Abstract
Background
Bullous skin lesions are characterised by the presence of intraepidermal or subepidermal bullae. Although inflammatory cell infiltrate is a constant feature in these lesions, their immunophenotypic characterisation is still incomplete.
Aim
To determine whether the development of bullous skin diseases is associated with changes in the inflammatory cell infiltrate.
Materials and methods
34 cases representing lesions with both intraepidermal and subepidermal bullae were examined using immunoperoxidase staining methods and antibodies targeting antigens for histiocytes (CD68), B cells (CD20+), T cells (CD3+), T cells with cytotoxic potential (T cell intracellular associated antigen, TIA1+) and activity (granzyme B, GRB+). The adjacent normal skin (lesions) and an additional five cases of normal skin were also examined (controls).
Results
The transition from normal skin to lesional skin (lesions with intraepidermal and subepidermal bullae) was associated with a significant increase (p⩽0.05) in the density of total inflammatory cell infiltrate, CD68+ cells, CD3+ T lymphocytes, CD20+ B lymphocytes, TIA1+‐resting cytotoxic T cells and GRB+ T cells with cytotoxic activity.
Conclusions
The increase in inflammatory cell infiltrate during the transition from normal to lesional skin may reflect the presence of an increased antigenicity of the lesional cells or a response to some basement membrane components. CD68+ and CD3+ cells, especially the resting cytotoxic ones, achieved numerical dominance in these lesions. Cell‐mediated immunity seems to have critical role in the development of these lesions.
Bullous skin lesions are groups of diseases characterised by localised or generalised occurrence of bullae. According to the site and mode of bullae formation, they are classified into intraepidermal and subepidermal lesions. Intraepidermal lesions include pathologies such as pemphigus foliaceus, pemphigus vulgaris, Darier's disease, familial cutaneous peeling, bullous drug eruption and pustular psoriasis. Subepidermal lesions include diseases such as bullous pemphigoid, dermatitis herpetiformis, bullous drug eruption, chronic bullous disease of childhood, familial benign pemphigus, lichen planus atrophicus, epidermolysis bullosa and chronic contact dermatitis. Some of these lesions are characterised by the presence of inflammatory cell infiltrate.1,2,3 This infiltrate includes cells such as lymphocytes, histiocytes, eosinophils, neutrophils and mast cells. To date, although reports have considered some aspects of inflammatory cell infiltrate in these lesions, their immunophenotypic characterisation is still incomplete.4,5
Cytotoxic T lymphocytes (CTLs) can destroy target cells through the release of cytoplasmic granules that contain T cell intracellular‐associated antigen (TIA1) and granzyme B (GRB). TIA1 is an intracellular 15‐kDa granule membrane‐associated protein that can induce apoptotic cell death. TIA1 can also act as a regulator of the alternative pre‐mRNA splicing pathway.6,7 Granzymes are exogenous serine proteinases released from cytoplasmic granules of CTLs and natural killer cells. These granules contain, besides granzymes, other proteins including a pore‐forming protein (perforin). On binding of the CTLs to a target cell, the contents of the granules are released in the intercellular space where perforin can “perforate” the target cell membrane by forming transmembrane pores. Through these pores, the granzymes can enter the cytosol of the target cell, activate the cascade of caspases, resulting in apoptosis.8,9 To date, the expression pattern of the CTL granules (TIA1 and GRB) in the bullous skin lesions is still unknown.
We hypothesised that the development of some bullous skin diseases is associated with changes in the inflammatory cell infiltrate. We carried out this study to explore our hypothesis and to fill this existing gap in the literature. We examined the inflammatory cell infiltrate, with particular emphasis on the lymphocytes and histiocytes, in 34 lesions representing some pathologies of the two main categories of bullous skin diseases—namely, intraepidermal and subepidermal lesions—using immunoperoxidase staining methods. We used antibodies targeting the antigens for B cells (CD20), T cells (CD3), histiocytes (CD68), T cells with cytotoxic potential (TIA1) and activity (GRB).
Materials and methods
Tissue specimens
Tissue samples fixed in formalin and embedded in paraffin wax were obtained from the Department of Pathology, Assiut University Hospitals, Assiut, Egypt. We obtained 39 specimens: 15 cases of intraepidermal lesions (pemphigus foliaceus, n = 6; pustular psoriasis, n = 2; familial cutaneous peeling, n = 1; pemphigus vulgaris, n = 2; Hailey–Hailey disease, n = 1; Darier's disease, n = 2; and bullous drug eruption, n = 1) and 19 cases of subepidermal lesions (dermatitis herpetiformis, n = 7; bullous pemphigoid, n = 5; chronic bullous disease of childhood, n = 2; epidermolysis bullosa, n = 2; chronic contact dermatitis, n = 1; bullous drug eruption, n = 1; and lichen planus atrophicus, n = 1). The adjacent normal skin and five more cases (normal skin) served as controls. Clinical data obtained from the clinical referral reports included the age and sex of the patient, type of lesions and the site, size and number of these lesions. All patients were Egyptians (Caucasian); no black individual was included in this study. The diagnosis of some of these diseases was confirmed by immunofluorescence when applicable.
Histological evaluation of the inflammatory cell infiltrate
The inflammatory cell infiltrate was evaluated histologically. The following strategy was used:
Inflammatory cell infiltrate was counted in serial sections in at least five different fields
The cells were counted in several sites including dermoepidermal junction, bullae and perivascular areas
Two different observers counted the inflammatory cell infiltrate
The areas of highest density of cells were chosen
The results were expressed as mean (standard error of mean (SEM)).10,11,12
Immunohistochemical evaluation of the inflammatory cell infiltrate
Immunostaining was carried out as described previously.13,14,15 Briefly, sections mounted on glass slides were deparaffinised and rehydrated through graded alcohols to water. Endogeneous peroxidase activity was blocked with 0.6% hydrogen peroxide. Sections were then immersed in the retrieval solution (10 mM sodium citrate buffer, pH 6.0 for CD68, GRB and CD20) and subjected to heat‐induced antigen retrieval for 15 min. The slides, in plastic Coplin jars containing retrieval solution, were microwaved in a microwave set at high (about 750 W) for four cycles of 5 min each. Antigen retrieval of CD3 was performed using trypsin (enzymatic retrieval; 0.062 g trypsin dissolved in 50 ml distilled water for 3 min at 37°C). Non‐specific protein binding was blocked with 10 min exposure to 10% normal goat serum. Sections were then incubated with mouse monoclonal antibodies for 30 min at room temperature (Clones PG‐M1, L26 and UCHL1 for CD68, CD20 and CD3, respectively, Dako Corporation, California, USA). Anti‐TIA1 antibody was provided by Immunotech (Marseilles, France). For TIA1 staining, the slides, in plastic Coplin jars containing retrieval solution (EDTA, pH 8), were microwaved in a microwave set at high (about 600 W) for 2 min, then three cycles of 5 min each at ∼100 W. Non‐specific protein binding was blocked with 10 min exposure to 10% normal goat serum. Sections were then incubated overnight with mouse monoclonal antibodies at 4°C. A secondary staining system (LSAB2, Dako) was used according to the manufacturer's instructions. Sections were then treated with peroxidase‐labelled streptavidin for 30 min at room temperature and incubated with 14‐diaminobenzidine and 0.06% H2O2 for 5 min. They were counterstained with haematoxylin, dehydrated in alcohol, cleared in xylene and cover slipped. The slides were independently evaluated by two observers (MRH and FNA‐Y). Tables 1 and 2 summarise the characteristics of the antibodies used.
Table 1 Antibodies used in the immunohistochemical evaluation of bullous skin lesions.
| Antibody | Specificity | Retrieval | Dilution | Incubation time |
|---|---|---|---|---|
| CD68 | Histiocytes | Citrate buffer, pH 6 | Ready to use | 30 min at 37°C |
| CD3 | Pan T cells | Trypsin | 1:100 | 30 min at 37°C |
| CD20 | Pan B cells | Citrate buffer, pH 6 | 1:200 | Overnight at 4°C |
| TIA1 | Cytotoxic T cells | EDTA | 1:100 | Overnight at 4°C |
| Granzyme B | Cytotoxic T cells | Citrate buffer, pH 6 | 1:25 | 30 min at 37°C |
TIA, T cell intracellular‐associated antigen.
Table 2 Immunohistochemical staining characteristics of the antibodies used in the positive control specimens .
| Antibody | Control tissue (lymph node) | Distribution of staining |
|---|---|---|
| CD68 | Sinusoidal | Cytoplasmic, granular, diffuse |
| CD3 | Paracortical | Membranous |
| CD20 | Follicular | Membranous |
| TIA1 | Paracortical | Cytoplasmic granular, focal and diffuse |
| Granzyme B | Paracortical | Cytoplasmic granular, focal and diffuse |
TIA, T cell intracellular‐associated antigen.
Positive controls
The positive control specimens consisted of lymph nodes with reactive lymphoid hyperplasia (CD68, CD3, CD20, GRB and TIA1).14,15
Negative controls
Additional sections, running in parallel but with omission of the primary antibody served as negative controls.14,15
Evaluation of immunostaining results
For evaluation of the percentage of positive cells with CD3, CD20, CD68, TIA1 and GRB immunostaining, the following rules were followed:
Corresponding sections stained using haematoxylin and eosin were examined along side the immunostained sections.
In each case, the entire section was histologically examined by bright‐field microscope at low magnification to detect the sites of antibody positivity and then at higher magnification to evaluate the immunostaining.
The sections were examined independently by the authors.
The positive staining was evaluated by counting 100 cells in the areas of the highest expression.
CD3 and CD20 signals were identified as a membranous brown rim around the basophilic nucleus. Staining with CD68, TIA1 and GRB gave diffuse and granular cytoplasmic signals.14,15
Table 2 summarises staining features of these antibodies.
Statistical analysis
Analysis of variance and differences were considered statistically significant at p<0.05.
Results
Clinical features of the bullous skin lesions
The study group consisted of 34 patients (14 males and 20 females), mean age 37.1 (SEM 3.5, range 5–70) years. Multiple vesiculobullous lesions with ulceration and itching were the most common presentation in all bullous skin lesions. Table 3 summarises the clinical characteristics of these lesions.
Table 3 Clinical characteristics of the bullous skin lesions.
| Aspects | Intraepidermal lesions n = 15 | Subepidermal lesions n = 19 | p Value |
|---|---|---|---|
| Age (years), mean (SEM) | 35.3 (3.4) | 39.8 (5.5) | NS |
| Sex, n (%) | |||
| Female | 10 (73) | 10 (58) | NS |
| Male | 5 (27) | 9 (42) | <0.05 |
| Duration (months), mean (SEM) | 6.4 (2.5) | 6.8 (3.3) | NS |
| Site, n (%) | |||
| Face | 10 (67) | 4 (21) | <0.05 |
| Trunk | 8 (53) | 17 (89) | <0.05 |
| Upper limb | 7 (47) | 16 (84) | <0.05 |
| Lower limb | 11 (73) | 17 (89) | NS |
| Mucous membranes | 4 (27) | 1 (5 ) | — |
The difference between male and female sex incidence was significant (p>0.05).
Histological evaluation of the inflammatory cell infiltrate in bullous skin lesions
Histological examination of inflammatory cell infiltrate in bullous skin lesions disclosed the following:
Compared with normal skin, there was a significant increase in the total numbers of inflammatory cell infiltrate in patients with bullous skin diseases (p<0).
Cell populations of inflammatory cell infiltrate included lymphocytes, histiocytes, neutrophils, eosinophils and plasma cells.
Neutrophils represented the most predominant cell population, followed by lymphocytes and histiocytes.
Both plasma cells and eosinophils were scarce in these lesions.
The positioning of the inflammatory cell infiltrate differed between the intraepidermal and subepidermal lesions.
Inflammatory cell infiltrates were dense both inside the blisters and in the perivascular areas.
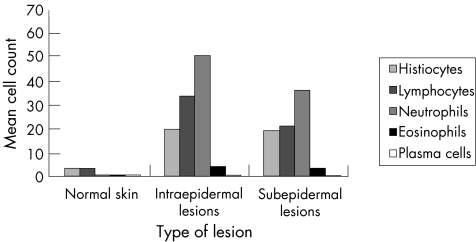
The mean numbers of inflammatory cell infiltrate were significantly higher in the lesional skin than in normal skin (p<0). Compared with normal skin, the total numbers of histiocytes, lymphocytes, neutrophils, eosinophils and plasma cells were significantly higher in each individual lesion. Compared with the non‐tumorigenic keratinocytic hyperproliferative lesions, these differences did not reach the level of statistical significance (p>0.05).13 Moreover, when inflammatory cell infiltrates were compared between intraepidermal and subepidermal lesions, the density of inflammatory cell infiltrates was higher in the intraepidermal lesions. However these differences did not reach significance (p>0.05). Other inflammatory cells (neutrophils, eosinophils and plasma cells) were absent. Figure 1 and tables 4–6 summarise these findings.
Figure 1 Histological evaluation of the inflammatory cell infiltrate in bullous skin lesions. The infiltrate was composed of lymphocytes, histiocytes, neutrophils, eosinophils and plasma cells. Total counts of inflammatory cell infiltrate in intraepidermal lesions were generally higher than those in subepidermal lesions.
Table 4 Histological evaluation of the inflammatory cell infiltrate in bullous skin lesions.
| Normal skin n = 5 | Intraepidermal lesions n = 15 | Subepidermal lesions n = 19 | ||
|---|---|---|---|---|
| Histiocytes | Total | 4.6 (1.4) | 26.8 (5.50)* | 25.7 (3.90)* |
| Epidermis | 0.2 (0.2) | 2.70 (1.00)* | 0.10 (0.10) | |
| Bullae (inside) | 0 (0) | 10.3 (2.40) | 10.2 (3.00) | |
| Bullae (around) | 0 (0) | 1.00 (0.50) | 0.60 (0.30) | |
| DEJ | 0.2 (0.2) | 5.00 (1.00)* | 3.80 (0.70)* | |
| Perivascular | 4.2 (1.5) | 14.1 (3.00)* | 12.5 (2.10)* | |
| Lymphocytes | Total | 4.2 (0.5) | 45.8 (8.60)* | 28.5 (3.90)* |
| Epidermis | 0 (0) | 0 (0) | 0 (0) | |
| Bullae (inside) | 0 (0) | 8.60 (5.40) | 6.00 (1.70) | |
| Bullae (around) | 0 (0) | 0 (0) | 0.70 (0.40) | |
| DEJ | 0 (0) | 5.40 (1.70)* | 1.60 (0.50)* | |
| Perivascular | 4.2 (0.5) | 34.3 (7.00)* | 21.5 (4.00)* | |
| Neutrophils | Total | 0 (0) | 68.7 (20.8)* | 49.2 (21.1)* |
| Epidermis | 0 (0) | 0 (0) | 0 (0) | |
| Bullae (inside) | 0 (0) | 76.3 (21.9) | 29.2 (11.4) | |
| Bullae (around) | 0 (0) | 6.60 (4.40) | 0.10 (0.10) | |
| DEJ | 0 (0) | 1.20 (0.90) | 1.60 (1.20) | |
| Perivascular | 0 (0) | 1.50 (0.60) | 20.5 (12.0)* | |
| Eosinophils | Total | 0 (0) | 5.50 (4.70)* | 4.20 (2.30)* |
| Epidermis | 0 (0) | 0 (0) | 0 (0) | |
| Bullae (inside) | 0 (0) | 2.90 (2.50) | 2.30 (1.40) | |
| Bullae (around) | 0 (0) | 0.50 (0.60) | 0.10 (0.10) | |
| DEJ | 0 (0) | 1.50 (1.50)* | 0.10 (0.10) | |
| Perivascular | 0 (0) | 1.50 (1.20) | 1.90 (1.00) | |
| Plasma cells | Total | 0 (0) | 0.30 (0.20) | 0 (0) |
| Epidermis | 0 (0) | 0 (0) | 0 (0) | |
| Bullae (inside) | 0 (0) | 0 (0) | 0 (0) | |
| Bullae (around) | 0 (0) | 0 (0) | 0 (0) | |
| DEJ | 0 (0) | 0 (0) | 0 (0) | |
| Perivascular | 0 (0) | 0.30 (0.20)* | 0 (0) | |
DEJ, dermoepidermal junction.
Inflammatory cells were counted in three different fields (sections stained with haematoxylin and eosin, 400×). Values are expressed as mean (SEM).
*Significant difference between the lesional tissues compared with normal skin.
Table 5 Histological evaluation of the inflammatory cells in bullous skin lesions with epidermal bullae.
| PF n = 6 | PP n = 2 | FCP n = 1 | PV, HH, DD, BDE n = 6 | ||
|---|---|---|---|---|---|
| Histiocytes | Total | 40.0 (10) | 30.0 (3.0) | 20 | 14.0 (6.20) |
| Epidermis | 3.30 (1.7) | 2.50 (1.5) | 0 | 2.50 (2.50) | |
| Bullae (inside) | 13.3 (4.0) | 6.00 | 0 | 9.30 (2.50) | |
| Bullae (around) | 1.30 (0.7) | 1.00 | 0 | 0 | |
| DEJ | 5.80 (1.9) | 4.50 (0.5) | 2.0 | 4.70 (1.30) | |
| Perivascular | 16.5 (6.1) | 22.0 (0) | 18 | 8.30 (8.50) | |
| Lymphocytes | Total | 51.0 (17) | 61.0 (0.5) | 8.0 | 41.0 (12.0) |
| Epidermis | 0 (0) | 0 (0) | 0 | 0 (0) | |
| Bullae (inside) | 8.30 (4.7) | 0 | 0 | 13.3 (12.3) | |
| Bullae (around) | 0 (0) | 0 | 0 | 0 | |
| DEJ | 5.50 (2.70) | 1.00 (0) | 0 | 8.80 (2.80) | |
| Perivascular | 36.7 (14.0) | 65.5 (0.5) | 8.0 | 25.8 (7.00) | |
| Neutrophils | Total | 86.0 (31.0) | 217 | 0 | 38.0 (24.0) |
| Epidermis | 0 (0) | 0 (0) | 0 | 0 (0) | |
| Bullae (inside) | 87.0 (31.1) | 200 | 0 | 48.3 (27.7) | |
| Bullae (around) | 6.70 (6.70) | 13.0 | 0 | 6.00 | |
| DEJ | 0 (0) | 0.50 (0.5) | 0 | 3.50 (2.90) | |
| Perivascular | 0.50 (0.50) | 1.50 (1.5) | 0 | 2.80 (1.30) | |
| Eosinophils | Total | 0.20 (0.20) | 0.50 (0.5) | 0 | 14.0 (12.0) |
| Epidermis | 0 (0) | 0 (0) | 0 | 0 (0) | |
| Bullae (inside) | 0 (0) | 4.00 | 0 | 9.30 (9.30) | |
| Bullae (around) | 0 (0) | 0 | 0 | 5.00 | |
| DEJ | 0 (0) | 0 | 0 | 5.00 (5.00) | |
| Perivascular | 0.20 (0.20) | 0.50 (0.5) | 0 | 3.50 (2.90) | |
| Plasma cells | Total | 0 (0) | 0 (0 | 0 | 0.70 (0.50) |
| Epidermis | 0 (0) | 0 (0 | 0 | 0 (0) | |
| Bullae (inside) | 0 (0) | 0 | 0 | 0 (0) | |
| Bullae (around) | 0 (0) | 0 | 0 | 0 | |
| DEJ | 0 (0) | 0 (0) | 0 | 0 (0) | |
| Perivascular | 0 (0) | 0 (0) | 0 | 0.70 (0.50) | |
BDE, bullous drug eruption (intradermal type); DD, Darier's disease; DEJ, dermoepidermal junction; FCP, familial cutaneous peeling; HH, Hailey–Hailey disease; PF, pemphigus foliaceus; PP, pustular psoriasis; PV, pemphigus vulgaris.
Inflammatory cells were counted in three different fields (sections stained with haematoxylin and eosin, 400×). Values are expressed as mean (SEM).
Table 6 Histological evaluation of the inflammatory cells in bullous skin lesions with subepidermal bullae.
| DH n = 7 | BP n = 5 | BDE n = 1 | CCD n = 1 | EB n = 2 | CBDC n = 2 | LPA n = 1 | ||
|---|---|---|---|---|---|---|---|---|
| Histiocytes | Total | 36.0 (5.2) | 12.0 (2.00) | 60 | 9.0 | 30.0 (6.00) | 22.0 (9.0) | 3.0 |
| Epidermis | 0.20 (0.2) | 0 (0) | 0 | 0 | 0.50 (0.50) | 0 (0) | 0 | |
| Bullae (inside) | 16.4 (2.9) | 3.00 (1.60) | 51 | 0 | 2.50 (0.50) | 3.50 (0.5) | 0 | |
| Bullae (around) | 0 (0) | 1.00 (0.60) | 0 | 0 | 0 | 3.00 | 0 | |
| DEJ | 3.20 (1) | 3.40 (0.40) | 0 | 5.0 | 6.00 (1.00) | 7.00 (4.0) | 0 | |
| Perivascular | 20.2 (2.7) | 5.40 (1.30) | 9.0 | 4.0 | 20.5 (4.50) | 10.0 (6.0) | 3.0 | |
| Lymphocytes | Total | 28.0 (8) | 19.0 (5.40) | 35 | 39 | 24.0 (19.0) | 36.0 (6.0) | 50 |
| Epidermis | 0 (0) | 0 (0) | 0 | 0 | 0 (0) | 0 (0) | 0 | |
| Bullae (inside) | 4.90 (2.7) | 4.80 (2.80) | 19 | 7.0 | 11.5 (10.5) | 3.50 (3.5) | 0 | |
| Bullae (around) | 0 (0) | 1.80 (1.10) | 0 | 1.0 | 0 | 0 | 0 | |
| DEJ | 1.40 (1.00) | 2.00 (1.00) | 0 | 1.0 | 1.50 (1.50) | 3.50 (1.5) | 0 | |
| Perivascular | 26.3 (9.30) | 10.6 (2.90) | 16 | 30 | 11.0 (7.00) | 29.0 (1.0) | 50 | |
| Neutrophils | Total | 98.0 (51.0) | 18.0 (12.0) | 3.0 | 1.0 | 59.0 (59.0) | 18.0 (18.0) | 0 |
| Epidermis | 0 (0) | 0 (0) | 0 | 0 | 0 (0) | 0 (0) | 0 | |
| Bullae (inside) | 62.6 (25.7) | 3.00 (3.00) | 3.0 | 1.0 | 34.0 (34.0) | 15.0 (15.0) | 0 | |
| Bullae (around) | 0 (0) | 0.40 (0.40) | 0 | 0 | 0 | 0 | 0 | |
| DEJ | 0.40 (0.40) | 0.80 (0.50) | 0 | 0 | 10.0 (10.0) | 1.00 (1.0) | 0 | |
| Perivascular | 41.2 (32.3 | 13.8 (12.6) | 0 | 0 | 30.0 | 1.50 (1.5) | 0 | |
| Eosinophils | Total | 7.30 (6.00) | 5.40 (3.00) | 1.0 | 0 | 0.50 (0.50) | 0 (0) | 0 |
| Epidermis | 0 (0) | 0 (0) | 0 | 0 | 0 (0) | 0 (0) | 0 | |
| Bullae (inside) | 4.40 (3.70) | 2.00 (2.00) | 1.0 | 0 | 0.50 (0.50) | 0 (0) | 0 | |
| Bullae (around) | 0 (0) | 0.40 (0.40) | 0 | 0 | 0 | 0 | 0 | |
| DEJ | 0 (0) | 0.40 (0.20) | 0 | 0 | 0 (0) | 0 (0) | 0 | |
| Perivascular | 3.30 (2.60) | 2.60 (1.30) | 0 | 0 | 0 | 0 (0) | 0 | |
| Plasma cells | Total | 0 (0) | 0 (0) | 0 | 0 | 0 (0) | 0 (0) | 0 |
| Epidermis | 0 (0) | 0 (0) | 0 | 0 | 0 (0) | 0 (0) | 0 | |
| Bullae (inside) | 0 (0) | 0 (0) | 0 | 0 | 0 | 0 (0) | 0 | |
| Bullae (around) | 0 (0) | 0 (0) | 0 | 0 | 0 | 0 | 0 | |
| DEJ | 0 (0) | 0 (0) | 0 | 0 | 0 (0) | 0 (0) | 0 | |
| Perivascular | 0 (0) | 0 (0) | 0 | 0 | 0 | 0 (0) | 0 | |
BDE, bullous drug eruption (with subepidermal bullae); BP, bullous pemphigoid; CBDC, chronic bullous disease of childhood; CCD, chronic contact dermatitis; DEJ, dermoepidermal junction; DH, dermatitis herpetiformis; EB, epidermolysis bullosa..
Inflammatory cells were counted in three different fields (sections stained with haematoxylin and eosin, 400×). Values are expressed as mean (SEM).
Immunohistochemical evaluation of the inflammatory cell infiltrate in bullous skin lesions
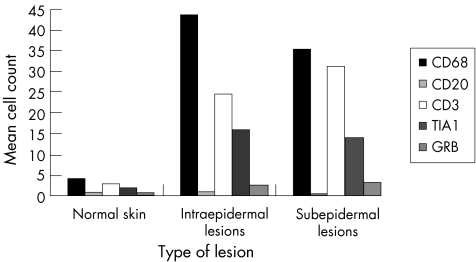
Immunohistological analysis showed that: (1) the inflammatory cell infiltrate was predominantly formed of histiocytes (CD68+ cells) and lymphocytes (CD3+ cells); (2) CD3+ T cells were more predominant than CD20+ B cells; and (3) both resting (TIA1+ cells) and active CTLs (GRB+ cells) were found. In normal skin, CD68+ cells were found around blood vessels and occasionally in the epidermis. The CD68+ cells were the predominant cell population in both intraepidermal and subepidermal bullous lesions. They were found both inside the blister and around blood vessels, with a slight increase around blood vessels. The blisters of intraepidermal lesions contained more CD68+ cells than those of subepidermal lesions. The increased number of CD68+ cells was directly proportional to that of CD3+ cells. CD20+cells were absent in normal skin and were scarce in bullous lesions. TIA1+ CTLs were present in normal skin in a perivascular location. In bullous lesions, they were found in a perivascular location and also inside the blisters. GRB+ cells were found in normal skin around blood vessels. In bullous lesions, GRB+ cells were greater in number than in normal skin, and were positioned mainly around blood vessels. Tables 7–9 and figs 2–4 summarise these findings.
Table 7 Immunohistochemical evaluation of the percentage of positive inflammatory cells in bullous skin lesions.
| Normal skin n = 5 | Intraepidermal lesions n = 15 | Subepidermal lesions n = 19 | ||
|---|---|---|---|---|
| CD68 | Total | 4.0 (1.0) | 43.8 (10.9) | 35.6 (4.1) |
| Epidermis | 0.2 (0.2) | 3.30 (1.20) | 0.20 (0.1) | |
| Bullae (inside) | 0 (0) | 19.6 (7.00) | 11.0 (3.0) | |
| Bullae (around) | 0 (0) | 1.10 (0.50) | 1.00 (0.7) | |
| DEJ | 0.2 (0.2) | 6.10 (1.30) | 4.80 (1.1) | |
| Perivascular | 3.6 (1.2) | 20.6 (3.60) | 20.7 (2.5) | |
| CD20 | Total | 0 (0) | 0.80 (0.50) | 0.40 (0.2) |
| Epidermis | 0 (0) | 0 (0) | 0 (0) | |
| Bullae (inside) | 0 (0) | 0.10 (0.10) | 0.10 (0.1) | |
| Bullae (around) | 0 (0) | 0 (0) | 0 (0) | |
| DEJ | 0 (0) | 0 (0) | 0 (0) | |
| Perivascular | 0 (0) | 1.00 (0.60) | 0.30 (0.2) | |
| CD3 | Total | 3.0 (1.1) | 24.7 (7.40) | 31.3 (5.0) |
| Epidermis | 0 (0) | 0.10 (0.10) | 0 (0) | |
| Bullae (inside) | 0 (0) | 5.50 (4.90) | 4.80 (1.5) | |
| Bullae (around) | 0 (0) | 0.30 (0.20) | 0 (0) | |
| DEJ | 0 (0) | 0.90 (0.30) | 1.10 (0.6) | |
| Perivascular | 3.0 (1.1) | 20.3 (5.30) | 25.5 (4.8) | |
| TIA1 | Total | 1.8 (0.4) | 16.1 (4.70) | 14.1 (3.2) |
| Epidermis | 0 (0) | 0 (0) | 0 (0) | |
| Bullae (inside) | 0 (0) | 4.60 (3.70) | 2.20 (0.6) | |
| Bullae (around) | 0 (0) | 0 (0) | 0.30 (0.2) | |
| DEJ | 0.2 (0.2) | 2.20 (1.10) | 1.20 (0.3) | |
| Perivascular | 1.6 (0.2) | 10.9 (3.70) | 11.3 (3.4) | |
| GRB | Total | 0.6 (0.4) | 2.50 (0.50) | 3.10 (0.9) |
| Epidermis | 0 (0) | 0 (0) | 0 (0) | |
| Bullae (inside) | 0 (0) | 0 (0) | 0.20 (0.1) | |
| Bullae (around) | 0 (0) | 0 (0) | 0 (0) | |
| DEJ | 0 (0) | 0.10 (0.10) | 0.10 (0.1) | |
| Perivascular | 0.6 (0.4)* | 2.40 (0.50)* | — |
DEJ, dermoepidermal junction; GRB, granzyme B; TIA1, T cell intracellular‐associated antigen 1.
The immune cells were counted in five different fields. Values are expressed as mean (SEM).
*Significant difference between values of lesional skin compared with the normal skin (p<0.05).
Table 8 Immunohistological evaluation of the inflammatory cells in bullous skin lesions.
| PF | PP | FCP | PV, HH, DD, BDE | ||
|---|---|---|---|---|---|
| CD68 | Total | 47.0 (14) | 58.5 (31.5) | 30 | 38.0 (22.8) |
| Epidermis | 4.00 (2.0) | 2.50 (1.50) | 0 | 5.00 | |
| Bullae (inside) | 11.2 (4.1) | 45.0 | 0 | 31.7 (19.2) | |
| Bullae (around) | 1.60 (0.7) | 1.00 | 0 | 0 | |
| DEJ | 7.00 (1.8) | 4.50 (0.50) | 2.0 | 7.50 (5.50) | |
| Perivascular | 21.6 (6.0) | 28.5 (6.50) | 28 | 15.0 (6.50) | |
| CD20 | Total | 0.50 (0.3) | 0 (0) | 0 | 1.60 (1.00) |
| Epidermis | 0 (0) | 0 (0) | 0 | 0 (0) | |
| Bullae (inside) | 0.30 (0.3) | 0 (0) | 0 | 0 (0) | |
| Bullae (around) | 0 (0) | 0 (0) | 0 | 0 (0) | |
| DEJ | 0 (0) | 0 (0) | 0 | 0 (0) | |
| Perivascular | 0.50 (0.3) | 0 (0) | 0 | 2.30 (1.40) | |
| CD3 | Total | 18.0 (2.7) | 49.0 (18.0) | 8.0 | 23.6 (15.7) |
| Epidermis | 0.30 (0.3) | 0 (0) | 0 | 0 | |
| Bullae (inside) | 1.00 (0.6) | 0 | 0 | 20.0 (20.0) | |
| Bullae (around) | 0 (0) | 0 | 0 | 1.00 (0) | |
| DEJ | 0.80 (0.5) | 1.50 (0.50) | 0 | 1.00) | |
| Perivascular | 16.0 (3.4) | 47.5 (17.5) | 8.0 | 15.2 (7.30) | |
| TIA1 | Total | 12.0 (4.8) | 21.5 (19.5) | 1.0 | 21.8 (8.40) |
| Epidermis | 0 (0) | 0 (0) | 0 | 0 | |
| Bullae (inside) | 1.80 (1.2) | 0 | 0 | 15.0 (15.0) | |
| Bullae (around) | 0 (0) | 0 | 0 | 0 | |
| DEJ | 3.00 (2.4) | 0.50 (0.50) | 0 | 3.50 (0.50) | |
| Perivascular | 6.80 (3.4) | 21.0 (19.0) | 1.0 | 12.5 (4.60) | |
| GRB | Total | 1.80 (0.5) | 3.00 (2.0) | 3.0 | 2.80 (1.10) |
| Epidermis | 0 (0) | 0 | 0 | 0 | |
| Bullae (inside) | 0 (0) | 0 | 0 | 0 (0) | |
| Bullae (around) | 0 (0) | 0 | 0 | 0 | |
| DEJ | 0 (0) | 0 | 0 | 0.50 (0.50) | |
| Perivascular | 1.80 (0.5) | 3.00 (2.00) | 3.0 | 2.60 (1.10) | |
BDE, bullous drug eruption (intradermal type); DD, Darier's disease; DEJ, dermoepidermal junction; FCP, familial cutaneous peeling; GRB, granzyme B; HH, Hailey–Hailey disease; PF, pemphigus foliaceus; PP, pustular psoriasis; PV, pemphigus vulgaris.
Inflammatory cells were counted in three different fields (sections stained with haematoxylin and eosin, 400×). Values are expressed as mean (SEM).
Immunoreactivity score was evaluated as by other groups12a,13. It was scored as mild, moderate or severe (scores 1, 2 or 3, respectively).
Table 9 Immunohistological evaluation of the inflammatory cells in bullous skin lesions.
| DH | BP | BDE | CCD | EB | CBDC | LPA | ||
|---|---|---|---|---|---|---|---|---|
| CD68 | Total | 42.4 (5.80) | 31.0 (6.8) | 64 | 11 | 29.5 (5.50) | 43.5 (7.5) | 3.0 |
| Epidermis | 0.20 (0.20) | 0.40 (0.4) | 0 | 0 | 0.50 (0.50) | 0 (0) | 0 | |
| Bullae (inside) | 15.9 (3.00) | 5.40 (3.8) | 51 | 0 | 2.50 (0.50) | 7.50 (3.5) | 0 | |
| Bullae (around) | 0 (0) | 1.00 (0.6) | 0 | 0 | 0 | 9.00 | 0 | |
| DEJ | 3.20 (1.00) | 6.60 (2.9) | 0 | 5.0 | 6.00 (1.00) | 11.0 | 0 | |
| Perivascular | 28.2 (3.40) | 17.6 (3.5) | 13 | 6.0 | 20.5 (4.50) | 26.0 (10) | 3.0 | |
| CD20 | Total | 0.40 (0.40) | 0.40 (0.4) | 1.0 | 0 | 0.50 (0.50) | 0 (0) | 0 |
| Epidermis | 0 (0) | 0 (0) | 0 | 0 | 0 (0) | 0 (0) | 0 | |
| Bullae (inside) | 0 (0) | 0 (0) | 1.0 | 0 | 0 (0) | 0 (0) | 0 | |
| Bullae (around) | 0 (0) | 0 (0) | 0 | 0 | 0 | 0 | 0 | |
| DEJ | 0 (0) | 0 (0) | 0 | 0 | 0 (0) | 0 (0) | 0 | |
| Perivascular | 0.50 (0.50) | 0.40 (0.4) | 0 | 0 | 0.50 (0.50) | 0 (0) | 0 | |
| CD3 | Total | 37.3 (12.0) | 19.8 (6.7) | 45 | 27 | 22.5 (18.5) | 37.0 (5.0) | 50 |
| Epidermis | 0 (0) | 0 (0) | 0 | 0 | 0 (0) | 0 (0) | 0 | |
| Bullae (inside) | 2.70 (0.90) | 2.40 (1.3) | 21 | 7.0 | 10.5 (9.50) | 4.50 (2.5) | 0 | |
| Bullae (around) | 0 (0) | 0 (0) | 0 | 0 | 0 | 0 | 0 | |
| DEJ | 0 (0) | 0.60 (0.4) | 0 | 0 | 4.50 (4.50) | 3.50 (1.5) | 0 | |
| Perivascular | 34.7 (11.6) | 16.8 (6.4) | 24 | 20 | 7.50 (4.50) | 29.0 (1.0) | 50 | |
| TIA1 | Total | 16.4 (8.20) | 10.4 (2.6) | 10 | 21 | 7.50 (2.50) | 12.5 (2.5) | 30 |
| Epidermis | 0 (0) | 0 (0) | 0 | 0 | 0 (0) | 0 (0) | 0 | |
| Bullae (inside) | 2.00 (0.80) | 2.00 (0.9) | 8.0 | 4.0 | 1.00 | 1.50 (1.5) | 0 | |
| Bullae (around) | 0 (0) | 0.60 (0.4) | 0 | 1.0 | 0 | 0 | 0 | |
| DEJ | 1.20 (0.60) | 1.20 (0.4) | 0 | 1.0 | 1.50 (1.50) | 2.00 (0) | 0 | |
| Perivascular | 15.8 (9.00) | 6.60 (3.1) | 2.0 | 15 | 5.50 (1.50) | 9.00 (4.0 | 30 | |
| GRB | Total | 3.90 (2.10) | 3.20 (1.7) | 1.0 | 2.0 | 2.00 (2.00) | 1.50 (0.5) | 6.0 |
| Epidermis | 0 (0) | 0 (0) | 0 | 0 | 0 (0) | 0 (0) | 0 | |
| Bullae (inside) | 0.30 (0.20) | 0.20 (0.2) | 0 | 0 | 0 | 0 (0) | 0 | |
| Bullae (around) | 0 (0) | 0 (0) | 0 | 0 | 0 | 0 | 0 | |
| DEJ | 0.20 (0.20) | 0 (0) | 0 | 0 | 0 (0) | 0.50 (0.5) | 0 | |
| Perivascular | 4.00 (2.30) | 3.00 (1.6) | 1.0 | 2.0 | 4.00 | 1.00 (0) | 6.0 | |
BDE, bullous drug eruption (with subepidermal bullae); BP, bullous pemphigoid; CBDC, chronic bullous disease of childhood; CCD, chronic contact dermatitis; DEJ, dermoepidermal junction; DH, dermatitis herpetiformis; EB, epidermolysis bullosa; GRB, granzyme B; TIA1, T cell intracellular‐associated antigen 1.
Inflammatory cells were counted in three different fields (sections stained with haematoxylin and eosin, 400×). Values are expressed as mean (SEM).
Immunoreactivity score was evaluated as by other groups.12a,13 It was scored as mild, moderate or severe (scores 1, 2 or 3, respectively).
Figure 2 Immunohistochemical evaluation of the percentage of positive inflammatory cells in bullous skin lesions. CD68+ and CD3+ cells were the predominant cell populations in both intraepidermal and subepidermal bullous lesions. GRB, granzyme B; TIA1, T cell intracellular‐associated antigen.
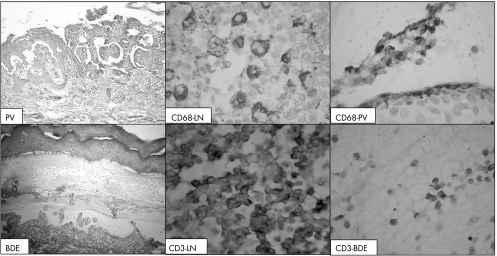
Figure 3 Immunohistochemical staining of T cells (CD3+) and histiocytes (CD68+) in pemphigus vulgaris (PV, upper panel) and bullous drug eruptions (BDE, lower panel). LN, lymph node. The reactivity for CD68 was granular and cytoplasmic, whereas that for CD3 was membranous.
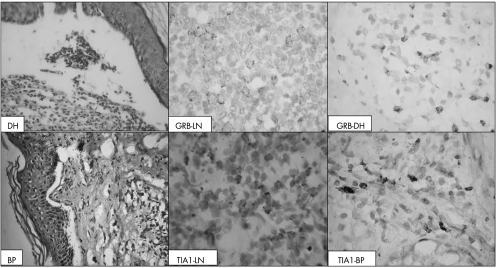
Figure 4 Immunohistochemical staining of cytotoxic T cell granules in dermatitis herpetiformis (DH, upper panel) and bullous pemphigoid (BP, lower panel). GRB, granzyme B; LN, lymph node; TIA1, T cell intracellular associated antigen 1. The reactivity for both GRB+ and TIA1+ cells was cytoplasmic and granular.
The transition from normal skin to lesional skin (lesions with intraepidermal and subepidermal bullae, respectively) was associated with a significant increase (p⩽0.05) in the density of the following:
CD20 B+ lymphocytes: 0 (0) v 0.80 (0.50) v 0.40 (0.2)
CD68+ cells: 4.0 (1.0) v 43.8 (10.9) v 35.6 (4.1)
CD3+ T lymphocytes: 3.0 (1.1) v 24.7 (7.40) v 31.3 (5.0)
TIA1+ resting CTLs: 1.8 (0.4) v 16.1 (4.70) v 14.1 (3.2)
GRB+ T cells with cytotoxic activity: 0.6 (0.4) v 2.50 (0.50) v 3.10 (0.9).
Tables 7–9 and figs 2–4 summarise these findings
Discussion
Bullous skin lesions are diseases characterised by the presence of blisters and inflammatory cell infiltrate. To date, immunophenotypic characterisation of inflammatory cell infiltrate, and particularly CTLs, is still incomplete in these bullous lesions. We hypothesised that that the development of bullous skin diseases is associated with changes in the inflammatory cell infiltrate in response to increased antigenicity of the lesional keratinocytic cells. We carried out this investigation to explore our hypothesis and to examine these issues. Compared with normal skin, the lesional skin showed the following:
An increased density of the inflammatory cell infiltrate
The inflammatory cell infiltrate was predominantly formed of neutrophils, lymphocytes (CD3+ cells) and histiocytes (CD68+ cells)
The density of CD3+ T cells was higher than that of CD20+ B cells
Both resting (TIA1+ cells) and active CTLs (GRB+ cells) were found.
Increased density of inflammatory cell infiltrate in bullous skin lesions compared with normal skin
Analysis of the inflammatory cell infiltrate in normal skin showed the presence of both lymphocytes and histiocytes as the predominant cell populations. Further immunophenotyping of these cells showed them to be exclusively of T cell lineage (CD3+ cells). No neutrophils, eosinophils, plasma cells or B lymphocytes were detected in normal skin. These findings agree with the previous findings and also support the notion that in normal skin, the immune response is essentially a cell‐mediated one.4,5,16
Our finding of increased inflammatory cell infiltrate in the bullous skin lesions is consistent with results from previous studies.17,18,19 This infiltrate consisted of an admixture of both acute (neutrophils, eosinophils and lymphocytes) and chronic (lymphocytes, histiocytes and plasma cells) inflammatory cells, with the acute cells being the predominant cell populations. In some lesions (pustular psoriasis, familial cutaneous peeling, chronic contact dermatitis and lichen planus atrophicus), this admixture of cells showed sharp peaking of one of the inflammatory cell components (neutrophils for pustular psoriasis and lymphocytes for the others). These acute and chronic inflammatory cells were present side by side in variable proportions. The presence of a pattern of inflammatory milieu agrees with the findings of others20 and also reflects the relapsing nature of the disease process in these lesions.21,22,23,24 It is well established that the bullous skin lesions are dynamic ones, with the inflammatory phenomenon spanning a spectrum from mild or moderate to severe (acute) chronic inflammation. The cause of this dynamic property is the cascade of inflammatory events that occur in succession which seems to be the hallmark of these lesions.
From a diagnostic point of view, the positioning of the inflammatory cell infiltrate may be helpful in distinguishing some lesions. For instance, pustular psoriasis is characterised by intense neurophilic infiltrate inside the blister, with minimal neutrophilic infiltrate around the blood vessels. In pemphigus foliaceus, this increase in neutrophils is proportional to lymphocyte increase both inside the blister and around blood vessels. In dermatitis herpetiformis, the infiltrate is mainly composed of neutrophils inside the blister, whereas in bullous pemphigoid blisters, the infiltrate is formed of an admixture of neutrophils, eosinophils, lymphocytes and histiocytes. Neutrophils are essentially minimal or absent in other lesions such as familial cutaneous peeling, chronic contact dermatitis and lichen planus atrophicus.
Increased density of both neutrophils and eosinophils in bullous skin lesions
We found the presence of polymorphs inside the blister to be a constant feature. This finding is in keeping with previous reports.5,17,19,25,26,27,28 The presence of a high density of polymorphs in the blisters may be a complement‐mediated or a chemokine (interleukin 8)‐mediated recruitment.4,29,30 We propose that polymorphs have a role in the intensity of the inflammatory phenomenon in the bullous skin lesions.31 The presence of eosinophilic infiltrate in the bullous skin lesions is in line with other studies.23,32,33,34 This infiltrate may be a result of increased eosinophil chemotaxis by complement components29,35 and eotaxin (chemokine).36 Eotaxin, a chemokine secreted by keratinocytes and phagocytes, can bind to chemokine receptors found on T helper (Th)2 cells, eosinophils and basophils.18 On the immunological level, the high density of neutrophils and eosinophils may reflect ongoing activation of both Th1 and Th2 immunity.5,16,17,19,27,37
Predominance of CD3+ T lymphocytes and CD68+ histiocytes in bullous skin lesions
The high density of CD3+ and CD68+ cells in the bullous skin lesions as well as their perivascular location is in line with previous reports.5,17,27,38,39,40 The numerical dominance of these cells strongly suggests their critical role in the development, exacerbation and remission of these lesions. The direct association (position) between CD3+ T cells and CD68+ macrophages reflects the close interaction between them in execution of the immune response. In this respect, the presence of CD68+ macrophages is essential for uptake, processing and presentation of the antigenic epitopes associated with the major histocompatibility complex class II to the CD3+ T cells.41,42,43,44,45 The increased density of CD3+ cells may be due to: (1) increased antigenicity of the lesional cells; (2) production of soluble factors (chemokines)17,19,23,46; (3) increased expression of adhesion molecules35,47,48; and (4) increased tissue accessibility for immune cells. Several antigens were reported in the bullous skin lesions, such as bullous pemphigoid antigen 2 (bullous pemphigoid),49 130‐kDa polypeptide pemphigus vulgaris29 and cutaneous lymphocyte‐associated antigen.40
Expression of cytotoxic molecules in the bullous skin lesions
This study is the first to report the presence of a high density of TIA1+ and GRB+ cells in bullous skin lesions. GRB, a cytotoxic molecule present in natural killer cells and active CTLs, can induce apoptosis in target cells. TIA1, a cytotoxic molecule that induces apoptosis in target cells, is present in the granules of CTLs and natural killer cells. The antibody used to target TIA1 antigen can decorate these granules in CTLs, natural killer cells, neutrophils, eosinophils and mast cells. Accordingly, the increased density of TIA1+ cells may not merely reflect the presence of CTLs but also the presence of other inflammatory cell infiltrate. The presence of both TIA1+ and GRB+ cells suggests the presence of both resting and active CTLs in these lesions. On the other hand, it is tempting to speculate that these cells have a role in blister formation by inducing apoptosis of keratinocytes. The ratio of GRB+ to total CD3+ cells in the bullous skin lesions was 3–10%. These findings agree with those of other studies5 reporting that CD8+ cells comprise 10% of the total T lymphocyte population in pemphigus vulgaris and bullous pemphigoid. The low density of GRB+ cells inside the blister may be a reason for their rapid degradation.5 The acantholytic cells had condensed eosinophilic cytoplasm and pyknotic nuclei, a picture resembling that of apoptotic cells. Further investigations are required to examine apoptosis in the bullous skin lesions. The low density of GRB+ cells inside the blister may be a reason for the rapid degranulation of cells containing this molecule.
High density of CD68+ cells in bullous skin lesions
The increased density of CD68+ cells in these lesions agrees with that in other reports.50 The lack of specific CD68+ cell positioning pattern (site distribution) in these lesions reflects their ubiquitous role as antigen‐presenting cells. Accordingly, their presence is essential as a sentinel for receiving the danger signals.12 The increased staining intensity for CD68+ cells may reflect their increased activity and also their contents of lysosomes. The numerical dominance of CD68+ cells in our study may be due to the broad expression of CD68 antigen in other cell types, including dendritic cells40,50,51,52 and fibroblasts. It is well known that CD68+ cells are scarce in the epidermis of normal skin. The marked increase in CD68+ histiocytes or dendritic cells in the epidermis and dermis of the bullous skin lesions is in line with previous findings in other skin dermatoses.40 Therefore, the presence of CD68+ signals in the epidermis of both normal (occasional) and lesional (frequent) skin may reflect the presence of dendritic cells or histiocytes. It is tempting to speculate that the CD68+ cells have a role in the evolution of these lesions, and also that these cells may be a prerequisite for the initiation of these pathologies. Should the second possibility be the case, then targeting these cells, by immunotherapy, may help abort the development of these blistering lesions.
CD20+ B lymphocytes are scarce in bullous skin lesions
In our series, the scarcity of both CD20+ B lymphocytes and plasma cells is in line with previous studies.4,5,53 Ambach et al53 examined 21 samples of bullous pemphigoid and 11 controls (five normal skin and six eczematous skin) and showed the absence of B lymphocytes, suggesting that autoantibodies alone are not capable of inducing blister formation. Schaller et al examined 15 specimens with bullous pemphigoid and five with pemphigus vulgaris using CD19 and CD21 (markers of B cells), and reported a scarce B lymphocyte cell population in these lesions.4,5 These findings suggest that CD20+ B lymphocytes are recruited to the site of inflammation, but once activated, they migrate to the bone marrow where they secrete antibodies that rapidly transit via the blood to the site of inflammation.
Take-home messages
Inflammatory cell infiltrate is a constant feature in the bullous skin lesions.
Inflammatory cell infiltrate in bullous skin lesions is predominantly composed of neutrophils, lymphocytes and histiocytes.
Both CD3+T‐ lymphocytes and CD68+ histiocytes achieved numerical dominance in the bullous skin lesions.
Both resting (TIA‐1+) and active (Granzyme‐B+) cytotoxic T‐lymphocytes are found in the infiltrate of bullous skin lesions.
Conclusion
Our investigation reports that the development of bullous skin lesions is associated with changes in the inflammatory cell infiltrate. We found a considerable increase in the counts of inflammatory cell infiltrate with transitions from normal skin to the lesional one, suggesting increased antigenicity of the lesional cells. Although both CD20 (humoral immunity) and CD3 (cell‐mediated immunity) were present in these lesions, CD3 was more prevalent. Also, relatively large numbers of T lymphocytes (CD3+ cells) had cytotoxic activity. The possible pathogenetic and prognostic ramifications of our findings are open for further investigations.
Abbreviations
CTL - cytotoxic T lymphocyte
GRB - granzyme B
TIA - T cell intracellular‐associated antigen
Footnotes
Competing interests: None declared.
References
- 1.Benbow M. Bullous skin disorders. Nurs Times 19969266–68. [PubMed] [Google Scholar]
- 2.Barnadas M A, Gelpi C, Curell R.et al Repeat direct immunofluorescence test, using, 1 M NaCl treated skin, in the subepidermal autoimmune bullous diseases that contain IgG at the dermal epidermal junction. J Cutan Pathol 19992637–41. [DOI] [PubMed] [Google Scholar]
- 3.Barnadas M A, Gonzalez M J, Planaguma M.et al Clinical, histopathologic, and therapeutic aspects of subepidermal autoimmune bullous diseases with IgG on the floor of salt‐split skin. Int J Dermatol 200140268–272. [DOI] [PubMed] [Google Scholar]
- 4.Schaerli P, Britschgi M, Keller M.et al Characterization of human T cells that regulate neutrophilic skin inflammation. J Immunol 20041732151–2158. [DOI] [PubMed] [Google Scholar]
- 5.Schaller J, Niedecken H W, Biwer E.et al Characterization of lymphocytic infiltrate cells in bullous pemphigoid and pemphigus vulgaris. Dermatol Monatsschr 1990176661–668. [PubMed] [Google Scholar]
- 6.Diaz J, Tubbs R, Stoler M.et al Cytolytic (TIA‐1+) tumor infiltrating lymphocytes in B cell non‐Hodgkin's lymphomas. SWOG Central Repository Members. Leuk Lymphoma 1993991–94. [DOI] [PubMed] [Google Scholar]
- 7.Taupin J L, Tian Q, Kedersha N.et al The RNA‐binding protein TIAR is translocated from the nucleus to the cytoplasm during Fas‐mediated apoptotic cell death. Proc Natl Acad Sci USA 1995921629–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amsterdam A, Keren‐Tal I, Aharoni D.et al Steroidogenesis and apoptosis in the mammalian ovary. Steroids 200368861–867. [DOI] [PubMed] [Google Scholar]
- 9.Amsterdam A, Sasson R, Keren‐Tal I.et al Alternative pathways of ovarian apoptosis: death for life. Biochem Pharmacol 2003661355–1362. [DOI] [PubMed] [Google Scholar]
- 10.Davidorf F H, Lang J R. Lymphocytic infiltration in choroidal melanoma and its prognostic significance. Trans Ophthalmol Soc UK 197797394–401. [PubMed] [Google Scholar]
- 11.Saito T, Tanaka R, Kouno M.et al Immunohistological analysis of infiltrating lymphocyte subpopulations in gliomas and metastatic brain tumors. No To Shinkei 198739339–345. [PubMed] [Google Scholar]
- 12.Hussein M R. Dendritic cells and melanoma tumorigenesis: an insight. Cancer Biol Ther 20054501–505. [DOI] [PubMed] [Google Scholar]
- 12a.Chan W Y, Cheung K</initia<initial>K, Scharge J O.et al Bcl‐2 and p53 protein expression, apoptosis, and p53 mutation in human epithelial ovarian cancers. Am J Pathol 2000156409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussein M R, Al‐Badaiwy Z H, Guirguis M N. Analysis of p53 and bcl‐2 protein expression in the non‐tumorigenic, pretumorigenic, and tumorigenic keratinocytic hyperproliferative lesions. J Cutan Pathol 200431643–651. [DOI] [PubMed] [Google Scholar]
- 14.Hussein M R, Hassan H I, Hofny E R.et al Alterations of mononuclear inflammatory cells, CD4/CD8+ T cells, interleukin 1beta, and tumour necrosis factor alpha in the bronchoalveolar lavage fluid, peripheral blood, and skin of patients with systemic sclerosis. J Clin Pathol 200558178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hussein M R, Al‐Sabae T M, Georgis M N. Analysis of the Bcl‐2 and p53 protein expression in the lymphoproliferative lesions in the Upper Egypt. Cancer Biol Ther200512305–358. [DOI] [PubMed] [Google Scholar]
- 16.Hunyadi J, Szabo I, Simon M., Jr Skin as an immune system. Orv Hetil 19941352749–2754. [PubMed] [Google Scholar]
- 17.Caproni M, Giomi B, Cardinali C.et al Further support for a role for Th2‐like cytokines in blister formation of pemphigus. Clin Immunol 200198264–271. [DOI] [PubMed] [Google Scholar]
- 18.Frezzolini A, Teofoli P, Cianchini G.et al Increased expression of eotaxin and its specific receptor CCR3 in bullous pemphigoid. Eur J Dermatol 20021227–31. [PubMed] [Google Scholar]
- 19.Fabbri P, Caproni M, Berti S.et al The role of T lymphocytes and cytokines in the pathogenesis of pemphigoid gestationis. Br J Dermatol 20031481141–1148. [DOI] [PubMed] [Google Scholar]
- 20.Heiligenhaus A, Schaller J, Mauss S.et al Eosinophil granule proteins expressed in ocular cicatricial pemphigoid. Br J Ophthalmol 199882312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuhns D B, DeCarlo E, Hawk D M.et al Dynamics of the cellular and humoral components of the inflammatory response elicited in skin blisters in humans. J Clin Invest 1992891734–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giacalone B, D'Auria L, Ferraro C.et al Bullous pemphigoid blisters of the same duration have similar cytokine concentrations which decrease in older blisters. Br J Dermatol 1998139158–159. [DOI] [PubMed] [Google Scholar]
- 23.Rico M J, Benning C, Weingart E S.et al Characterization of skin cytokines in bullous pemphigoid and pemphigus vulgaris. Br J Dermatol 19991401079–1086. [DOI] [PubMed] [Google Scholar]
- 24.Sun C C, Wu J, Wong T T.et al High levels of interleukin‐8, soluble CD4 and soluble CD8 in bullous pemphigoid blister fluid. The relationship between local cytokine production and lesional T‐cell activities. Br J Dermatol 20001431235–1240. [DOI] [PubMed] [Google Scholar]
- 25.Takemiya M, Shiraishi S, Miki Y. A vegetating variety of pemphigus foliaceus. Dermatologica 1990180102–105. [DOI] [PubMed] [Google Scholar]
- 26.Hoss D M, Shea C R, Grant‐Kels J M. Neutrophilic spongiosis in pemphigus. Arch Dermatol 1996132315–318. [PubMed] [Google Scholar]
- 27.Feliciani C, Toto P, Mohammad Pour S.et al A Th2‐like cytokine response is involved in bullous pemphigoid. The role of IL‐4 and IL‐5 in the pathogenesis of the disease. Int J Immunopathol Pharmacol 19991255–61. [PubMed] [Google Scholar]
- 28.Pappalardo E, Abramo F, Noli C. Pemphigus foliaceus in a goat. Vet Dermatol 200213331–336. [DOI] [PubMed] [Google Scholar]
- 29.Hashizume H, Iwatsuki K, Takigawa M. Epidermal antigens and complement‐binding anti‐intercellular antibodies in pemphigus vegetans, Hallopeau type. Br J Dermatol 1993129739–743. [DOI] [PubMed] [Google Scholar]
- 30.O'Toole E A, Mak L L, Guitart J.et al Induction of keratinocyte IL‐8 expression and secretion by IgG autoantibodies as a novel mechanism of epidermal neutrophil recruitment in a pemphigus variant. Clin Exp Immunol 2000119217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hietanen J. Acantholytic cells in pemphigus. A scanning and transmission electron microscopic study. Acta Odontol Scand 198240257–273. [DOI] [PubMed] [Google Scholar]
- 32.Buslau M, Marsch W C. Pemphigus herpetiformis—the value of simple diagnostic measures. Z Hautkr 198964729–30, 356. [PubMed] [Google Scholar]
- 33.Muramatsu T, Iida T, Honoki K.et al Pemphigus vulgaris preceded by herpetiform‐like skin lesions with negative immunofluorescence findings. J Dermatol 199926154–159. [DOI] [PubMed] [Google Scholar]
- 34.Muramatsu T, Ko T, Honoki K.et al Intraepidermal expression of basement membrane components in the lesional skin of a patient with dystrophic epidermolysis bullosa. J Dermatol 199926106–110. [DOI] [PubMed] [Google Scholar]
- 35.Lin M S, Mascaro J M, Jr, Liu Z.et al The desmosome and hemidesmosome in cutaneous autoimmunity. Clin Exp Immunol 1997107(Suppl 1)9–15. [PubMed] [Google Scholar]
- 36. Talanin NY, Shelley WB, Shelley ED. IgE bullous disease. Clin Exp Dermatol 19972282–86. [PubMed] [Google Scholar]
- 37.Veldman C M, Gebhard K L, Uter W.et al T cell recognition of desmoglein 3 peptides in patients with pemphigus vulgaris and healthy individuals. J Immunol 20041723883–3892. [DOI] [PubMed] [Google Scholar]
- 38.Zillikens D, Ambach A, Zentner A.et al Evidence for cell‐mediated immune mechanisms in the pathology of pemphigus. Br J Dermatol 1993128636–643. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt E, Bastian B, Dummer R.et al Detection of elevated levels of IL‐4, IL‐6, and IL‐10 in blister fluid of bullous pemphigoid. Arch Dermatol Res 1996288353–357. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura K, Yasaka N, Asahina A.et al Increased numbers of CD68 antigen positive dendritic epidermal cells and upregulation of CLA (cutaneous lymphocyte‐associated antigen) expression on these cells in various skin diseases. J Dermatol Sci 199818170–180. [DOI] [PubMed] [Google Scholar]
- 41.Nepom G T, Erlich H. MHC class‐II molecules and autoimmunity. Annu Rev Immunol 19919493–525. [DOI] [PubMed] [Google Scholar]
- 42.Day M J. Expression of major histocompatibility complex class II molecules by dermal inflammatory cells, epidermal Langerhans cells and keratinocytes in canine dermatological disease. J Comp Pathol 1996115317–326. [DOI] [PubMed] [Google Scholar]
- 43.Miyagawa S, Amagai M, Iida T.et al Late development of antidesmoglein 1 antibodies in pemphigus vulgaris: correlation with disease progression. Br J Dermatol 19991411084–1087. [DOI] [PubMed] [Google Scholar]
- 44.Hertl M, Veldman C. T‐cellular autoimmunity against desmogleins in pemphigus, an autoantibody‐mediated bullous disorder of the skin. Autoimmun Rev 20032278–283. [DOI] [PubMed] [Google Scholar]
- 45.Loewenthal R, Slomov Y, Gonzalez‐Escribano M F.et al Common ancestral origin of pemphigus vulgaris in Jews and Spaniards: a study using microsatellite markers. Tissue Antigens 200463326–334. [DOI] [PubMed] [Google Scholar]
- 46.D'Auria L, Pimpinelli F, Ferraro C.et al Relationship between theoretical molecular weight and blister fluid/serum ratio of cytokines and five other molecules evaluated in patients with bullous pemphigoid. J Biol Regul Homeost Agents 19981276–80. [PubMed] [Google Scholar]
- 47.Miyagawa S, Amagai M, Niizeki H.et al HLA‐DRB1 polymorphisms and autoimmune responses to desmogleins in Japanese patients with pemphigus. Tissue Antigens 199954333–340. [DOI] [PubMed] [Google Scholar]
- 48.Tenaud I, Leroy S, Chebassier N.et al Modulation in vitro of keratinocyte integrins by interferon‐alpha and interferon‐gamma. Int J Dermatol 200241836–840. [DOI] [PubMed] [Google Scholar]
- 49.Budinger L, Borradori L, Yee C.et al Identification and characterization of autoreactive T cell responses to bullous pemphigoid antigen 2 in patients and healthy controls. J Clin Invest 19981022082–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petzelbauer P, Fodinger D, Rappersberger K.et al CD68 positive epidermal dendritic cells. J Invest Dermatol 1993101256–261. [DOI] [PubMed] [Google Scholar]
- 51.Djemadji‐Oudjiel N, Goerdt S, Kodelja V.et al Immunohistochemical identification of type II alternatively activated dendritic macrophages (RM 3/1+3, MS‐1+/−, 25F9−) in psoriatic dermis. Arch Dermatol Res 1996288757–764. [DOI] [PubMed] [Google Scholar]
- 52.Emile J F, Fraitag S, Leborgne M.et al Langerhans' cell histiocytosis cells are activated Langerhans cells. J Pathol 199417471–76. [DOI] [PubMed] [Google Scholar]
- 53.Ambach A, Zillikens D, Klingert B.et al Immune phenotyping of mononuclear infiltrate in bullous pemphigoid. Hautarzt 19924381–85. [PubMed] [Google Scholar]