Abstract
Gastrocystoplasty is a form of surgical bladder augmentation or neobladder used to restore bladder capacity and compliance in children and in patients with neurogenic bladder. Other forms of bladder augmentation include ileocystoplasty and colocystoplasty. Reported complications of gastrocystoplasty include post‐operative bleeding, haematuria, stricture, metabolic alkalosis and rupture of the gastric segment. There are reports of adenocarcinomas arising in the setting of ileocystoplasty and colocystoplasty. However, the first case of adenocarcinoma arising in the setting of a gastrocystoplasty is reported.
Gastrocystoplasty is an uncommon form of bladder augmentation (neobladder), where portions of the stomach are surgically attached to the urinary bladder to increase bladder capacity and compliance. Other forms of bladder augmentation include ileocystoplasty and colocystoplasty. Gastrocystoplasty is commonly carried out in children and in adults with neurogenic bladder. Reported complications include postoperative bleeding, rupture of the gastric segment, haematuria, stricture and metabolic alkalosis.1 Although there are reports of carcinomas arising in other forms of neobladders,2 we report the first case of adenocarcinoma arising in the gastric segment of a gastrocystoplasty.
Case history
A 64‐year‐old man with a history of spina bifida and right‐sided weakness underwent a gastrocystoplasty for neurogenic bladder in 1991. Approximately 10 years after the surgery, he developed haematuria, which was investigated with cystoscopy and biopsy. The biopsy specimen showed moderately differentiated adenocarcinoma arising in the gastric mucosa. The patient underwent a cystoprostatectomy that showed an infiltrative (3 cm) moderately to poorly differentiated adenocarcinoma arising from the gastric portion of the gastrocystoplasty. Since the surgery, the patient has had multiple metastases to the perineal area and is currently undergoing palliative chemotherapy
Materials and methods
The cystoprostatectomy specimen was fixed in 10% neutral buffered formalin and processed to paraffin‐wax blocks in a routine fashion. The tissue sections prepared from these blocks were stained with haematoxylin and eosin for histological examination. The gastric portions of the bladder were stained with the Genta stain (combined argyrophil/alcian blue method) for Helicobacter pylori.3 Representative sections of the adenocarcinoma were also stained with antibodies to Cdx‐2, villin, p63, high‐molecular‐weight cytokeratin (clone 34bE12), cytokeratin 5/6, cytokeratin 7 and cytokeratin 20, using a modified avidin–biotin peroxidase technique after microwave antigen retrieval in 0.01 M citrate buffer (pH 6.0).
Results
Gross findings
The surgical specimen consisted of a cystoprostatectomy specimen with the neobladder measuring 10.5×6.5×4.2 cm and the prostate measuring 3.5×3.5×2.2 cm. Both gastric and urothelial mucosa were present on the mucosal surface of the bladder. At the gastric portion of the neobladder, a partially ulcerated and exophytic mass measuring 3.0×2.7 cm was present. Sectioning of the mass showed infiltration into the muscularis propria and extension to the inked resection margin.
Microscopic features
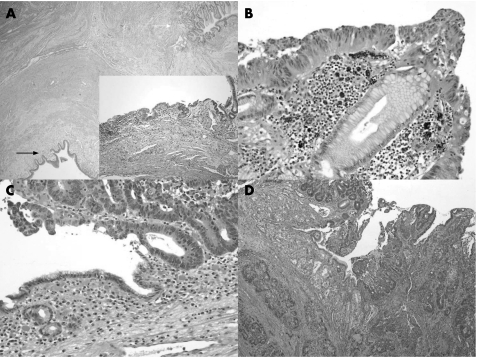
Histological sections showed both urothelial and gastric mucosa and their junction (fig 1A). The tumour showed an exophytic, moderately to poorly differentiated adenocarcinoma (fig 1C,D) arising in low‐grade (fig 1A inlet) and high‐grade (fig 1B) dysplastic gastric epithelium. These sections confirmed the gross impression that the tumour infiltrated into muscularis propria with extension to the inked margin. Lymphovascular space and perineural invasion were identified. The prostate gland was not involved. In addition, patchy areas of chronic gastritis without intestinal metaplasia were identified. There was no active inflammation. Genta stain was negative for H pylori. The urothelial epithelium exhibited both acute and chronic inflammation, but there was no evidence of dysplasia. To further characterise the pattern of epithelial differentiation in this neoplasm, we conducted a limited panel of immunohistochemical staining. The neoplastic cells were strongly positive for Cdx‐2 and villin (the villin stained with a focal brush‐border pattern), focally positive for keratin 20 and high‐molecular‐weight keratin, but negative for keratin 7, p63, and keratin 5/6. These results suggest an enteric pattern of differentiation in this neoplasm.
Figure 1 (A) Low magnification of gastrocystoplasty showing both the urothelial (black arrow) and gastric mucosa (white arrow). Haematoxylin and eosin, original magnification ×20. Inset: Low‐grade dysplasia arising in the gastric mucosa. Haematoxylin and eosin, original magnification ×100. (B,C) High‐grade dysplasia and intramucosal adenocarcinoma arising in the gastric mucosa. Haematoxylin and eosin, original magnification ×200. (D) Invasive adenocarcinoma infiltrates the gastric mucosa and muscularis propria of the neobladder. Haematoxylin and eosin, original magnification ×40.
Discussion
Epithelial malignancies are a commonly documented, late complication of bladder augmentation procedures such as ileocystoplasties and colocystoplasties. In these cases, the overall incidence of tumour development is 1.5%; the carcinomas develop in an average of 18 years after augmentation surgery.2 These tumours usually occur on the enteric side of the anastomosis or at the enterovesicle junction. All such lesions have either been adenocarcinomas or urothelial carcinomas.
In this study, we have described the first case of adenocarcinoma arising in the gastric portion of a gastrocystoplasty. The English literature contains only one other case report of a malignancy arising in a gastrocystoplasty,4 in which a 73‐year‐old woman developed transitional cell carcinoma 14 years after a gastrocystoplasty for a neurogenic bladder. The carcinoma was present in the distal gastric segment of the gastrocystoplasty, adjacent to the surgical anastomosis, with extension into the native bladder. Our patient is unique in that the malignancy developed in the gastric segment of the neobladder and showed an adenocarcinoma with gastrointestinal phenotype.
Retrospective and prospective studies of gastrocystoplasties in animal models have shown varying degrees of active and chronic inflammation, metaplasia and dysplasia, as well as both benign and malignant neoplasms. The neoplasms have been located in the gastric, vesicle and anastomotic sites with a predilection for the anastomotic area.5,6,7 Multiple theories have been put forth for the development of neoplasms in the setting of both clinical and experimental gastrocystoplasty, including chronic bladder irritation, urinary‐tract infection (including the formation of carcinogens such as nitrosamines by bacterial catalysis) and the induction of tissue proliferation by the apposition of tissues of different origin.2,7 Common to many of the hypotheses is a chronic inflammatory background, on which there is a development of metaplasia or dysplasia, and malignancy. The scenario of chronic inflammation leading to metaplasia or dysplasia, and development of a malignancy is, of course, not unique to this surgical setting; indeed, it is typical of the relationship between underlying disease and neoplastic transformation in gastro‐oesophageal reflux disease, chronic gastritis and ulcerative colitis.8,9
Long‐term studies on gastrocystoplasties in the paediatric and adult populations have shown atrophic gastric mucosa, acute and chronic inflammation, and metaplasia, but no report of malignancy.10,11 The absence of dysplasia or malignancy in these studies can be attributed to the relatively short follow‐up time (range of 4–10 years) as well as the relatively young mean age of the patient at the time of gastrocystoplasty (adults, 39.5 years; children, 13 years). These two studies also showed a low level of bladder infections and permanent bacterial colonisation, two factors (as noted earlier) that have been implicated as potential risk factors for the development of malignancies in neobladders.2,7
The role of H pylori in the development of gastric adenocarcinoma is well established.12 However, in the setting of gastrocystoplasty, the carcinogenic potential of H pylori has not been explored. Case reports in the paediatric literature have documented the existence of H pylori in the gastric biopsy specimens of the neobladder, as well as positive serology for this organism. Subsequent biopsy specimens from children have shown various degrees of chronic gastritis, chronic active gastritis and the development of ulcers in the adjacent bladder mucosa.13,14,15 In this report, adenocarcinoma was identified in the setting of chronic gastritis, but the ulcers were not accompanied by identifiable H pylori organisms. This could be due to previous use of antibiotics or to the lack of colonisation of the stomach in this patient. In either case, it is notable that the process of neoplastic transformation did not appear to include a passage through a phase of intestinal metaplasia, although dysplastic gastric epithelium clearly flanked and was admixed with malignant elements.
Although the lesion in this case is unequivocally adenocarcinoma in type, it is not possible to determine with certainty, on morphological or immunohistochemical grounds, that the lesion exhibits features of gastric (to the exclusion of urothelial) mucosal differentiation. Adenocarcinoma of the urinary bladder is virtually indistinguishable from similar tumours arising in the gastrointestinal tract, especially those arising in colonic mucosal or intestinal metaplasia. Immunohistochemical analysis in this case clearly identified an intestinal pattern of differentiation, including immunoreactivity for keratin 20 and Cdx‐2, with a brush border pattern of reactivity for villin, whereas keratin 7, CK5/6 and p63 were lacking. Keratin 7, 5/6 and p63 argue effectively against urothelial differentiation, as urothelial carcinomas typically coexpress keratins 7 and 20, lack Cdx‐2 and show elements of a phenotype that is shared with squamous neoplasia (keratin 5/6 and p63). However, adenocarcinoma arising in urothelium may express an intestinal phenotype as well, including immunoreactivity for Cdx‐2 and villin. Hence, the putative gastric nature of this neoplasm is based primarily on its location and the coexistence of dysplasia in gastric mucosa.
In conclusion, we report the first case of an adenocarcinoma arising in a gastrocystoplasty. Similar to neoplasms arising in other augmentation cystoplasties, this adenocarcinoma arose in disordered gastric mucosa and affected the anastomotic site, developing as a long‐term complication. Chronic inflammation secondary to infection or some form of irritation is known to predispose to malignancy in bladder augmentations and probably influences the development of malignancies in the gastrocystoplasty. Patients with gastrocystoplasty must be followed up in the long term keeping in mind the possibility of development of malignant disease in mind.
Footnotes
Competing interests: None declared.
References
- 1.Mingin G, Stock J A. Gastrocystoplasty: long‐term complications in 22 patients. J Urol 19991621122–1125. [DOI] [PubMed] [Google Scholar]
- 2.Filmer R B, Spencer J R. Malignancies in bladder augmentations and intestinal conduits. J Urol 1990143671–678. [DOI] [PubMed] [Google Scholar]
- 3.Genta R M, Robason G O, Graham D Y. Simultaneous visualization of Helicobacter pylori and gastric morphology: a new stain. Hum Pathol 199425221–226. [DOI] [PubMed] [Google Scholar]
- 4.Qiu H, Kordunskaya S, Yantiss R. Transitional cell carcinoma arising in the gastric remnant following gastrocystoplasty. Int J Surg Pathol 200311143–147. [DOI] [PubMed] [Google Scholar]
- 5.Buson H, Diaz D C, Manivel C. The development of tumors in experimental gastroenterocystoplasty. J Urol 1993150730–733. [DOI] [PubMed] [Google Scholar]
- 6.Klee L, Hoover D M, Mitchell M E. Long‐term effects of gastrocystoplasty in rats. J Urol 19901441283–1287. [DOI] [PubMed] [Google Scholar]
- 7.Little J S, Klee L W. Long‐term histopathological changes observed in rats subjected to augmentation cystoplasty. J Urol 1994152720–724. [DOI] [PubMed] [Google Scholar]
- 8.Haggitt R C. Barrett's esophagus, dysplasia, and adenocarcinoma. Hum Pathol 199425982–993. [DOI] [PubMed] [Google Scholar]
- 9.Riddell R H, Goldman H. Ransohoff DF, et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications, Hum Pathol 198314931–936. [DOI] [PubMed] [Google Scholar]
- 10.Ngan J H K, Lau J, Lim S. Long‐term results of antral gastrocystoplasty. J Urol 1993149731–734. [DOI] [PubMed] [Google Scholar]
- 11.Vajda P, Kaiser L. Histological findings after colocystoplasty and gastrocystoplasty. J Urol 2002168698–701. [PubMed] [Google Scholar]
- 12.Forman D, Newell D G, Fullerton F.et al Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ 19913021302–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Celayir S, Goksel S.Helicobacter pylori infection in a child with gastric augmentation. J Pediatr Surg 1997321757–1758. [DOI] [PubMed] [Google Scholar]
- 14.Celayir S, Goksel S. The relationship between Helicobacter pylori infection and acid‐hematuria syndrome in pediatric patients with gastric augmentation II. J Pediatr Surg 199934532–535. [DOI] [PubMed] [Google Scholar]
- 15.Muraishi O, Ogawa A. Bladder peptic ulcer after gastrocystoplasty for radiation cystitis. J Urol 1994152473–474. [DOI] [PubMed] [Google Scholar]