Abstract
Over the past two decades, major advances have been made in the understanding of the immune system and disease pathogenesis. This has coincided with the development of biologic therapies—monoclonal antibodies and fusion proteins. The decision of when to use such treatment in the clinic is not always straightforward. In addition to immune biology, the focus of this review will be on the application of these treatments to immune‐mediated diseases and the molecular targets involved in pathogenesis, specifically those that have US Food and Drug Administration/European Medicines Agency approval. Brief comments will be made on biologics that have approval for non‐immune disorders.
An underlying immune pathogenesis has been identified for a wide spectrum of diseases. Those in which specific immune components have a central role lend themselves to targeted therapy. The aim of such treatment is to modify the disease course when applied early, limit the complications of conventional treatment and provide options for otherwise treatment‐refractory disease. Biologic therapies, both monoclonal antibodies and fusion proteins, incorporate specific recognition components with antibody function.
The immune system
The immune system is designed to protect the host against infection. It can be divided into innate and adaptive components. Innate immunity includes all those features that are constitutive and ready to act immediately on exposure to pathogens. It is not antigen specific, and the response is the same on repeat exposure to pathogens.
By contrast, lymphocyte‐driven adaptive immunity has both specificity and memory as central features. Each lymphocyte clone carries a unique receptor on the cell surface for interaction with the antigen. Specific antigen recognition is required before activation, proliferation and differentiation into effector or memory cells. B lymphocytes are able to recognise native antigens, whereas T cells require antigen processing.
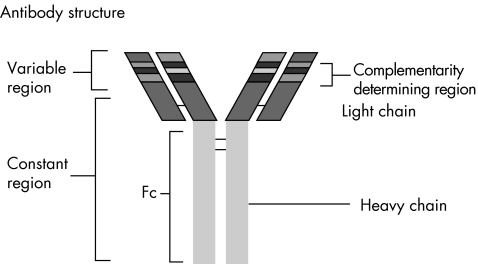
After activation, B cells proliferate and differentiate into antibody‐secreting plasma cells. Each B cell clone produces an antibody with a single antigen‐binding specificity—that is, a monoclonal antibody. It is this antigen specificity that is exploited in the generation of biologic therapy. Figure 1 shows the antibody structure. Each antibody consists of two identical heavy and light chains, composed of variable and constant regions. In the variable domain, the amino acid composition of the complementarity‐determining regions (CDRs) determines the antigen‐binding specificity. Variations in the amino acid sequence at this site lead to the huge diversity of antibodies that can be produced. The constant domain (Fc) determines the antibody function, which can include complement activation, immune complex clearance and antibody‐dependent cellular cytotoxicity. Antibody‐dependent cellular cytotoxicity is a potent mechanism by which cellular targets labelled with therapeutic monoclonal antibodies undergo lysis.
Figure 1 Antibody structure: antibodies consist of two identical heavy and light chains, arranged as a Y‐shaped tetramer. Each chain is arranged into variable and constant regions. The variable regions are the sites of antigen binding, the amino acid sequence of the complementarity‐determining regions at this site confers the antigen‐binding specificity. The heavy chain constant region determines the antibody function.
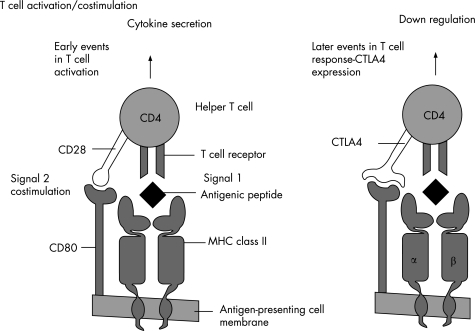
T cells require antigen to be taken up by an antigen‐presenting cell, processed and presented in the context of a self‐major histocompatibility complex molecule. Naive T cells also require a second “costimulatory” signal for full activation (fig 2). Absence of this second signal leads to T cell anergy, a state of unresponsiveness.
Figure 2 T cell activation: for full activation, T cells require interaction between the antigen‐specific T cell receptor and the antigenic peptide presented in the context of self major histocompatibility complex (MHC) by the antigen‐presenting cell. A “costimulatory” second signal is then required, delivered by the interaction between cluster of differentiation (CD)28 on the T cell with CD80/86 on the antigen‐presenting cell. Later, the T cell expresses CTLA4, which has higher affinity for CD80/86, and delivers a down‐regulatory signal, thereby “switching off” the immune response when the pathogen is cleared. Fusion proteins based on the CTLA4 structure have been developed to block the costimulatory pathway, for use in autoimmunity and transplant rejection (fig 4).
Lymphocytes express a variety of surface glycoproteins—for example, T cells express CD4 or CD8, separating them largely into cytokine‐secreting “helper” cells or cytotoxic cells, respectively. In addition, they express adhesion molecules, which change during their progression from naive to effector cells. These molecules direct the lymphocytes to the relevant tissue. The homing mechanism is again specific, with certain adhesion molecules directing specific lymphocytes to certain sites—for example, α4β7 integrins directing gut‐specific lymphocytes.
There are many potential targets for the therapeutic manipulation of immune‐based disease—for example, a specific cytokine, an activation pathway, costimulatory pathway or adhesion molecule.
Monoclonal antibodies and fusion proteins
The development of hybridoma technology by Köhler and Milstein in the early 1970s1 set the scene for the development of monoclonal antibody therapeutics. They were awarded the Nobel Prize for Medicine in 1984,2 and cannot have anticipated the enormous impact their work would have.
Conventional hybridomas were developed by the fusion of B cells from immunised mice with murine myeloma cell lines. The immortalised clones could then be selected for those producing the antibody of interest, and antibody produced in unlimited quantities. Despite early interest, it took almost another 10 years before therapeutic monoclonal antibodies reached the clinic.
The development of human anti‐mouse antibodies was an important limiting factor for some of the early murine treatments. Advances in technology have opened new perspectives for the selection and production of monoclonal antibodies.
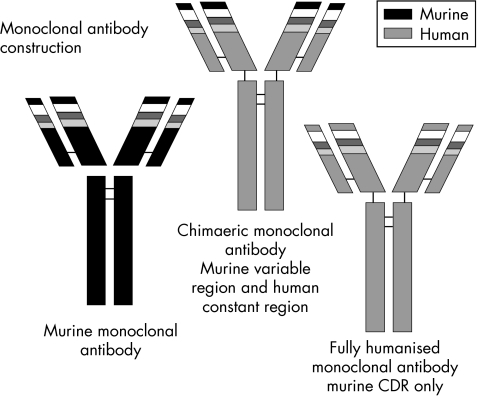
Genetic manipulation allows the combination of genes from different B lymphocyte sources. Chimaeric antibodies are derived from the combination of murine variable‐region genetic material with human constant‐region genes. Fully humanised antibodies are derived from the combination of murine CDR genetic material with the remainder of the antibody of human origin, and finally, fully human monoclonal antibodies are derived entirely from human sources—for example, transgenic mice or phage display (fig 4).3 The advantages of humanising the preparations include improved interaction with human Fc receptors on effector cells and hence improved effector function and reduced immunogenicity, so prolonging the therapeutic half life and reducing adverse reactions particularly on repeat exposure. The selection of a specific Fc component allows the function of the antibody to be determined—for example, target cell lysis versus inhibition of a cell surface molecule. Monoclonal antibodies can be further modified—for example, labelled with toxins—for use in cancer immunotherapy. Table 1 sets out biologic nomenclature.
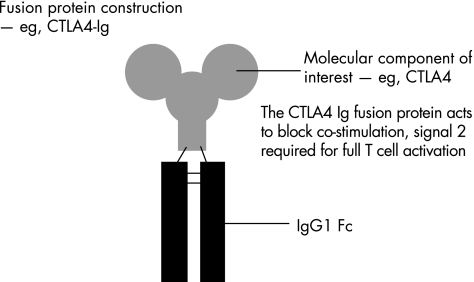
Figure 4 Fusion protein construction: combination of the molecular component of interest with the constant region (Fc) of an antibody molecule, usually immunoglobulin (Ig)G1 Fc, imparts the Fc function on to the molecular component for therapeutic use. The example given is of CTLA4‐Ig, derivatives of which have shown clinical efficacy in the treatment of rheumatoid arthritis and transplant rejection (see text).
Table 1 Nomenclature of monoclonal antibodies.
| Prefix | Disease or target class | Animal source | Suffix |
|---|---|---|---|
| To create a unique name, a distinct syllable is selected as the prefix | Viral:vir‐ | The following letters identify the animal sourceu: human | ‐mab is used both for monoclonal antibodies and fragments |
| Bacterial: bac‐ | |||
| Immune: lim‐ | |||
| Infectious lesions: les‐ | o: mouse | ||
| Cardiovascular: cir‐ | a: rat | ||
| Tumours | e: hamster | ||
| Colon: col‐ | i: primate | ||
| Melanoma: mel‐ | xi: chimaeric | ||
| Mammary: mar‐ | zu: humanised | ||
| Testis: got‐ | |||
| Ovary: gov‐ | |||
| Prostate: pr(o)‐ | |||
| Miscellaneous: tum‐ |
The nomenclature of monoclonal antibodies is based on the following convention:
Prefix‐disease or target class‐animal source‐suffix.
When combining the target or disease infix with the source stem for chimaeric monoclonal antibodies, the last consonant of the target disease syllable is dropped—for example, abciximab; target = cir, source = xi, ‐mab stem.
Fusion protein nomenclature includes the suffix ‐cept
Engineering the Fc region of a human antibody molecule, usually immunoglobulin (Ig)G1, on to a specific molecular component, generates a fusion protein. This integrates the antibody effector function into the molecular component of interest (fig 3). An example is etanercept, a recombinant fusion protein of human tumour necrosis factor (TNF) receptor 2 with human IgG1 Fc.
Figure 3 Monoclonal antibody construction: the different components of the antibody structure can be derived from different genetic sources of B cells. Chimaeric monoclonal antibodies are derived from murine variable‐region genetic sequences combined with constant‐region genes of human origin. Fully humanised monoclonal antibodies are composed of murine complementarity‐determining regions (CDRs), the rest of the antibody being of human genetic origin. Fully human monoclonal antibodies are produced from entirely human genetic sequences.
Immunological targets of approved biologic therapy
Tumour necrosis factor α
TNFα is a pro‐inflammatory cytokine that has an important role in inflammatory disease. In Crohn's disease and rheumatoid arthritis, for example, anti‐TNFα therapies have become well established as an effective treatment option. Three biological anti‐TNFα agents are approved for clinical use: two monoclonal antibodies, infliximab and adalimumab, and the fusion protein etanercept.
In Crohn's disease, randomised controlled trials of infliximab showed clinical benefit in patients with moderate to severely active intestinal disease,4 some of whom were resistant to conventional treatments. Infliximab has also shown efficacy in the treatment of enterocutaneous fistulas complicating Crohn's disease.5 Those with fistulising Crohn's disease having a response to induction therapy have an increased likelihood of sustained response over a 54‐week period if infliximab is repeated every 8 weeks.6 Infliximab is now licensed for patients whose disease is resistant to conventional treatments. The Active Ulcerative Colitis Trials 1 and 2 (ACT 1 and 2, respectively) each studied 364 adult patients with moderate to severe active ulcerative colitis, evaluating the efficacy of infliximab for induction and maintenance therapy. Patients were followed up for 54 weeks in the ACT 1 and 30 weeks in the ACT 2. In both studies, patients receiving infliximab were more likely to have a clinical response than those receiving placebo.7 Infliximab is not yet approved for use in ulcerative colitis.
In 1997, a 3‐month trial of etanercept in rheumatoid arthritis reported that etanercept was safe, well tolerated, associated with improvement in the inflammatory symptoms and that therapeutic effects were dose related.8 The combination of etanercept with methotrexate has been shown to be safe, well tolerated and efficacious in patients with persistently active rheumatoid arthritis, and provides considerably greater efficacy than methotrexate alone.9 In a trial of 632 patients with early active rheumatoid arthritis, etanercept acted more rapidly to decrease symptoms and slow joint damage as compared with oral methotrexate.10 Etanercept is now licensed to use alone or in combination with methotrexate in patients with moderate to severely active rheumatoid arthritis, including those not previously treated with methotrexate. In patients with persistently active rheumatoid arthritis despite methotrexate treatment, repeated doses of infliximab at 4–8‐week intervals in combination with methotrexate provided clinical benefit and halted the progression of joint damage.11 Infliximab is licensed for use in conjunction with methotrexate. In view of the cost implications, National Institute for Clinical Excellence (NICE) issued guidance on the use of these agents for rheumatoid arthritis in 200212 that they should be used only if adult patients with active disease have failed at least two standard disease‐modifying anti‐rheumatic drugs including methotrexate (unless contraindicated). Adalimumab, a fully human monoclonal antibody, is also licensed and can be used as monotherapy in cases of methotrexate intolerance or when continued methotrexate is inappropriate.
Anti‐TNFα therapies are licensed when conventional treatment has proved inadequate or poorly tolerated for the treatment of active juvenile chronic arthritis, and in adults with active psoriatic arthritis, ankylosing spondylitis and psoriasis, supporting TNFα as a pathogenic cytokine in these disorders (table 2).
Table 2 US Food and Drug Administration‐approved/European Medicines Agency‐authorised therapeutic biologicals.
| Biological (trade name) | Biological type | Molecular target | Current summary of product characteristics indications |
|---|---|---|---|
| Adalimumab (Humira) | Recombinant human IgG1 monoclonal antibody | TNFα | Rheumatoid arthritis |
| In combination with methotrexate for | |||
| (a) Treatment of moderate to severe active RA in adults when the response to DMARDs including methotrexate has been inadequate | |||
| (b) Treatment of severe, active and progressive RA in adults not previously treated with methotrexate | |||
| Can be given as monotherapy in cases of methotrexate intolerance or when continued methotrexate is inappropriate | |||
| Psoriatic arthritis | |||
| Treatment of active, progressive psoriatic arthritis in adults when response to previous DMARDs has been inadequate | |||
| Etanercept (Enbrel) | Recombinant fusion protein of human TNF receptor 2 with IgG1 Fc | TNFα | Rheumatoid arthritis |
| (a) Alone or in combination with methotrexate for the treatment of active RA in adults when the response to DMARDs including methotrexate has been inadequate | |||
| (b) Treatment of severe, active and progressive RA in adults not previously treated with methotrexate | |||
| Juvenile chronic arthritis | |||
| Treatment of active poly‐articular course JCA in children aged 4–17 years who have had an inadequate response to, or are intolerant of methotrexate | |||
| Psoriatic arthritis | |||
| Treatment of active, progressive psoriatic arthritis in adults with inadequate response to prior DMARDs | |||
| Ankylosing spondylitis | |||
| Treatment of adults with severe active ankylosing spondylitis who have had an inadequate response to conventional treatment | |||
| Psoriasis | |||
| Treatment of adults with moderate to severe plaque psoriasis who failed to respond to, or who have a contraindication to, or are intolerant to other systemic therapy including ciclosporin, methotrexate or PUVA | |||
| Infliximab (Remicade) | Chimaeric IgG1 monoclonal antibody | TNFα | Rheumatoid arthritis |
| In combination with methotrexate for reduction in signs and symptoms, improved physical function in patients with | |||
| (a) Active disease when the response to DMARDs including methotrexate has been inadequate | |||
| (b) Patients with severe, active and progressive RA not previously treated with methotrexate or DMARDs | |||
| Crohn's disease | |||
| (a) Treatment of severe active Crohn's disease, in patients who have not responded despite a full and adequate course of therapy with a steroid and an immunosuppressant, or who are intolerant to or have medical contraindications for such therapy | |||
| (b) Treatment of fistulising, active Crohn's disease in patients who have not responded despite a full and adequate course of therapy with conventional treatment (including antibiotics, drainage and immunosuppression) | |||
| Ankylosing spondylitis | |||
| Treatment of patients with severe axial symptoms, raised serological markers of inflammatory activity and who have responded inadequately to conventional treatment | |||
| Psoriatic arthritis | |||
| In combination with methotrexate for the treatment of active, progressive psoriatic arthritis in patients who have responded inadequately to DMARDs | |||
| Psoriasis | |||
| Treatment of moderate to severe plaque psoriasis in adults who have a contraindication to, or are intolerant to other systemic therapy including ciclosporin, methotrexate or PUVA | |||
| Alefacept (Amevive) | Recombinant fusion protein of human LFA‐3‐IgG1 Fc | CD2 on T lymphocytes | US FDA approval for treatment of adults with moderate to severe chronic plaque psoriasis who are candidates for systemic therapy or phototherapy |
| Does not have EMEA authorisation | |||
| Muromonab—CD3 (Orthoclone) | Murine monoclonal antibody | CD3 glycoprotein on T lymphocytes | US FDA approval for the treatment of acute renal allograft rejection, treatment of acute rejection after heart or liver transplantation in patients resistant to standard steroidal therapy |
| Does not have EMEA authorisation | |||
| Rituximab (MabThera) | Chimaeric IgG1 monoclonal antibody | CD20 | Treatment of patients with stage III–IV follicular lymphoma who are chemo‐resistant or in their second or subsequent relapse after chemotherapy |
| Treatment of previously untreated patients with stage III–IV follicular lymphoma in combination with CVP chemotherapy | |||
| Treatment of patients with CD20‐positive diffuse large B‐cell NHL in combination with CHOP chemotherapy | |||
| Ibritumomab tiuxetan (Zevalin) | Immunoconjugate of murine IgG1 monoclonal antibody conjugated to tiutexan | CD20 | The 90Y‐radiolabelled product is indicated for treatment of adult patients with rituximab relapsed or refractory CD20‐positive follicular B‐cell NHL |
| Tositumomab and 131I tositumomab (Bexxar) | Murine IgG2a monoclonal antibody and 131I radio‐iodinated derivative | CD20 | US FDA approval and EMEA “orphan drug” designation for treatment of patients with CD20‐positive follicular NHL, with and without transformation, whose disease is refractory to rituximab and has relapsed after chemotherapy |
| Basiliximab (Simulect) | Chimaeric IgG1 monoclonal antibody | CD25 (IL2Rα chain) on activated T cells | Prophylaxis of acute organ transplant in de novo allogeneic renal transplantation in adult and paediatric patients. Used with ciclosporin for microemulsion and steroid‐based immunosuppression, in patients with panel reactive antibodies <80%, or in a triple maintenance immunosuppressive regimen containing ciclosporin, steroid and either azathioprine or mycophenolate mofetil |
| Daclizumab (Zenapax) | Recombinant humanised IgG1 monoclonal antibody | CD25 (IL2Rα chain) on activated T cells | Prophylaxis of acute organ rejection in de novo allogeneic renal transplantation in combination with an immunosuppressive regime including ciclosporin and steroids in patients who are not highly immunised |
| Gemtuzumab (Mylotarg) | Humanised monoclonal antibody | CD33 on myeloid cells | US FDA approval and EMEA “orphan drug” designation for treatment of patients with CD33‐positive acute myeloid leukaemia in first relapse who are ⩾60 years and who are not considered candidates for cytotoxic chemotherapy |
| Alemtuzumab (MabCampath) | Recombinant humanised IgG1 monoclonal antibody | CD52 surface glycoprotein on lymphocytes | Treatment of patients with CLL who have been treated with alkylating agents and who have failed to achieve a complete or partial response or achieved only a short remission (<6/12) after fludarabine therapy |
| Efalizumab (Raptiva) | Recombinant humanised IgG1 monoclonal antibody | CD11a (α subunit of LFA‐1) | Treatment of adult patients with moderate to severe chronic plaque psoriasis who have failed to respond to, or who have a contraindication to, or are intolerant of other systemic therapies including ciclosporin, methotrexate and PUVA |
| Natalizumab (Tysabri) | Recombinant humanised IgG4 monoclonal antibody | α4 subunit of the α4β1 and α4β7 integrins | US FDA approval in relapsing multiple sclerosis (see text) |
| Omalizumab (Xolair) | Recombinant humanised IgG1 monoclonal antibody | IgE | Additional therapy to improve asthma control in adult and adolescent patients with severe persistent allergic asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen and who have reduced lung function (FEV1<80%) as well as frequent daytime symptoms or awakenings and who have had multiple documented severe asthma exacerbations despite daily high‐dose inhaled steroid and long‐acting β2‐agonist |
| Cetuximab (Erbitux) | Chimaeric IgG1 monoclonal antibody | EGFR | In combination with irinotecan for the treatment of patients with EGFR expressing metastatic colorectal cancer after failure of irinotecan‐including cytotoxic chemotherapy. US FDA approval March 06 for use in combination with radiotherapy to treat patients with squamous cell carcinoma of the head and neck that cannot be removed surgically and as monotherapy to treat patients with head and neck cancer that has metastasised despite the use of standard chemotherapy |
| Bevacizumab (Avastin) | Recombinant humanised IgG1 monoclonal antibody | VEGF | First‐line treatment for patients with metastatic colorectal carcinoma in combination with intravenous 5‐flourouracil/folinic acid or intravenous 5‐floururacil/folinic acid/irinotecan |
| Trastuzumab (Herceptin) | Recombinant humanised IgG1 monoclonal antibody | HER2 | Treatment of patients with metastatic breast cancer whose tumours overexpress HER2: |
| (a) As monotherapy for those who have received at least two chemotherapy regimes for metastatic disease. Prior chemotherapy must have included at least an anthracycline and a taxane unless patients are unsuitable for these treatments. Hormone receptor‐positive patients must also have failed hormonal therapy unless unsuitable for these treatments. | |||
| (b) In combination with paclitaxel for the treatment of those patients who have not received chemotherapy and for whom an anthracycline is unsuitable | |||
| (c) In combination with docetaxel for those patients who have not received chemotherapy for their metastatic disease | |||
| Abciximab (ReoPro) | Fab fragment of a chimaeric IgG1 monoclonal antibody | Glycoprotein IIb/IIIa receptor of human platelets | As an adjunct to heparin and aspirin for: |
| (1) The prevention of ischaemic cardiac complications in patients undergoing percutaneous coronary intervention | |||
| (2) Short‐term reduction of the risk of myocardial infarction in patients with unstable angina, not responding to full conventional treatment who have been scheduled for percutaneous coronary intervention | |||
| Palivizumab (Synagis) | Recombinant humanised IgG1 monoclonal antibody | The A epitope of the RSV F protein | Prevention of serious lower respiratory tract disease requiring hospitalisation caused by RSV in children at high risk for RSV disease |
| (a) Children born at ⩽35 weeks and <6/12 of age at the onset of the RSV season | |||
| (b) Children <2 years requiring treatment for bronchopulmonary dysplasia within the past 6 months | |||
| (c) Children <2 years with haemodynamically relevant congenital heart disease |
CHOP, cyclophosphamide, doxorubicin, vincristine and prednisone; CLL, chronic lymphocytic leukaemia; DMARDs, disease‐modifying anti‐rheumatic drugs; EGFR, epidermal growth factor receptor; EMEA, European Medicines Agency; FDA, Food and Drug Administration; FEV1, forced expiratory volume in one second; HER2, human epidermal growth factor receptor 2; IL, interleukin; JCA, juvenile chronic arthritis; NHL, non‐Hodgkin's lymphoma; PUVA, Psoralen/ultraviolet A therapy; RA, rheumatoid arthritis; RSV, respiratory syncitial virus; TNF, tumour necrosis factor; VEGF, vascular endothelial growth factor.
TNFα is also thought to have a pathogenic role in anti‐neutrophil cytoplasmic antibody (ANCA)‐associated vasculitis. Treatment with TNFα inhibitors has been reported to be effective in uncontrolled studies.13,14 The Wegener's Granulomatosis Etanercept Trial, evaluating etanercept and standard treatment for Wegener's granulomatosis, reported that etanercept was not effective in maintaining remission in patients with Wegener's granulomatosis, durable remissions were only achieved in a minority and there was a high rate of treatment‐related complications.15 The rate of solid tumours was increased in the etanercept‐treated group. The authors postulated that the combination of TNFα inhibition and cyclophosphamide may heighten the risk of cancer beyond that of cyclophosphamide alone. Etanercept does not have a role in managing active Wegener's granulomatosis with this protocol. Infliximab has been used successfully in ANCA‐associated vasculitis,16 but infectious complications are a concern.
Vasculitis remains an unlicensed indication for this agent, and data from larger trials are required.
Leucocyte surface glycoproteins
CD2
Alefacept, a fusion protein of human leucocyte functional antigen 3–IgG1 Fc, targeting CD2 on T lymphocytes, has US Food and Drug Administration (FDA) approval for the treatment of adults with moderate to severe chronic plaque psoriasis who are candidates for systemic therapy or phototherapy. In a randomised, double‐blind placebo‐controlled trial involving 229 patients, 12 weeks of alefacept treatment was associated with improvement in chronic plaque psoriasis and some patients had a sustained response after cessation of treatment.17 Alefacept does not have European Medicines Agency (EMEA) authorisation.
CD3
Muromonab‐CD3, a murine monoclonal antibody directed against T cell CD3, was approved for treatment of acute renal transplant rejection in 1986. Its licence was extended to acute cardiac or hepatic transplant rejection in patients resistant to standard treatment. Muromonab‐CD3 does not have EMEA authorisation and has been superseded by monoclonal antibodies directed against CD25.
A phase 1–2 study of a humanised anti‐CD3 monoclonal antibody in new‐onset type‐1 diabetes mellitus was reported in 2002.18 In a randomised controlled trial of 24 patients diagnosed with type‐1 diabetes mellitus in the preceding 6 weeks, treatment mitigated the deterioration in insulin production and improved metabolic control during the first year in most patients. A larger study has since reported that short‐term treatment with anti‐CD3 monoclonal antibody preserves residual β‐cell function for at least 18 months in patients with recent‐onset type‐1 diabetes mellitus.19 In view of the frequency of type‐1 diabetes mellitus, further trial data are required to determine the role of monoclonal therapy in this context.
CD20
Rituximab, a chimaeric monoclonal antibody directed against CD20, a B lymphocyte surface glycoprotein, selectively targeting B cells, was shown to be effective in relapsed low‐grade non‐Hodgkin's lymphoma in 1997.20 It is licensed and widely used for treatment of patients with chemoresistant or relapsed stage III–IV follicular lymphoma, treatment of previously untreated patients in combination with chemotherapy and treatment of patients with CD20‐positive diffuse large B cell non‐Hodgkin's lymphoma in combination with cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) chemotherapy. NICE guidance was issued in 2002 and 2003.
Rituximab is of increasing interest in the treatment of disorders in which auto‐antibodies are believed to have a pathogenic role. It has been successful in the treatment of cryoglobulinaemic21,22 and ANCA‐associated vasculitis.23 In patients with active rheumatoid arthritis despite treatment with methotrexate, a single course of two rituximab infusions, alone or in combination with cyclophosphamide or continued methotrexate, provided notable benefit at weeks 24 and 48.24 A total of 100 patients with severe, treatment‐refractory systemic lupus erythematosus treated with rituximab has been reported, suggesting that 80% achieved marked and rapid reductions in global disease activity.25 However, because of the clinical heterogeneity, dosing differences and concomitant treatments, a proper evaluation of the clinical efficacy was difficult, and double‐blind studies comparing rituximab with existing immunosuppression are needed. We have reported benefit of rituximab as adjuvant therapy in treatment‐refractory pemphigus foliaceus.26
Long‐term B cell depletion is a concern. Based on the summary of product characteristic data, the median peripheral B cell count declines below the normal range after completion of the first dose of rituximab. Recovery begins after 6 months, with B cell levels returning to normal between 9 and 12 months after completion of treatment. Obviously, there will be interindividual variability in B cell recovery, confounded by the additional treatment required for the underlying disease.
Radiolabelled monoclonal antibodies, given systemically, can potentially deliver cytotoxic doses of irradiation to sites of disseminated disease, targeted by the specific antigen‐recognition properties. An example is 131I‐tositumomab, which targets CD20 on normal and malignant B cells. This has shown efficacy in patients with relapsed or refractory follicular lymphoma. A single 1‐week course of 131I‐tositumomab as initial treatment was reported in 76 patients with advanced follicular lymphoma. Prolonged clinical and molecular remissions were achieved.27
CD25
Daclizumab and basiliximab, monoclonal antibodies directed against CD25, the α‐subunit of the interleukin 2 (IL2) receptor expressed on activated T lymphocytes, were approved for use in 1997 and 1998, respectively. They prevent ongoing lymphocyte activation and hence T cell immune responses. In a study of 260 patients receiving their first cadaveric renal transplant, daclizumab, in addition to standard immunosuppression, reduced the incidence of biopsy‐confirmed acute rejection from 35% in the placebo group to 22% in the active treatment group; p = 0.03.28 Induction therapy with daclizumab also reduces the incidence and severity of cardiac allograft rejection during the induction period.29 Daclizumab is associated with an increased risk of fatal infection when used with concomitant cytolytic treatment such as muromonab‐CD3.30 In view of the confirmed efficacy, anti‐CD25 monoclonal antibody therapy is being increasingly incorporated into standard post‐transplant immunosuppression.
CD33
The CD33 glycoprotein is expressed on myeloid progenitor cells and monocytes. A humanised monoclonal antibody directed against CD33 has US FDA approval and EMEA “orphan drug” designation for the treatment of patients with CD33‐positive acute myeloid leukaemia in the first relapse who are ⩾60 years and who are not considered candidates for cytotoxic chemotherapy.
CD52
Rheumatoid arthritis was the focus of early application of monoclonal antibody therapy. CD52 is a pan‐lymphocyte surface glycoprotein, and CamPath IH (anti‐CD52), a lymphocyte‐depleting monoclonal antibody that depletes T and B lymphocytes, was investigated for rheumatoid arthritis in the late 1990s.31 It did not penetrate the synovium sufficiently to suppress disease activity without causing severe and protracted peripheral blood lymphopenia. It has been superseded by the introduction of methotrexate and anti‐TNFα biologicals for rheumatoid arthritis.
Alemtuzumab was first approved in 2001 for the treatment of patients with chronic lymphocytic leukaemia who have been treated with an alkylating agent and who have failed to achieve a complete or partial response or achieved only a short remission after treatment with fludarabine. It is used in a variety of protocols for conditioning pre‐bone marrow transplants, although this use remains unlicensed.
Adhesion Molecules
CD11a
Efalizumab, a humanised monoclonal antibody that binds to the α subunit of leucocyte functional antigen‐1, inhibiting T cell activation, has approval for use in adults with moderate to severe chronic plaque psoriasis who have failed to respond to, have a contraindication for or are intolerant of other systemic therapies (table 2). It is associated with remarkable improvements in plaque psoriasis in patients with moderate to severe disease.32 Despite efalizumab having EMEA authorisation, cost is potentially a limiting factor to the use of biologic agents for chronic plaque psoriasis in the UK.
α4 Integrin
Monoclonal antibodies against α4 integrins as “selective adhesion molecule inhibitors”, preventing leucocyte migration into inflamed tissue, have been used in Crohn's disease, ulcerative colitis and multiple sclerosis.33,34,35,36 The International Efficacy of Natalizumab as Active Crohn's Therapy (ENACT 1) and the Evaluation of Natalizumab as Continuous Therapy (ENACT 2) trials found that induction therapy with natalizumab for Crohn's disease resulted in small, unremarkable improvements in response and remission rates. Patients who had a response had increased rates of sustained response and remission if treatment was repeated every 4 weeks; however, the benefit needed to be weighed against the serious adverse events.33 All administrations of natalizumab were voluntarily suspended in early 2005 after three reports of progressive multifocal leucoencephalopathy.37,38,39 However, outcome data after a 2‐year trial of natalizumab in 942 patients with relapsing multiple sclerosis found that natalizumab reduced the risk of sustained progression of disability and the rate of clinical relapse.35 Addition of natalizumab to interferon β‐1a was found to be considerably more efficacious than interferon β‐1a alone in relapsing disease.36 A detailed evaluation of 3116 patients treated with natalizumab suggested a risk of progressive multifocal leucoencephalopathy in the order of 1/1000 patients treated for a mean of 17.9 months.40 The longer‐term risk is obviously unknown. In February 2006, the US FDA confirmed that Biogen‐IDEC could resume administration of natalizumab to patients with relapsing–remitting multiple sclerosis who had previously been treated with the drug in clinical trials.
Costimulatory blockade
Costimulatory signals are important for the activation of naive T cells (fig 2), and down‐regulatory signals are later delivered by CTLA4. Fusion proteins incorporating CTLA4 have been developed to block costimulation and although as yet unlicensed, show promise as an immunomodulatory treatment. Patients with active rheumatoid arthritis receiving methotrexate, when treated with CTLA4–Ig fusion protein showed remarkable improvement in the signs and symptoms of rheumatoid arthritis.41 The modified CTLA4–Ig fusion protein, abatacept, has since been reported to have efficacy in anti‐TNFα treatment‐refractory rheumatoid arthritis.42 Belatacept, a fusion protein derived from abatacept, has shown similar efficacy to ciclosporin as a means of preventing acute rejection after renal transplantation.43
Immunoglobulin E
IgE is the major antibody isotype involved in type‐1 hypersensitivity. A study of 317 patients found that a humanised anti‐IgE monoclonal antibody had potential benefit for patients with moderate to severe allergic asthma. More patients in the active treatment arms were able to decrease or discontinue their use of corticosteroids than in the placebo group.44 Omalizumab, a humanised monoclonal antibody targeting IgE, is now licensed for use in adults and adolescents with severe persistent atopic asthma, whose symptoms are inadequately controlled with inhaled corticosteroids and long‐acting β2‐agonists. It can make possible the reduction or withdrawl of inhaled steroid therapy, and is effective as an adjunct to conventional treatment to reduce asthma exacerbations, but the clinical benefits have to be balanced against the high cost.
Growth factor targets of approved biologic therapy
Epidermal growth factor receptor
Epidermal growth factor receptor is commonly expressed by colorectal cancer cells. Cetuximab, a chimaeric monoclonal antibody specifically blocking epidermal growth factor receptor, is licensed for use in combination with irinotecan in metastatic colorectal malignancy. There is also evidence that cetuximab has clinically relevant activity when given alone or in combination with irinotecan in patients with irinotecan‐refractory colorectal cancer.45
Vascular endothelial growth factor
Vascular endothelial growth factor (VEGF) stimulates the growth of endothelial cells and seems to be central to angiogenesis in tumour growth. Bevacizumab, a monoclonal antibody directed against VEGF, is the other licensed monoclonal for the treatment of metastatic colorectal cancer. It has also been reported to have clinical benefit in metastatic renal cell carcinoma in which mutations in the tumour suppressor gene VHL cause oversecretion of VEGF.46
Human epidermal growth factor receptor 2
Human epidermal growth factor receptor 2 (HER2) is amplified in 25–30% of patients with breast cancer. The encoded protein is then overexpressed in the malignant cells. Women with cancers that overexpress HER2 have aggressive disease with significantly shortened disease‐free survival. Trastuzumab, a humanised monoclonal antibody directed against HER2 increases the clinical benefit of first‐line chemotherapy in metastatic breast cancer that overexpresses HER2,47 and 1 year of treatment with trastuzumab after adjuvant chemotherapy has been shown to improve disease‐free survival among women with HER2‐positive disease.48
Other receptor targets of approved biological therapy
Glycoprotein IIb/IIIa receptor
Platelets have an important role in ischaemic complications after coronary angiography. Abciximab, a chimaeric monoclonal antibody Fab fragment directed against platelet glycoprotein IIb/IIIa receptor was shown to reduce the incidence of acute ischaemic events by 35% among patients undergoing “high‐risk” percutaneous coronary revascularisation in the EPIC Trial.49 However, this was at the expense of increased haemorrhagic complications. The EPILOG Study50 subsequently found that abciximab together with low‐dose, weight‐adjusted heparin markedly reduced the risk of acute ischaemic complications in patients undergoing percutaneous coronary revascularisation. NICE guidance recommends that a glycoprotein IIb/IIIa inhibitor should be considered in the management of unstable angina or non‐ST segment‐elevation myocardial infarction and as an adjunct to percutaneous intervention when early intervention is indicated but delayed in patients with diabetes or if the procedure is complex.
Infectious disease targets of approved biologic therapy
Respiratory syncitial virus F protein
Palivizumab, a humanised monoclonal antibody directed against the A epitope of the F protein of respiratory syncitial virus, is currently the only monoclonal antibody licensed for the prevention of infectious disease. It is indicated for the prevention of respiratory syncitial virus infection in infants at high risk.51
Unlicensed cytokine targets for immune‐based disease
Cytokines have a major role in pathogenesis across a wide disease spectrum. Additional anti‐cytokine therapies are under development and are likely to be important therapeutics in the future. For example, anti‐IL12 monoclonal therapy has been investigated in Crohn's disease and associated with a decrease in T helper cell 1 inflammatory cytokines (IL12, IFNγ, TNFα) at the site of disease,52 and mepolizumab, a humanised monoclonal antibody directed against IL5, has reported efficacy in patients with dermatological manifestations of hypereosinophilic syndrome.53 Efficacy data for these agents are currently based on small patient numbers, and further trial data will be required before consideration can be given to licensing.
Therapeutic considerations
As with other systemic immunomodulatory treatments, there are considerations and warnings that apply to biologic therapy (table 3). For example, tuberculosis has been recognised as a particular risk factor in the use of anti‐TNFα therapies. The British Society for Rheumatology (BSR) established the BSR Biologics Registry in 200054 with the aim of following up both short‐term and long‐term safety issues associated with anti‐TNFα biologic therapy. Registration for etanercept was completed in May 2005. Even when patient registration is complete for national purposes, units using biologic therapies should consider maintaining their own databases for recording and reporting adverse events.
Table 3 Biologicals warnings—consult summary of product characteristics.
| Biological agent | Molecular target | Specific warnings |
|---|---|---|
| Adalimumab | TNFα | Risk of infection, especially TB |
| Patients should be assessed for active/latent TB before treatment. Treatment of latent TB should be initiated before treatment with adalimumab, contraindicated in active TB | ||
| Etanercept | TNFα | Infection |
| Infliximab | TNFα | Risk of infection, especially TB. |
| Patients should be assessed for active/latent TB and treatment should be initiated before treatment with infliximab, contraindicated in active TB | ||
| Invasive fungal and other opportunist infections. | ||
| Hypersensitivity reactions | ||
| Rituximab | CD20 | Infusion reactions |
| Tumour lysis syndrome | ||
| Severe mucocutaneous reactions | ||
| Ibritumomab tiuxetan | CD20 | Fatal infusion reactions |
| Prolonged and severe cytopenias | ||
| Tositumomab and 131I tositumomab | CD20 | Hypersensitivity reactions |
| Prolonged and severe cytopenias | ||
| Basiliximab | CD25 | Only administered by doctors experienced in immunosuppressive treatment and management of organ transplantation. Facilities must be equipped and staffed with adequate laboratory and supportive medical resources |
| Daclizumab | CD25 | Only administered by doctors experienced in immunosuppressive treatment and management of organ transplantation. Facilities must be equipped and staffed with adequate laboratory and supportive medical resources |
| Alemtuzumab | CD52 | Haematological toxicity (ITP alert, November 2005) |
| Infusion reactions | ||
| Infection/opportunist infection | ||
| Cetuximab | EGFR | Infusion reactions |
| Bevacizumab | VEGF | Gastrointestinal perforation |
| Wound‐healing complications | ||
| Haemorrhage | ||
| Trastuzumab | HER2 | Cardiomyopathy especially when given with anthracyclines and cyclophosphamide |
| Palivizumab | RSV F protein | Anaphylaxis (rare) |
CD, cluster of differentiation; EGFR, epidermal growth factor receptor; HER2, human epidermal growth factor receptor 2; RSV, respiratory syncitial virus; TB, tuberculosis; TNF, tumour necrosis factor; VEGF, vascular endothelial growth factor.
The concerns over natalizumab highlight the importance of safety monitoring as the application of biologics extends to phase 2 or 3 clinical trials. Safety issues at an earlier stage of drug development have been focused on by the recent TeGenero TGN1412 trial. This phase 1 trial of a fully humanised CD28 agonistic monoclonal antibody had to be stopped after life‐threatening adverse events occured in all six of the healthy volunteers given the active drug. This not only highlights safety concerns but also the need for careful selection of immune components for modification by biologic therapy. The drug was designed to expand the regulatory T cell population but clearly had a much wider role in immune activation. An investigation by the Medicines and Healthcare Products Regulatory Agency is under way.
An understanding of regulatory and licensing processes of drugs is important for healthcare professionals and patients to avoid confusion surrounding access to drugs.55 Media attention focused on individual cases needs to be carefully assessed—for example, the controversy surrounding access to trastuzumab (Herceptin). There are inevitable (arguably excessive) delays between the publication of trial data, licensing of drugs by the regulatory authorities and the subsequent decisions on health services funding. However, these processes are required to ensure that new medicines meet standards of efficacy and safety that will benefit a population as a whole.
Subsequent to approval, cost is a major factor when broadening the applications of biologic therapy. These agents are not only expensive to develop and subject to the necessary rigours of clinical trials but once approved and licensed are also expensive to use in the clinic. The fact that a treatment has approval does not necessarily translate into unrestricted use. Application to funding agencies may be required; certain indications are explicit concerning treatment‐refractory disease, and NICE guidance has been developed for those biologicals that have potential for widespread use. Despite this, the cost of not treating also has to be considered, and treatment‐refractory disease makes this all the more important.
Application of either a licensed or unlicensed biologic therapy to an unlicensed indication may need to be submitted for approval through the local drugs and therapeutics committee, with appropriate supporting evidence. If approval is granted for an individual case, careful explanation to the patient and explicit patient consent are considered good medical practice (box 1).
Box 1: Summary of important considerations for biologic therapy
Is the underlying disease pathogenesis known?
Is there a biologic therapy that could be appropriately applied?
What is the evidence of benefit?
Has the patient failed conventional treatment?
Is there guidance to help in the decision—eg, NICE?
Is there a suitable alternative?
Is the product licensed in context?
If used for an unlicensed disease:
Is it safe?
Does the patient understand and consent to unlicensed treatment?
What is the cost of
Using the biologic?
Not using the biologic?
What are the selection/registration/monitoring requirements?
Take‐home messages
Specific immune components having a central role in disease pathogenesis lend themselves to targeted therapy.
Biological therapies can theoretically be designed against an unlimited number of such pathogenic components.
The aim of biologic therapy is to modify the disease course when applied early, limit the complications of conventional treatment and provide options for otherwise treatment‐refractory disease.
These agents are costly and not without risk.
Careful patient selection is crucial.
Summary
Biologic therapies have been developed on the basis of a greater understanding of the immune system and disease pathogenesis. Targeting of a specific molecular pathway to influence disease activity is an attractive approach, aiming to limit the side effects commonly associated with conventional immunomodulation. These agents are potentially hazardous and costly, hence careful patient and disease selection are important. The long‐term safety issues with many of these agents is still unknown, and ongoing monitoring is an important part of patient care.
Abbreviations
ACT - Active Ulcerative Colitis Trial
ANCA - anti‐neutrophil cytoplasmic antibody
CDR - complementarity‐determining region
EMEA - European Medicines Agency
FDA - Food and Drug Administration
HER2 - human epidermal growth factor receptor 2
TNF - tumour necrosis factor
VEGF - vascular endothelial growth factor
Footnotes
Competing interests: None.
References
- 1.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975256495–497. [DOI] [PubMed] [Google Scholar]
- 2.Uhr J W. The 1984 Nobel Prize in Medicine. Science 19842261025–1028. [DOI] [PubMed] [Google Scholar]
- 3.Reichert J M. Monoclonal antibodies in the clinic. Nature Biotechnol 200119819–822. [DOI] [PubMed] [Google Scholar]
- 4.Bell S, Kamm M A. Antibodies to tumour necrosis factor α as treatment for Crohn's disease. Lancet 2000355858–860. [DOI] [PubMed] [Google Scholar]
- 5.Present D H, Rutgeerts P, Targan S.et al Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med 19993401398–1405. [DOI] [PubMed] [Google Scholar]
- 6.Sands B E, Anderson F H, Bernstein C N.et al Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med 2004350876–885. [DOI] [PubMed] [Google Scholar]
- 7.Rutgeerts P, Sandborn W J, Faegan B G.et al Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 20053532462–2476. [DOI] [PubMed] [Google Scholar]
- 8.Moreland L W, Baumgartner S W, Schiff M H.et al Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)‐Fc fusion protein. N Engl J Med 1997337141–147. [DOI] [PubMed] [Google Scholar]
- 9.Weinblatt M E, Kremer J M, Bankhurst A D.et al A trial of etanercept, a recombinant tumor necrosis factor receptor: Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med 1999340253–259. [DOI] [PubMed] [Google Scholar]
- 10.Bathon J M, Martin R W, Fleischmann R M.et al A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med 20003431586–1593. [DOI] [PubMed] [Google Scholar]
- 11.Lipsky P E, Van Der Heijde D M F M, St Clair E W.et al Infliximab and methotrexate in the treatment of rheumatoid arthritis. N Engl J Med 20003431594–1602. [DOI] [PubMed] [Google Scholar]
- 12.National Institute for Clinical Excellence Guidance on the use of etanercept and infliximab for the treatment of rheumatoid arthritis. Technology appraisal guidance number 36. London: National Institute for Clinical Excellence, 2002
- 13.Lamprecht P, Voswinkel J, Lilienthal T.et al Effectiveness of TNFα blockade with infliximab in refractory Wegener's granulomatosis. Rheumatology 2002411303–1307. [DOI] [PubMed] [Google Scholar]
- 14.Stone J H, Uhlfelder M L, Hellmann D B.et al Etanercept combined with conventional treatment in Wegener's granulomatosis: a six‐month open‐label trial to evaluate safety. Arthritis Rheum 2001441149–1154. [DOI] [PubMed] [Google Scholar]
- 15.The Wegener's Granulomatosis Etanercept Trial (WGET) Research Group Etanercept plus standard therapy for Wegener's granulomatosis. N Engl J Med 2005352351–361. [DOI] [PubMed] [Google Scholar]
- 16.Booth A, Harper L, Hammad T.et al Prospective study of TNFα blockade with infliximab in anti‐neutrophil cytoplasmic antibody‐associated systemic vascultis. J Am Soc Nephrol 200415717–721. [DOI] [PubMed] [Google Scholar]
- 17.Ellis C N, Krueger G G, for the Alefacept Clinical Study Group Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. N Engl J Med 2001345248–255. [DOI] [PubMed] [Google Scholar]
- 18.Herold K C, Hagopian W, Auger J A.et al Anti‐CD3 monoclonal antibody in new‐onset type 1 diabetes mellitus. N Engl J Med 20023461692–1698. [DOI] [PubMed] [Google Scholar]
- 19.Keymeulen B, Vandemeulebroucke E, Ziegler A G.et al Insulin needs after CD3‐antibody therapy in new‐onset type 1 diabetes. N Engl J Med 20053522598–2608. [DOI] [PubMed] [Google Scholar]
- 20.Maloney D G, Grillo‐López A J, White C A.et al IDEC‐C2B8 (rituximab) anti‐CD20 monoclonal antibody therapy in patients with relapsed low‐grade non‐Hodgkin's lymphoma. Blood 1997902188–2195. [PubMed] [Google Scholar]
- 21.Zaja F, De Vita S, Russo D.et al Rituximab for the treatment of type II mixed cryoglobulinaemia. Arthritis Rheum 2002462252–2254. [DOI] [PubMed] [Google Scholar]
- 22.Ferri C, Mascia M T. Cryoglobulinemic vasculitis. Curr Opin Rheumatol 20061854–63. [DOI] [PubMed] [Google Scholar]
- 23.Buhaescu I, Covic A, Levy J. Systemic vasculitis: still a challenging disease. Am J Kidney Dis 200546173–185. [DOI] [PubMed] [Google Scholar]
- 24.Edwards J C W, Szczepański L, Szechiński J.et al Efficacy of B‐cell‐targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 20043502572–2581. [DOI] [PubMed] [Google Scholar]
- 25.Sfikakis P P, Boletis J N, Tsokos G C. Rituximab anti‐B‐cell therapy in systemic lupus erythematosus: pointing to the future. Curr Opin Rheumatol 200517550–557. [DOI] [PubMed] [Google Scholar]
- 26.Johnston S, Kennedy C. Pemhigus foliaceus. N Engl J Med 20053532589. [DOI] [PubMed] [Google Scholar]
- 27.Kaminski M S, Tuck M, Estes J.et al131I‐Tositumomab therapy as initial treatment for follicular lymphoma. N Engl J Med 2005352441–449. [DOI] [PubMed] [Google Scholar]
- 28.Vincenti F, Kirkman R, Light S.et al Interleukin‐2‐receptor blockade with daclizumab to prevent acute rejection in renal transplantation. N Engl J Med 1998338161–165. [DOI] [PubMed] [Google Scholar]
- 29.Beniaminovitz A, Itescu S, Lietz K.et al Prevention of rejection in cardiac transplantation by blockade of the interleukin‐2‐receptor with a monoclonal antibody. N Engl J Med 2000342613–619. [DOI] [PubMed] [Google Scholar]
- 30.Hershberger R E, Starling R C, Eisen H J.et al Daclizumab to prevent rejection after cardiac transplantation. N Engl J Med 20053522705–2713. [DOI] [PubMed] [Google Scholar]
- 31.Isaacs J D, Manna V K, Rapson N.et al Campath‐1H in rheumatoid arthritis—an intravenous dose‐ranging study. Br J Rheumatol 199635231–240. [DOI] [PubMed] [Google Scholar]
- 32.Lebwohl M, Tyring S K, Hamilton T K.et al A novel targeted T‐cell modulator, efalizumab, for plaque psoriasis. N Engl J Med 20033492004–2013. [DOI] [PubMed] [Google Scholar]
- 33.Sandborn W J, Colombel J F, Enns R.et al Natalizumab induction and maintenance therapy for Crohn's disease. N Engl J Med 20053531912–1925. [DOI] [PubMed] [Google Scholar]
- 34.Faegen B G, Greenberg G R, Wild G.et al Treatment of ulcerative colitis with a humanized antibody to the α4β7 integrin. N Engl J Med 20053522499–2507. [DOI] [PubMed] [Google Scholar]
- 35.Polman C H, O'Connor P W, Havrdova E.et al A randomized, placebo‐controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006354899–910. [DOI] [PubMed] [Google Scholar]
- 36.Rudick R A, Stuart W H, Calabresi P A.et al Natalizumab plus interferon beta‐1a for relapsing multiple sclerosis. N Engl J Med 2006354911–923. [DOI] [PubMed] [Google Scholar]
- 37.Van Assche G, Van Ranst M, Sciot R.et al Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. N Engl J Med 2005353362–368. [DOI] [PubMed] [Google Scholar]
- 38.Langer‐Gould A, Atlas S W, Green A J.et al Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med 2005353375–381. [DOI] [PubMed] [Google Scholar]
- 39.Kleinschmidt‐DeMasters B K, Tyler K L. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta‐1a for multiple sclerosis. N Engl J Med 2005353369–374. [DOI] [PubMed] [Google Scholar]
- 40.Yousry T A, Major E O, Ryschkewitsch C.et al Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med 2006354924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kremer J M, Westhovens R, Leon M.et al Treatment of rheumatoid arthritis by selective inhibition of T‐cell activation with fusion protein CTLA4Ig. N Engl J Med 20033491907–1915. [DOI] [PubMed] [Google Scholar]
- 42.Genovese M C, Becker J C, Schiff M.et al Abatacept for rheumatoid arthritis refractory to tumor necrosis factor α inhibition. N Engl J Med 20053531114–1123. [DOI] [PubMed] [Google Scholar]
- 43.Vincenti F, Larsen C, Durrbach A.et al Costimulation blockade with belatacept in renal transplantation. N Engl J Med 2005353770–781. [DOI] [PubMed] [Google Scholar]
- 44.Milgrom H, Fick R B, Su J Q.et al Treatment of allergic asthma with monoclonal anti‐IgE antibody. N Engl J Med 19993411966–1973. [DOI] [PubMed] [Google Scholar]
- 45.Cunningham D, Humblet Y, Siena S.et al Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan‐refractory metastatic colorectal cancer. N Engl J Med 2004351337–345. [DOI] [PubMed] [Google Scholar]
- 46.Yang J C, Haworth L, Sherry R M.et al A randomized trial of bevacizumab, an anti‐vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 2003349427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slamon D J, Leyland‐Jones B, Shak S.et al Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001344783–792. [DOI] [PubMed] [Google Scholar]
- 48.Piccart‐Gebhart M J, Procter M, Leyland‐Jones B.et al Trastuzumab after adjuvant chemotherapy in HER2‐positive breast cancer. N Engl J Med 20053531659–1672. [DOI] [PubMed] [Google Scholar]
- 49.The EPIC Investigators Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high‐risk coronary angioplasty. N Engl J Med 1994330956–961. [DOI] [PubMed] [Google Scholar]
- 50.The EPILOG Investigators Platelet glycoprotein IIb/IIIa receptor blockade and low‐dose heparin during percutaneous coronary revascularization. N Engl J Med 19973361689–1696. [DOI] [PubMed] [Google Scholar]
- 51.The IMpact‐RSV Study Group Palivizumab, a humanized respiratory syncitial virus monoclonal antibody, reduces hospitalization from respiratory syncitial virus infection in high‐risk infants. Pediatrics 1998102531–537. [PubMed] [Google Scholar]
- 52.Mannon P J, Fuss I J, Mayer L.et al Anti‐interleukin‐12 antibody for active Crohn's disease. N Engl J Med 20043512069–2079. [DOI] [PubMed] [Google Scholar]
- 53.Plötz S G, Simon H U, Darsow U.et al Use of an anti‐interleukin‐5 antibody in the hypereosinophilic syndrome with eosinophilic dermatitis. N Engl J Med 20033492334–2339. [DOI] [PubMed] [Google Scholar]
- 54.Griffiths I, Silman A, Symmons D.et al BSR biologics registry. Rheumatology 2004431463–1464. [DOI] [PubMed] [Google Scholar]
- 55.Mayor S. The public needs better understanding of drug regulation. BMJ 2006332990. [DOI] [PMC free article] [PubMed] [Google Scholar]