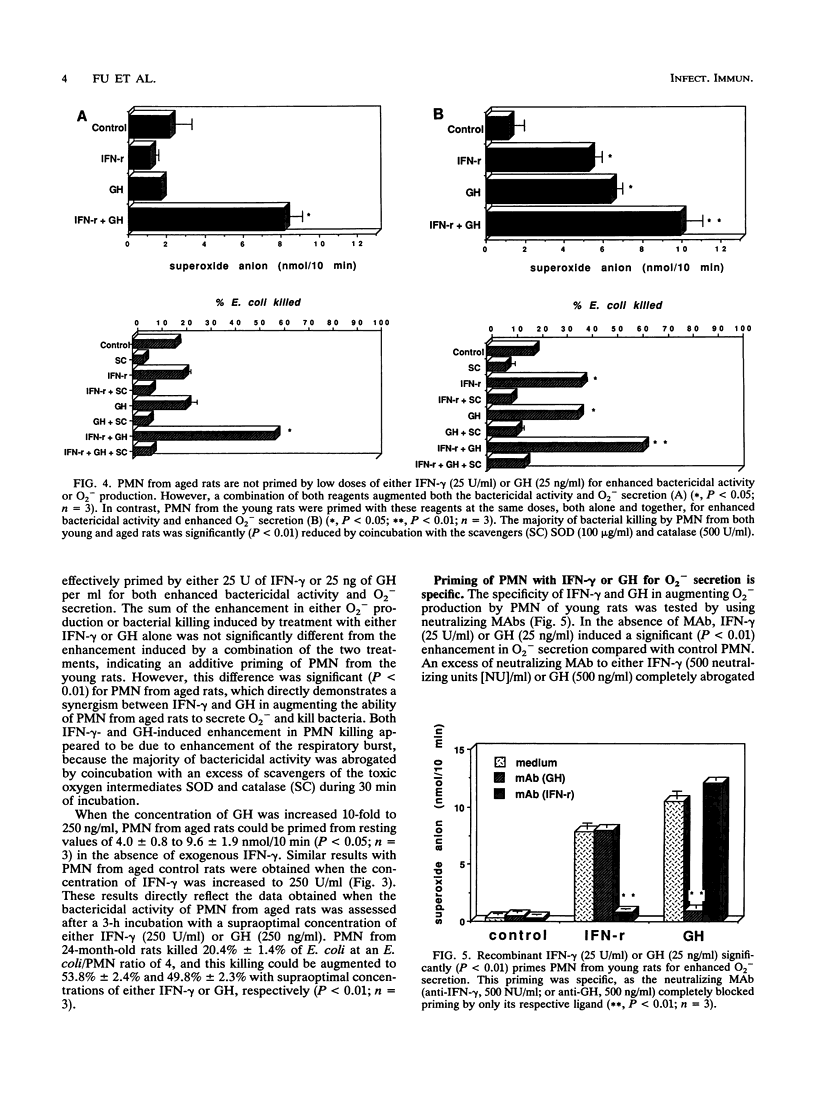
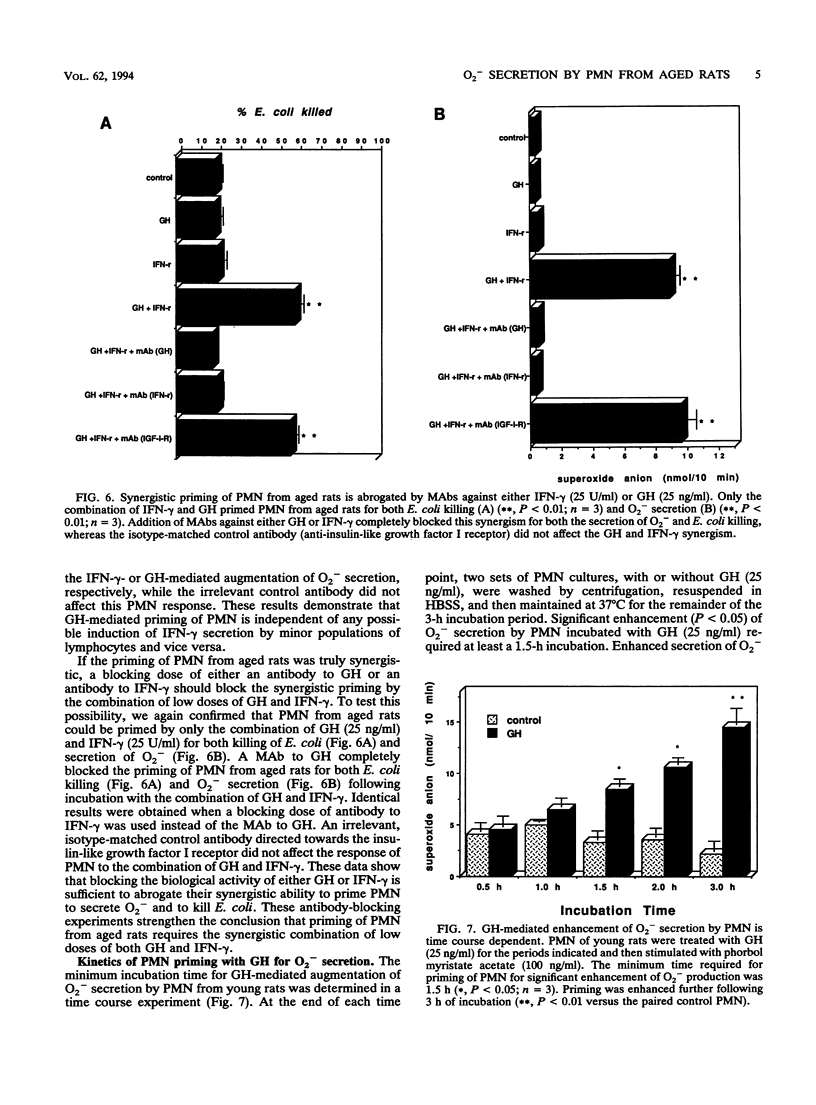
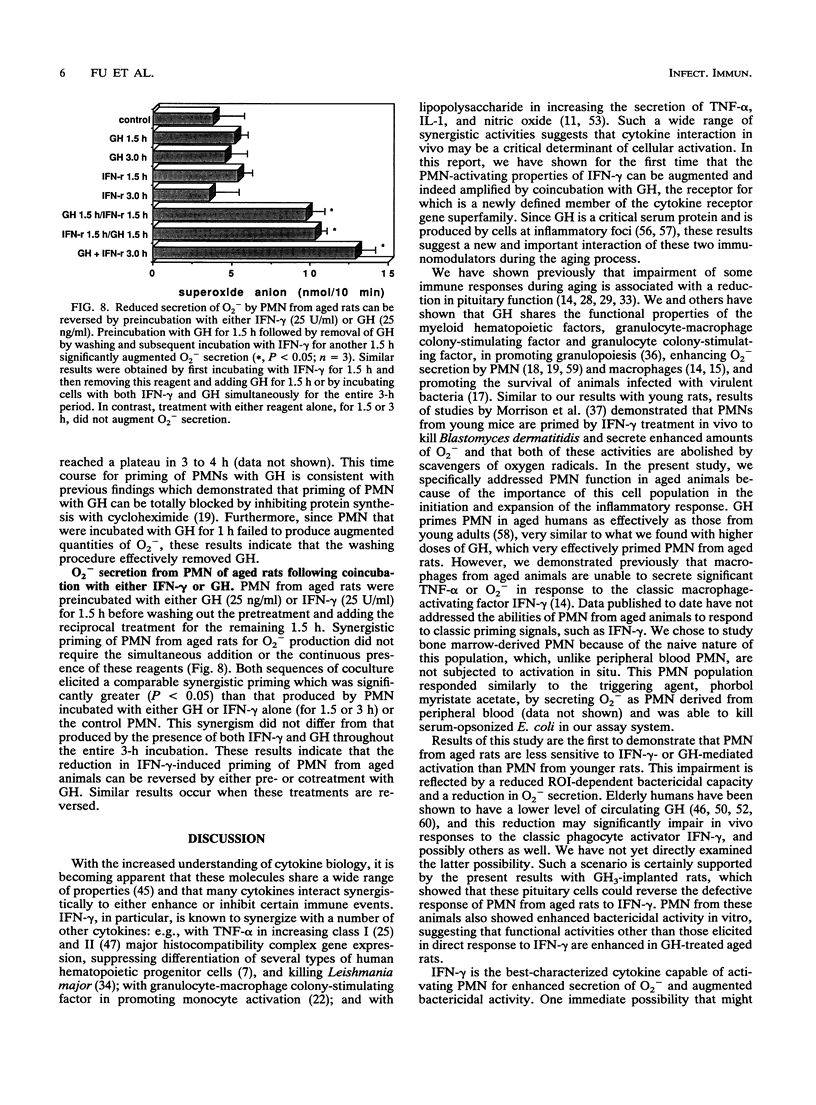
Polymorphonuclear neutrophils (PMN) from bone marrow of 24-month-old rats kill Escherichia coli less efficiently than PMN from 3-month-old rats. Secretion of O2- and killing of E. coli by PMN from both young and old rats can be significantly augmented by preincubation with either 250 U of gamma interferon (IFN-gamma) or 250 ng of growth hormone (GH) per ml. This priming is specific, because neutralizing monoclonal antibodies against either IFN-gamma or GH completely abrogate the enhanced O2- secretion by PMN from young rats. However, in contrast to PMN from young rats, PMN from aged rats are not primed to kill E. coli by 10-fold-lower concentrations of either IFN-gamma (25 U/ml) or GH (25 ng/ml). To explore the mechanism for the reduction in bacterial killing by PMN from old rats, a syngeneic GH-secreting pituitary cell line (GH3) was implanted in vivo. PMN from GH3-treated aged rats, but not control aged rats, could now be primed in vitro for O2- secretion by IFN-gamma (25 U/ml). Although PMN from aged rats do not respond to the lower doses of either IFN-gamma or GH, the combination of both reagents totally restores the ability of PMN to secrete O2- and to kill E. coli. This synergistic priming is observed with PMN from aged rats, but not with those from young rats, and can be detected when both reagents are added simultaneously or when they are added in either sequence. Furthermore, addition of a monoclonal antibody against either IFN-gamma or GH abrogates the synergism of these two molecules. Collectively, these data identify an important alteration in myeloid cells from aged rodents by showing that their PMN are intrinsically unable to respond to low concentrations of IFN-gamma by secreting O2- and killing bacteria. The results also define a previously unrecognized synergism in PMN from aged animals by showing that GH synergizes with IFN-gamma both in vivo and in vitro to restore these suppressed responses.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Walz A., Kunkel S. L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989 Oct;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan J. F. Neuropoietic cytokines in the hematopoietic fold. Neuron. 1991 Aug;7(2):197–208. doi: 10.1016/0896-6273(91)90258-2. [DOI] [PubMed] [Google Scholar]
- Bazan J. F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton G., Zeni L., Cassatella M. A., Rossi F. Gamma interferon is able to enhance the oxidative metabolism of human neutrophils. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1276–1282. doi: 10.1016/s0006-291x(86)80421-1. [DOI] [PubMed] [Google Scholar]
- Boockfor F. R., Hoeffler J. P., Frawley L. S. Cultures of GH3 cells are functionally heterogeneous: thyrotropin-releasing hormone, estradiol and cortisol cause reciprocal shifts in the proportions of growth hormone and prolactin secretors. Endocrinology. 1985 Jul;117(1):418–420. doi: 10.1210/endo-117-1-418. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H. E., Williams D. E., Lu L., Cooper S., Anderson S. L., Beyer G. S., Hoffman R., Rubin B. Y. The suppressive influences of human tumor necrosis factors on bone marrow hematopoietic progenitor cells from normal donors and patients with leukemia: synergism of tumor necrosis factor and interferon-gamma. J Immunol. 1986 Jun 15;136(12):4487–4495. [PubMed] [Google Scholar]
- Cassatella M. A., Cappelli R., Della Bianca V., Grzeskowiak M., Dusi S., Berton G. Interferon-gamma activates human neutrophil oxygen metabolism and exocytosis. Immunology. 1988 Mar;63(3):499–506. [PMC free article] [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E. Superoxide generation by digitonin-stimulated guinea pig granulocytes. A basis for a continuous assay for monitoring superoxide production and for the study of the activation of the generating system. J Clin Invest. 1978 Apr;61(4):1081–1087. doi: 10.1172/JCI109007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corberand J., Ngyen F., Laharrague P., Fontanilles A. M., Gleyzes B., Gyrard E., Senegas C. Polymorphonuclear functions and aging in humans. J Am Geriatr Soc. 1981 Sep;29(9):391–397. doi: 10.1111/j.1532-5415.1981.tb02376.x. [DOI] [PubMed] [Google Scholar]
- Corradin S. B., Mauël J. Phagocytosis of Leishmania enhances macrophage activation by IFN-gamma and lipopolysaccharide. J Immunol. 1991 Jan 1;146(1):279–285. [PubMed] [Google Scholar]
- Cosman D., Lyman S. D., Idzerda R. L., Beckmann M. P., Park L. S., Goodwin R. G., March C. J. A new cytokine receptor superfamily. Trends Biochem Sci. 1990 Jul;15(7):265–270. doi: 10.1016/0968-0004(90)90051-c. [DOI] [PubMed] [Google Scholar]
- Cross A. S., Sadoff J. C., Kelly N., Bernton E., Gemski P. Pretreatment with recombinant murine tumor necrosis factor alpha/cachectin and murine interleukin 1 alpha protects mice from lethal bacterial infection. J Exp Med. 1989 Jun 1;169(6):2021–2027. doi: 10.1084/jem.169.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila D. R., Edwards C. K., 3rd, Arkins S., Simon J., Kelley K. W. Interferon-gamma-induced priming for secretion of superoxide anion and tumor necrosis factor-alpha declines in macrophages from aged rats. FASEB J. 1990 Aug;4(11):2906–2911. doi: 10.1096/fasebj.4.11.2165948. [DOI] [PubMed] [Google Scholar]
- Edwards C. K., 3rd, Ghiasuddin S. M., Schepper J. M., Yunger L. M., Kelley K. W. A newly defined property of somatotropin: priming of macrophages for production of superoxide anion. Science. 1988 Feb 12;239(4841 Pt 1):769–771. doi: 10.1126/science.2829357. [DOI] [PubMed] [Google Scholar]
- Edwards C. K., 3rd, Ghiasuddin S. M., Yunger L. M., Lorence R. M., Arkins S., Dantzer R., Kelley K. W. In vivo administration of recombinant growth hormone or gamma interferon activities macrophages: enhanced resistance to experimental Salmonella typhimurium infection is correlated with generation of reactive oxygen intermediates. Infect Immun. 1992 Jun;60(6):2514–2521. doi: 10.1128/iai.60.6.2514-2521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C. K., 3rd, Yunger L. M., Lorence R. M., Dantzer R., Kelley K. W. The pituitary gland is required for protection against lethal effects of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2274–2277. doi: 10.1073/pnas.88.6.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y. K., Arkins S., Fuh G., Cunningham B. C., Wells J. A., Fong S., Cronin M. J., Dantzer R., Kelley K. W. Growth hormone augments superoxide anion secretion of human neutrophils by binding to the prolactin receptor. J Clin Invest. 1992 Feb;89(2):451–457. doi: 10.1172/JCI115605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y. K., Arkins S., Wang B. S., Kelley K. W. A novel role of growth hormone and insulin-like growth factor-I. Priming neutrophils for superoxide anion secretion. J Immunol. 1991 Mar 1;146(5):1602–1608. [PubMed] [Google Scholar]
- Fülöp T., Jr, Fóris G., Wórum I., Leövey A. Age-dependent alterations of Fc gamma receptor-mediated effector functions of human polymorphonuclear leucocytes. Clin Exp Immunol. 1985 Aug;61(2):425–432. [PMC free article] [PubMed] [Google Scholar]
- Goldberg M., Belkowski L. S., Bloom B. R. Regulation of macrophage function by interferon-gamma. Somatic cell genetic approaches in murine macrophage cell lines to mechanisms of growth inhibition, the oxidative burst, and expression of the chronic granulomatous disease gene. J Clin Invest. 1990 Feb;85(2):563–569. doi: 10.1172/JCI114473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. H., Whitty G. A., Piccoli D. S., Hamilton J. A. Synergistic activation of human monocytes by granulocyte-macrophage colony-stimulating factor and IFN-gamma. Increased TNF-alpha but not IL-1 activity. J Immunol. 1988 Sep 1;141(5):1516–1521. [PubMed] [Google Scholar]
- Havell E. A. Evidence that tumor necrosis factor has an important role in antibacterial resistance. J Immunol. 1989 Nov 1;143(9):2894–2899. [PubMed] [Google Scholar]
- Johnson D. R., Pober J. S. Tumor necrosis factor and immune interferon synergistically increase transcription of HLA class I heavy- and light-chain genes in vascular endothelium. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5183–5187. doi: 10.1073/pnas.87.13.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E. L., Laxdal T., Quie P. G. Studies of polymorphonuclear leukocytes from patients with chronic granulomatous disease of childhood: bactericidal capacity for streptococci. Pediatrics. 1968 Mar;41(3):591–599. [PubMed] [Google Scholar]
- Kelley K. W., Arkins S., Li Y. M. Growth hormone, prolactin, and insulin-like growth factors: new jobs for old players. Brain Behav Immun. 1992 Dec;6(4):317–326. [PubMed] [Google Scholar]
- Kelley K. W., Brief S., Westly H. J., Novakofski J., Bechtel P. J., Simon J., Walker E. B. GH3 pituitary adenoma cells can reverse thymic aging in rats. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5663–5667. doi: 10.1073/pnas.83.15.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley K. W. Growth hormone, lymphocytes and macrophages. Biochem Pharmacol. 1989 Mar 1;38(5):705–713. doi: 10.1016/0006-2952(89)90222-0. [DOI] [PubMed] [Google Scholar]
- Khansari D. N., Gustad T. Effects of long-term, low-dose growth hormone therapy on immune function and life expectancy of mice. Mech Ageing Dev. 1991 Jan;57(1):87–100. doi: 10.1016/0047-6374(91)90026-v. [DOI] [PubMed] [Google Scholar]
- Kharazmi A., Nielsen H., Rechnitzer C., Bendtzen K. Interleukin 6 primes human neutrophil and monocyte oxidative burst response. Immunol Lett. 1989 May;21(2):177–184. doi: 10.1016/0165-2478(89)90056-4. [DOI] [PubMed] [Google Scholar]
- Laharrague P., Corberand J., Fillola G., Nguyen F., Fontanilles A. M., Gleizes B., Gyrard E., Jean C. Impairment of polymorphonuclear functions in hospitalized geriatric patients. Gerontology. 1983;29(5):325–331. doi: 10.1159/000213134. [DOI] [PubMed] [Google Scholar]
- Li Y. M., Brunke D. L., Dantzer R., Kelley K. W. Pituitary epithelial cell implants reverse the accumulation of CD4-CD8- lymphocytes in thymus glands of aged rats. Endocrinology. 1992 May;130(5):2703–2709. doi: 10.1210/endo.130.5.1572290. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Li Y., Millott S. Tumor necrosis factor-alpha synergizes with IFN-gamma in mediating killing of Leishmania major through the induction of nitric oxide. J Immunol. 1990 Dec 15;145(12):4306–4310. [PubMed] [Google Scholar]
- McColl S. R., Beauseigle D., Gilbert C., Naccache P. H. Priming of the human neutrophil respiratory burst by granulocyte-macrophage colony-stimulating factor and tumor necrosis factor-alpha involves regulation at a post-cell surface receptor level. Enhancement of the effect of agents which directly activate G proteins. J Immunol. 1990 Nov 1;145(9):3047–3053. [PubMed] [Google Scholar]
- Merchav S., Tatarsky I., Hochberg Z. Enhancement of human granulopoiesis in vitro by biosynthetic insulin-like growth factor I/somatomedin C and human growth hormone. J Clin Invest. 1988 Mar;81(3):791–797. doi: 10.1172/JCI113385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison C. J., Brummer E., Stevens D. A. In vivo activation of peripheral blood polymorphonuclear neutrophils by gamma interferon results in enhanced fungal killing. Infect Immun. 1989 Oct;57(10):2953–2958. doi: 10.1128/iai.57.10.2953-2958.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy W. J., Durum S. K., Longo D. L. Differential effects of growth hormone and prolactin on murine T cell development and function. J Exp Med. 1993 Jul 1;178(1):231–236. doi: 10.1084/jem.178.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel J. E., Han K., Coon P. J., Adler W. H., Bender B. S. Age differences in phagocytosis by polymorphonuclear leukocytes measured by flow cytometry. J Leukoc Biol. 1986 Apr;39(4):399–407. doi: 10.1002/jlb.39.4.399. [DOI] [PubMed] [Google Scholar]
- Nagel J. E., Pyle R. S., Chrest F. J., Adler W. H. Oxidative metabolism and bactericidal capacity of polymorphonuclear leukocytes from normal young and aged adults. J Gerontol. 1982 Sep;37(5):529–534. doi: 10.1093/geronj/37.5.529. [DOI] [PubMed] [Google Scholar]
- Nakane A., Minagawa T., Kato K. Endogenous tumor necrosis factor (cachectin) is essential to host resistance against Listeria monocytogenes infection. Infect Immun. 1988 Oct;56(10):2563–2569. doi: 10.1128/iai.56.10.2563-2569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newburger P. E., Ezekowitz R. A., Whitney C., Wright J., Orkin S. H. Induction of phagocyte cytochrome b heavy chain gene expression by interferon gamma. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5215–5219. doi: 10.1073/pnas.85.14.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmblad J., Haak A. Ageing does not change blood granulocyte bactericidal capacity and levels of complement factors 3 and 4. Gerontology. 1978;24(5):381–385. doi: 10.1159/000212275. [DOI] [PubMed] [Google Scholar]
- Patthy L. Homology of a domain of the growth hormone/prolactin receptor family with type III modules of fibronectin. Cell. 1990 Apr 6;61(1):13–14. doi: 10.1016/0092-8674(90)90208-v. [DOI] [PubMed] [Google Scholar]
- Paul W. E. Pleiotropy and redundancy: T cell-derived lymphokines in the immune response. Cell. 1989 May 19;57(4):521–524. doi: 10.1016/0092-8674(89)90121-9. [DOI] [PubMed] [Google Scholar]
- Prinz P. N., Weitzman E. D., Cunningham G. R., Karacan I. Plasma growth hormone during sleep in young and aged men. J Gerontol. 1983 Sep;38(5):519–524. doi: 10.1093/geronj/38.5.519. [DOI] [PubMed] [Google Scholar]
- Pujol-Borrell R., Todd I., Doshi M., Bottazzo G. F., Sutton R., Gray D., Adolf G. R., Feldmann M. HLA class II induction in human islet cells by interferon-gamma plus tumour necrosis factor or lymphotoxin. Nature. 1987 Mar 19;326(6110):304–306. doi: 10.1038/326304a0. [DOI] [PubMed] [Google Scholar]
- Saltzman R. L., Peterson P. K. Immunodeficiency of the elderly. Rev Infect Dis. 1987 Nov-Dec;9(6):1127–1139. doi: 10.1093/clinids/9.6.1127. [DOI] [PubMed] [Google Scholar]
- Seo H., Refetoff S., Fang V. S. Induction of hypothyroidism and hypoprolactinemia by growth hormone producing rat pituitary tumors. Endocrinology. 1977 Jan;100(1):216–226. doi: 10.1210/endo-100-1-216. [DOI] [PubMed] [Google Scholar]
- Sonntag W. E., Steger R. W., Forman L. J., Meites J. Decreased pulsatile release of growth hormone in old male rats. Endocrinology. 1980 Dec;107(6):1875–1879. doi: 10.1210/endo-107-6-1875. [DOI] [PubMed] [Google Scholar]
- Spadoni G. L., Spagnoli A., Cianfarani S., Del Principe D., Menichelli A., Di Giulio S., Boscherini B. Enhancement by growth hormone of phorbol diester-stimulated respiratory burst in human polymorphonuclear leukocytes. Acta Endocrinol (Copenh) 1991 May;124(5):589–594. doi: 10.1530/acta.0.1240589. [DOI] [PubMed] [Google Scholar]
- Spik K. W., Boyd R. L., Sonntag W. E. Effect of aging on GHRF-induced growth hormone release from anterior pituitary cells in primary culture. J Gerontol. 1991 Mar;46(2):B72–B77. doi: 10.1093/geronj/46.2.b72. [DOI] [PubMed] [Google Scholar]
- Szefler S. J., Norton C. E., Ball B., Gross J. M., Aida Y., Pabst M. J. IFN-gamma and LPS overcome glucocorticoid inhibition of priming for superoxide release in human monocytes. Evidence that secretion of IL-1 and tumor necrosis factor-alpha is not essential for monocyte priming. J Immunol. 1989 Jun 1;142(11):3985–3992. [PubMed] [Google Scholar]
- Teahan C., Rowe P., Parker P., Totty N., Segal A. W. The X-linked chronic granulomatous disease gene codes for the beta-chain of cytochrome b-245. 1987 Jun 25-Jul 1Nature. 327(6124):720–721. doi: 10.1038/327720a0. [DOI] [PubMed] [Google Scholar]
- Van Epps D. E., Goodwin J. S., Murphy S. Age-dependent variations in polymorphonuclear leukocyte chemiluminescence. Infect Immun. 1978 Oct;22(1):57–61. doi: 10.1128/iai.22.1.57-61.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigent D. A., Baxter J. B., Wear W. E., Smith L. R., Bost K. L., Blalock J. E. Production of immunoreactive growth hormone by mononuclear leukocytes. FASEB J. 1988 Sep;2(12):2812–2818. doi: 10.1096/fasebj.2.12.3044906. [DOI] [PubMed] [Google Scholar]
- Weigent D. A., Blalock J. E. The production of growth hormone by subpopulations of rat mononuclear leukocytes. Cell Immunol. 1991 Jun;135(1):55–65. doi: 10.1016/0008-8749(91)90253-8. [DOI] [PubMed] [Google Scholar]
- Wiedermann C. J., Niedermühlbichler M., Beimpold H., Braunsteiner H. In vitro activation of neutrophils of the aged by recombinant human growth hormone. J Infect Dis. 1991 Nov;164(5):1017–1020. doi: 10.1093/infdis/164.5.1017. [DOI] [PubMed] [Google Scholar]
- Wiedermann C. J., Niedermühlbichler M., Geissler D., Beimpold H., Braunsteiner H. Priming of normal human neutrophils by recombinant human growth hormone. Br J Haematol. 1991 May;78(1):19–22. doi: 10.1111/j.1365-2141.1991.tb04376.x. [DOI] [PubMed] [Google Scholar]
- Zadik Z., Chalew S. A., McCarter R. J., Jr, Meistas M., Kowarski A. A. The influence of age on the 24-hour integrated concentration of growth hormone in normal individuals. J Clin Endocrinol Metab. 1985 Mar;60(3):513–516. doi: 10.1210/jcem-60-3-513. [DOI] [PubMed] [Google Scholar]


